Abstract
It is estimated from twin studies that heritable factors account for at-least half of asthma-risk, of which genetic variants identified through population studies explain only a small fraction. Multi-generation large families with high asthma prevalence can serve as a model to identify highly penetrant genetic variants in closely related individuals that are missed by population studies. To achieve this, a four-generation Indian family with asthma was identified and recruited for examination and genetic testing. Twenty subjects representing all generations were selected for whole genome genotyping, of which eight were subjected to exome sequencing. Non-synonymous and deleterious variants, segregating with the affected individuals, were identified by exome sequencing. A prioritized deleterious missense common variant in the olfactory receptor gene OR2AG2 that segregated with a risk haplotype in asthma, was validated in an asthma cohort of different ethnicity. Phenotypic tests were conducted to verify expected deficits in terms of reduced ability to sense odors. Pathway-level relevance to asthma biology was tested in model systems and unrelated human lung samples. Our study suggests that OR2AG2 and other olfactory receptors may contribute to asthma pathophysiology. Genetic studies on large families of interest can lead to efficient discovery.
Similar content being viewed by others
Introduction
Asthma is a chronic disorder of the airways caused by a strong genetic predisposition, in addition to environmental insults1. Previous genetic studies in asthma such as linkage analysis, twin studies, candidate gene approaches and GWAS (Genome Wide Association Studies), have identified numerous candidate genes and variants for asthma, but all the associated variants can explain less than 15% of the genetic risk2. Thus, a large piece of the puzzle is missing. The gap may be reduced by gaining information of genetic variants that are missed by the population studies, but may have strong effects in closely related individuals that share the variants. Discovery of such highly penetrant variants associated with asthma-risk is more efficient through the study of closely related individuals, such as multi-generation large families, rather than population based studies. Next generation sequencing has shown a promise in identifying novel targets which were previously missed by the candidate gene based approaches3. Exome sequencing i.e. sequencing the coding regions of the genome in a familial set up has proven to be highly informative in the context of other complex diseases such as cardiovascular diseases and Alzheimer’s disease4,5
Earlier, in Indian population, using candidate gene and genome wide candidate gene approaches, several genes including those for cytokines, FcεR1, STAT6 and INPP4A were found to be associated with asthma6,7. In India, there exists a rich reservoir of large families owing to endogamy and the concept of compound families. Also, cases of consanguinity and inter-clan marriages provide an excellent ground for well-designed genetic studies. Since asthma is a complex and heterogeneous disorder, we hypothesized that a study design that can enable identification of highly penetrating genetic variants could be achieved by studying large families with multiple generations affected with asthma. For this, a subset of subjects belonging to a four generation family from southern India with clinical history of asthma and atopy over four generations were selected for exome sequencing. In addition to this, all available samples from the family were subjected to whole genome genotyping for linkage and haplotype analysis.
Post bioinformatics analysis of exome sequencing and sequencing validation, a genetic variant from one candidate gene OR2AG2, encoding an olfactory receptor, was found in our multi-generational family to be novel in asthma. Literature suggests the expression of olfactory receptors on the airways similar to bitter taste receptors, and having roles in mucociliary clearance on activating stimuli by strong odor8. Studies in recent past, including a GWAS in a Korean population reported significant importance of olfactory receptors with respect to lung function9. We hypothesized that defect in sensing and clearance by these receptors may trigger exacerbations, which is a commonly observed phenomenon in asthmatics in presence of strong/volatile odor. Olfactory receptors having a pertinent role in the human lungs is a relatively new concept. We, therefore, explored the putative role of olfactory receptor gene, OR2AG2 in the context of allergic asthma using in vitro systems and human lung samples.
Results
Exome sequencing and analysis pipeline
A four generation pedigree was constructed with information provided by the family (Fig. 1). Clinical details of participating subjects are provided in Table 1. Eight samples were subjected to exome sequencing using the Illumina platform. A couple with consanguinity along with a distant member of the family were chosen for sequencing since their shared genome will be less, thus providing higher chances of finding variants that truly segregate with the disease.
Pedigree and sample information of family 1. Family 1 is a four generation family with approximately 40% individuals affected with asthma and atopy. Samples collected are marked with * symbol. Black square/circle denotes affected members while clear square/circle denotes unaffected subjects. No clear inheritance pattern could be identified from the pedigree. Interestingly, this family also has cases of consanguinity (depicted by double-lines=). Eight subjects- II:5, III:7, III:9, III:10, IV:31, IV:34, V:27, V:42 (five cases and three controls) were selected for Exome Sequencing.
Data obtained after sequencing was subjected to an analysis pipeline (Supplementary Fig. E1A). The average coverage of sequencing was ≈50X. A total of 115463 variants were identified, post annotation. We aimed at identifying variants that segregated with only affected subjects and were absent in unaffected subjects. For this, a model free approach was used, since asthma is a complex disease and the pedigree showed a multifarious mode of disease inheritance. 910 variants were seen to segregate with the affected members of the family. These variants were further subjected to prioritization, before technical validation.
Variant prioritization strategies
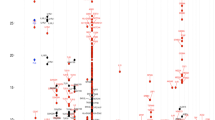
To understand the possible implication of all the identified segregating variants, the following parameters were used: 1. In-silico prediction tools (explained in Supplementary and Supplementary Fig. E1B) were exploited to understand the possible implication of the variants in human physiology and disease, and the ones predicted to have damaging consequences were considered. 2. Genetic variants from genes unreported in asthma (novel) that co-segregated with the disease were given priority. The idea to focus on these variants was in concordance with our initial hypothesis that a fraction of missing heritability for asthma is attributable to deleterious and highly penetrating genetic variants that are more likely to be identified in a large family based set-up. In-silico predicted deleterious variants that segregated only with asthma cases and belonged to novel genes of asthma were prioritized and validated using Sanger sequencing. Sanger sequencing based confirmation was conducted on all available samples from Family 1. Indels were confirmed by Sanger sequencing of the region surrounding the variant of interest, while SNPs were validated by SNaPshot sequencing. Fig. 2 shows the list of variants from 18 genes selected for Sanger sequencing and the final list of confirmed variants that co-segregated with the disease. GWAS database identified 5 of these variants in previously published asthma and lung reports (Details are provided in Supplementary Text and Table E3).
List of variants for validation of Family 1. The figure shows the list of novel variants (left) that were subjected to validation in additional members from family 1 to filter out false positives from exome sequencing, using Sanger (in italics) and SNaPshot sequencing reactions, (MAF: Minor allele frequency). The list of validated variants is shown in right.
Of the confirmed variants, one common variant in the gene OR2AG2, rs10839616, (NM_001004490.1:c.161 G > C) was found to belong to a risk haplotype that co-segregated with all the affected members of the large family, as observed by whole genome genotyping of all the subjects of the family (Supplementary Fig. E3). In addition to this, whole genome genotyping study was also performed on an ongoing cohort of 141 pediatric-asthma cases with 130 controls for which we had clinical data (Tables 2 and 3). This information was then used to check if any of the disease co-segregating variants were associated with asthma in the general population. In line with the idea that missing variants may have small effects at a population level, lost during statistical corrections in small underpowered studies, we used unadjusted associations for the intersection. Out of the six disease co-segregating variants, the OR2AG2 genetic variant rs10839616 was found to be significantly associated with asthma (OR = 1.579, p = 0.0132; MAF = 0.42). The variant rs10839616 was also observed in a previously reported GWAS on pulmonary function parameters (Supplementary Table E3).
Supplementary Fig. E4A shows the impact of OR2AG2 genetic variation on the protein sequence of OR2AG2. Multiple sequence alignment depicts conservation of the wild type amino acid across different species of non-human primates suggesting the evolutionary importance of the variant of interest (Fig. E4B). Furthermore, in silico prediction suggests possible loss of function of the OR2AG2 gene in the affected subjects carrying the genetic variant, rs10839616 (c.161 G > C) (Fig. E4C).
Phenotypic correlation of genotypic findings
To determine whether OR2AG2 genetic variations in the subjects were associated with altered olfactory function or other asthma-related phenotypes, we conducted subjective and objective testing. Affected and unaffected members of the family were subjected to olfactory identification and threshold tests using different concentrations of 2-phenylethyl alcohol (PEA) for sweet odor10,11. While the ability to identify and differentiate between the different odors were found to be uncompromised between the cases and controls of Family 1 (data not shown), subjects with asthma were able to detect some odors only at higher concentrations, indicating olfactory dysfunction (Fig. 3A). The affected family members also self-reported a diminished olfactory capacity on direct questioning. Control subjects from the family however showed no such olfactory dysfunction. This finding suggested relative functional hyposmia in patients as compared to the control subjects from the same family.
Threshold tests for phenotypic correlation in Family 1, OR2AG2 levels in human lung samples and in-vitro epithelial and fibroblast cells, with or without induction with IL-13. (A) Plot showing significant decrease in mean score for odor threshold in subjects with asthma as compared to control subjects from the same family when different concentration of 2-phenylethyl alcohol (PEA) was tested (sweet odor); n = 6 control, n = 9 asthmatic subjects. (B) The bar graph represents relative mRNA expression of OR2AG2 in normal human subjects (control) and asthmatic patients (Asthma), using Real time-PCR., n = 6 asthmatics and n = 10 normal subjects. (C) The representative western blot showing a decrease in the protein level of OR2AG2 in human alveolar epithelial cell line (A549), induced with rIL-13 for 24 hours. (D) The representative western blot showing decrease in the protein level of OR2AG2 in human lung fibroblast cells (HFL1), induced with rIL-13 for 24 hours. (E,F) Densitometric quantification of the experiment performed in (C,D) respectively, values are normalized to α-tubulin levels. All results are expressed as the mean ± SEM. *P < 0.05 and **P < 0.01 (t-test).
However, we could not clearly ascertain whether the differences were due to asthma or the genotype. In subjects from the pediatric asthma cohort, for whom genotypes were known, there was a general reduction of olfactory performance comparable to asthmatics from the large family, but there was no significant difference related to genotype. The asthma phenotypes of the three groups were also broadly similar, with no clinically important differences being noted in lung function or other relevant parameters (Table 3, Fig. 4). As an alternate explanation of similarity between asthmatic subjects with or without OR2AG2 variants, we hypothesized that OR2AG2 may be suppressed during molecular pathogenesis of asthma. The hypothesis that asthma may be associated with decline in OR2AG2 was therefore explicitly tested in human samples and in-vitro experimental set up with IL-13 induction.
Temporal changes in lung function parameters, exhaled NO and Frequency of exacerbation amongst different genotype asthmatic subjects from the pediatric cohort. (A) Line plots showing average percentage of predicted values of different lung function tests for different genotypes (CC, CG and GG) over a period of fifteen follow-ups from baseline amongst asthmatic subjects. (B) Line plot showing actual/adjusted exhaled nitric oxide levels in ppb over a period of fifteen follow-ups amongst asthmatic subjects, grouped according to their genotypes. (C) Line plot showing actual/adjusted frequency of exacerbation over a period of fifteen follow-ups amongst asthmatic subjects, grouped according to their genotypes.
OR2AG2 transcripts are significantly lower in lungs of asthmatics and are inhibited by IL-13
To validate our hypothesis, we compared the levels of OR2AG2 in RNA from lung lysates of asthma patients and normal subjects. OR2AG2 enrichment in lung tissues or resident cells has not been reported in previous studies on olfactory receptors12,13,14. However, in our study, a significant decrease in the level of OR2AG2 was seen across asthmatics at the RNA level, as measured by real time PCR (Fig. 3B). As expected from the MAF of the variant, the presence of natural variation in the control dataset can be observed from low level of OR2AG2 in few samples. Asthmatics, on the other hand show consistent decline in the level of OR2AG2. We next examined the possibility that OR2AG2 is downstream of established asthma-related molecular pathways in cell culture studies. Treatment with (recombinant) IL13, a prototype cytokine for asthma15,16, led to suppression of OR2AG2 in human lung cells, as shown in Fig. 3(C–F). Together the data supported the hypothesis that OR2AG2 may be a convergence point for asthma pathways at lung level.
Discussion
Asthma, being a well-known heritable complex disease, has been a target for genomic studies for decades. However, focussing on variants of high importance in families, but not frequent enough to explain population level asthma risk, may help in better understanding asthma genetics.
To achieve the same, a large family with four generations affected with asthma was selected and subjected to exome sequencing and whole genome genotyping. Of the validated disease co-segregating genetic variants, one of the variants in gene OR2AG2 (c.161 G > C, rs10839616) belonging to the family of olfactory receptors, was chosen for further validation for three reasons: 1) it showed significant association with asthma in a small asthma case-control cohort from a different population signifying relevance in general population, 2) patients from the family self-reported inability to sense odors and fumes, and 3) the possibility of the gene’s novel role in asthma biology. This was particularly interesting since the genetic variant was found to be a part of a risk haplotype that segregated with all the affected members of the family in addition to being significantly associated in a separate study on pediatric cohort of asthma (unpublished data from an ongoing project). Our observation that the genetic variant identified from a familial set-up was found to be associated with asthma in a cohort of subjects coming from a different population signifies the relevance of such a study design in the general population. Similar to bitter taste receptors, olfactory receptors are known to be expressed in the airways. They are hypothesized to play role in sensing strong odorant molecules from the environment which in turn can activate ciliary movements for clearance of mucus8. This process, when defective, may activate sensory neurons in the airways ultimately triggering exacerbation in asthmatics. Recent reports that suggest that olfactory receptor OR2AG1 (paralog of OR2AG2) are expressed in airway smooth muscle (ASM) cells. While one study shows activation of the receptor leads to histamine induced contraction in ASM cells17, other recent reports suggest that its inhibition hinders smooth muscle cell relaxation by triggering transient increase in Ca2+ via cAMP dependent signalling cascade12,18. We hypothesized that defect in the olfactory receptor gene OR2AG2 in asthmatics can possibly trigger exacerbation in response to strong odor stimuli, by influencing either the contractility of smooth muscle cells or the mucus clearance ability of the receptor-expressing cells in the lungs and airways.
We observed remarkable phenotypic correlation with our genotypic findings in the family. Most asthmatics would be sensitive to the presence of increased irritants including allergens in the environment and tend to avoid situations where they are increased, since an exposure to higher concentrations of irritants and allergens can lead to severe asthma attacks. Patients from this family self-reported inability to sense odors and fumes, representing a hostile environment, until the content in air is markedly high, after which they experienced severe symptoms and even asthma exacerbations. However, the control subjects of the family remain unaffected. This observation was confirmed by performing odor threshold test using different dilutions of PEA (sweet odor) solution on subjects from family 1. The ability to detect odor was seen to be significantly reduced in asthmatic subjects, demonstrating relative hyposmia as compared to controls from the same family. Although the genotype to phenotype correlation in the cohort was less pronounced, we observed ~80% of the asthmatics from the cohort to carry a copy of the disease allele. While the differences in airflow limitations were modest, these were young asthmatics with mild to moderate forms of asthma. The genotype related differences in the pediatric asthma cohort may emerge over a longer period of time. Additionally, larger sample size of the asthma cohort and more extensive genomic analysis, such as imputation of the non-genotyped variants, can provide further information. It is notable that the index family where the investigation started had drawn clinical attention for the large number of asthmatics as well as more difficult to control asthma. Future investigation follow up of the childhood asthma subjects may also shed more light upon this subject.
Together the data is consistent with a model where either innate genetic defects in OR2AG2 or acquired cytokine mediated suppression of OR2AG2 contributes to asthma pathogenesis. Since olfactory function is relevant to the asthma phenotype, with strong odors being known to trigger asthma, the mechanisms may be related to abnormal olfaction or impaired relaxation of airway smooth muscle cells. However, other possibilities cannot be excluded and the relevance of OR2AG2 in asthma genetics needs to be probed further. To end, our study highlights the value of large family studies in accelerating genetic discovery of complex diseases.
Methods
Subjects for the study
A multi-generation family (Family 1) with asthma was identified for this study (Fig. 1). Subjects were diagnosed by a primary physician familiar to the family for generations, based on history of episodic chest symptoms along with pulmonary functions satisfying American Thoracic Society (ATS) criteria19. Approximately 40% of the family members were diagnosed with allergic asthma and atopy based on Global Initiative for Asthma (GINA) guidelines. We performed spirometry pre and post bronchodilator treatment for all twenty subjects who provided written consent for the study (Table 1). Subjects with atopy without asthma e.g. allergic rhinitis/dermatitis were excluded from the analysis (III-13, IV-5, IV-29, V21, and V41). In addition to this, 141 asthmatic subjects from a pediatric cohort and 130 controls were used for performing whole genome genotyping. Pediatric subjects were part of an ongoing prospective asthma cohort at All India Institute of Medical Sciences (AIIMS), New Delhi, India, for which multidimensional genotype and phenotype data is being collected. Asthmatic children were recruited based on American Thoracic Society/European Respiratory Society criteria (ATS/ERS)20 using patient history, presenting symptoms and spirometric evaluation along with informed consent. Control normal adults were healthy non-smoking volunteers with no history of chest diseases/symptoms and thus chosen for the purpose of representing people with low risk for respiratory disease.
Informed consent and ethics
Written informed consent was obtained from all participating subjects (in case of children, parent/guardian’s consent was obtained). The nature of the study and its purpose was explained to participants and their parents. The study was designed and performed in accordance with the relevant guidelines approved by the Institutional Human Ethics Committee of CSIR-Institute of Genomics and Integrative Biology, Delhi, India under ethics approval number: IHEC/2015/06 and All India Institute of Medical Sciences (AIIMS) Ethics Committee for the asthma cohort study, with approval number A-15/5.5.2008.
DNA isolation and exome sequencing
The family pedigree depicted cases of consanguinity but no clear pattern of inheritance. Thus for exome sequencing, affected subjects from distant branches of pedigree were chosen. Detail of sample selection criteria for exome sequencing has been explained in supplementary. DNA was isolated from blood using Qiagen DNA blood mini kit. Eight subjects- II:5, III:7, III:9, III:10, IV:31, IV:34, V:27, V:42 (five cases and three controls) were selected for exome sequencing using the Illumina TruSeq DNA exome kit for the capture of the exonic region as per manufacturer’s protocol. 6 samples were multiplexed and loaded on a single lane of the Hiseq. 2000 flow cell for sequencing. Post sequencing, data was subjected to quality check (QC) and bioinformatics analysis21 with minor modifications as described in Supplementary Methods (Supplementary Fig. E1A).
Variant prioritization
To understand the possible implication of the variants in human physiology and disease, in-silico prediction tools such as SIFT22, Polyphen-223, CADD24, GERP25 and MutationTaster26 were exploited to prioritize and select deleterious variants with implications in asthma biology (Supplementary Fig. E1B). Apart from this, variants from genes unreported in asthma (i.e. novel) that co-segregated with all the affected subjects i.e. under disease condition were given priority. The minor allele frequency (MAF) of each variant was obtained using the Exome Variant Server27 and 1000Genome variants from the dbSNP database28. NHGRI, NCBI, Genecards and SNP4diseases were used to list known asthma genes.
Confirmation of genetic findings
Sanger and SNaPshot sequencing
To remove false positives, genetic variants shortlisted from exome data were confirmed in all twenty members of the family using SNaPshot (for Single nucleotide variations) and Sanger sequencing (for indels) approaches. Primer details are provided in Supplementary Table E1.
Whole genome genotyping of family
In DNA from 20 subjects of the family, whole genome genotyping was performed using Illumina Infinium Global Screening Array kit, version 2.0 following manufacturer’s protocol. The genotypes were called using Genome Studio 2.0. Linkage analysis was performed using Haploview29 and Haplotype analysis was performed using PHASE tool30, version 2.1.1 (shown in Supplementary Fig. E3).
Asthma case control cohort
In DNA obtained from an ongoing case-control cohort (as explained in the next sub-topic) of 271 individuals (141 asthmatics and 130 control), whole genome genotyping was performed using IlluminaOmni1-Quad SNP kit following manufacturer’s protocol. Subject details are provided in Table 2. The data was subjected to quality control and analysis using PLINK software31. The dataset was used to check the association of the validated variants from exome sequencing to asthma in population.
Pediatric cohort of asthma
141 subjects from pediatric cohort of asthma at AIIMS subjected to a 3-month follow-up for 5 years. Pediatric subjects were part of an ongoing prospective asthma cohort at All India Institute of Medical Sciences (AIIMS), New Delhi, India, for which multidimensional genotype and phenotype data is being collected (this study was approved by the Ethics Committee of AIIMS). Asthmatic children were recruited based on American Thoracic Society/European Respiratory Society criteria (ATS/ERS)20 using patient history, presenting symptoms and spirometric evaluation. Control normal adults were healthy non-smoking volunteers with no history of chest diseases/symptoms and thus chosen for the purpose of representing people with low risk for respiratory disease. Sample details are provided in Table 2. The Lung function values, Exhaled Nitric Oxide (FeNO), Exacerbation frequencies were recorded during these follow-ups. Predicted values for lung function were plotted over the period of 15 follow-ups. Exhaled nitric oxide values (FeNO) and exacerbation frequencies were adjusted for age, gender and height. Actual values for both of them were normalised with the adjusted values. All the plots have been shown in Fig. 4. The cohort characteristics were derived for three genotype groups GG, CG and CC. For comparison of FeNO value and exacerbation frequency between the genotypes, mean value of Actual/adjusted value of FeNO/exacerbation frequencies were computed and plotted for three genotypes from followup-1 to followup-15, respectively, as shown in Table 3 and Fig. 4. The statistical significance for the difference between the genotypes for Lung-function, exhale nitric oxide and exacerbation-frequency was calculated using ANOVA (parametric) and Kruskal-wallis test (non-parametric) after testing for the normality assumptions using Shapiro-Wilk test. The multiple testing correction was performed using Bonferroni adjustment (P- value is ≤α/n), 10 comparisons were made so results were considered to be significant for α = 0.05, when P-value is ≤0.005. All the analysis was carried out using R statistical software.
Multiple sequence alignment
3D structure of the protein OR2AG2 was obtained using Chimera tool32. Multiple sequence alignment was performed using NCBI blast tool and t-coffee33 to investigate the conservation of the protein sequence in the regions flanking the variant of interest.
Human lung samples
Human lung specimens were obtained with informed consent from patients undergoing thoracic surgery at St. Mary’s Hospital, Mayo Clinic Rochester after approval from institutional review board (from our collaborator Dr. Y.S. Prakash). The patients from whom the samples were derived are Caucasian. Details of human subjects is provided in Supplementary Table E2.
Protocols for cell culture, total cell/tissue lysate, cDNA synthesis, real time PCR and immunoblotting are mentioned in the Online Supplementary.
Olfactory test
Identification and threshold test using 2-phenylethyl alcohol (PEA, sweet odor) was performed using Sniffin’ Sticks, purchased from Burghart Messtechnik GmbH, Germany. Manufacturer’s protocol was used for the tests11. The experimenters were blinded to patient assignment during the tests. Subjects with rhinitis/sinusitis were excluded from the test. Odor score is defined as the tube number at which the subject was able to identify the odorant molecule. The mean score of the odor threshold is an inverse estimate to detect odorant molecules, i.e. lower the mean score in a subject, poorer is their ability to detect odor since activation of the olfactory receptor would require higher concentration of the odorant ligand.
Statistical analysis
Comparison between two groups were performed using unpaired student’s t-test (parametric) and Wilcoxon’s test (Non-parametric), after testing for normality using Shapiro-Wilk test. Similarly, for comparison between more than two groups, ANOVA or Kruskal wallis was performed based on the normality testing. All data is represented as mean ± SEM, unless stated otherwise.
Data availability
All results are available in the manuscript or in the supplementary file. Additional information will be available from the corresponding author upon reasonable request.
References
Mukherjee, A. B. & Zhang, Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem 286, 32883–32889, https://doi.org/10.1074/jbc.R110.197046 (2011).
Ober, C. Asthma Genetics in the Post-GWAS Era. Ann Am Thorac Soc 13 Suppl 1, S85–90, doi:10.1513/AnnalsATS.201507-459MG (2016).
Almoguera, B. et al. Application of Whole Exome Sequencing in Six Families with an Initial Diagnosis of Autosomal Dominant Retinitis Pigmentosa: Lessons Learned. PloS one 10, e0133624, https://doi.org/10.1371/journal.pone.0133624 (2015).
Wells, Q. S. et al. Whole exome sequencing identifies a causal RBM20 mutation in a large pedigree with familial dilated cardiomyopathy. Circ Cardiovasc Genet 6, 317–326, https://doi.org/10.1161/CIRCGENETICS.113.000011 (2013).
Cruchaga, C. et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature 505, 550–554, https://doi.org/10.1038/nature12825 (2014).
Sharma, M. et al. A genetic variation in inositol polyphosphate 4 phosphatase a enhances susceptibility to asthma. Am J Respir Crit Care Med 177, 712–719, https://doi.org/10.1164/rccm.200705-781OC (2008).
Kumar, A., Das, S., Agrawal, A., Mukhopadhyay, I. & Ghosh, B. Genetic association of key Th1/Th2 pathway candidate genes, IRF2, IL6, IFNGR2, STAT4 and IL4RA, with atopic asthma in the Indian population. J Hum Genet 60, 443–448, https://doi.org/10.1038/jhg.2015.45 (2015).
Gu, X. & Ben-Shahar, Y. Olfactory receptors in human airway epithelia. Methods Mol Biol 1003, 161–169, https://doi.org/10.1007/978-1-62703-377-0_12 (2013).
Lee, B. Y., Cho, S., Shin, D. H. & Kim, H. Genome-wide association study of copy number variations associated with pulmonary function measures in Korea Associated Resource (KARE) cohorts. Genomics 97, 101–105, https://doi.org/10.1016/j.ygeno.2010.11.001 (2011).
Hummel, T., Kobal, G., Gudziol, H. & Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264, 237–243, https://doi.org/10.1007/s00405-006-0173-0 (2007).
Croy, I. et al. Comparison between odor thresholds for phenyl ethyl alcohol and butanol. Chem Senses 34, 523–527, https://doi.org/10.1093/chemse/bjp029 (2009).
Aisenberg, W. H. et al. Defining an olfactory receptor function in airway smooth muscle cells. Scientific reports 6, 38231, https://doi.org/10.1038/srep38231 (2016).
Flegel, C., Manteniotis, S., Osthold, S., Hatt, H. & Gisselmann, G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PloS one 8, e55368, https://doi.org/10.1371/journal.pone.0055368 (2013).
Gu, X. et al. Chemosensory functions for pulmonary neuroendocrine cells. Am J Respir Cell Mol Biol 50, 637–646, https://doi.org/10.1165/rcmb.2013-0199OC (2014).
Wills-Karp, M. et al. Interleukin-13: central mediator of allergic asthma. Science 282, 2258–2261 (1998).
Mabalirajan, U. et al. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PloS one 8, e62916, https://doi.org/10.1371/journal.pone.0062916 (2013).
Kalbe, B. et al. Olfactory Receptors Modulate Physiological Processes in Human Airway Smooth Muscle Cells. Front Physiol 7, 339, https://doi.org/10.3389/fphys.2016.00339 (2016).
An, S. S. & Liggett, S. B. Taste and smell GPCRs in the lung: Evidence for a previously unrecognized widespread chemosensory system. Cell Signal 41, 82–88, https://doi.org/10.1016/j.cellsig.2017.02.002 (2018).
Miller, M. R. et al. Standardisation of spirometry. Eur Respir J 26, 319–338, https://doi.org/10.1183/09031936.05.00034805 (2005).
Reddel, H. K. et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 180, 59–99, https://doi.org/10.1164/rccm.200801-060ST (2009).
Faruq, M. et al. Novel mutations in typical and atypical genetic loci through exome sequencing in autosomal recessive cerebellar ataxia families. Clin Genet 86, 335–341, https://doi.org/10.1111/cge.12279 (2014).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4, 1073–1081, https://doi.org/10.1038/nprot.2009.86 (2009).
Adzhubei, I. A. et al. A method and server for predicting damaging missense mutations. Nat Methods 7, 248–249, https://doi.org/10.1038/nmeth0410-248 (2010).
Kircher, M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46, 310–315, https://doi.org/10.1038/ng.2892 (2014).
Davydov, E. V. et al. Identifying a high fraction of the human genome to be under selective constraint using GERP. PLoS Comput Biol 6, e1001025, https://doi.org/10.1371/journal.pcbi.1001025 (2010).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7, 575–576, https://doi.org/10.1038/nmeth0810-575 (2010).
Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP),(Seattle, WA, 2016).
Genomes Project, C. et al. A global reference for human genetic variation. Nature 526, 68–74, https://doi.org/10.1038/nature15393 (2015).
Barrett, J. C., Fry, B., Maller, J. & Daly, M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265, https://doi.org/10.1093/bioinformatics/bth457 (2005).
Stephens, M. & Scheet, P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet 76, 449–462, https://doi.org/10.1086/428594 (2005).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559–575, https://doi.org/10.1086/519795 (2007).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234, 779–815, https://doi.org/10.1006/jmbi.1993.1626 (1993).
Notredame, C., Higgins, D. G. & Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302, 205–217, https://doi.org/10.1006/jmbi.2000.4042 (2000).
Desai, U., Joshi, J. M., Chhabra, S. K. & Rahman, M. U. Prediction equations for spirometry in adults in western India. Indian J Tuberc 63, 176–182, https://doi.org/10.1016/j.ijtb.2016.08.005 (2016).
Acknowledgements
The authors would like to acknowledge the participating subjects, doctors and staff from JSS medical College, Mysore, and AIIMS, New Delhi for their cooperation. We thank Dr. Rajesh Pandey for his help in NGS run and members of Dr. M. Mukerji, Dr. M. Faruq and Dr. D. Dash’s lab, CSIR-IGIB, for assistance in NGS data analysis. We also thank Dr. Arjun Ray for his help with 3D structure of OR2AG2 protein. This work was supported by Grants- MLP-5502, CLP-0026, BSC0116 (A.A., B.G.) from the Council of Scientific and Industrial research, India, GAP0124 (A.A.) from Wellcome trust DBT India Alliance Senior Fellowship, GAP84 (B.G.) from the Department of Science and Technology, India and NIH grants R01 HL088029 and R01 HL056470 (Y.S.P.).
Author information
Authors and Affiliations
Contributions
S.C., P.A.M., B.G. and A.A. conceived the study; S.C., A.S., S.V., S.S., P.A.M. and A.A. were involved in patient recruitment and sample collection; S.C. and A.A. designed the experiments; S.C., P.D. and M.F. analysed the data; Y.S.P. was involved in collection of the human samples for the study; A.N., R.L. and S.K.K helped in sample collection and data analysis from the asthma cohort, S.C. B.G. and A.A. wrote the manuscript. All authors contributed in editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chakraborty, S., Dakle, P., Sinha, A. et al. Genetic variations in olfactory receptor gene OR2AG2 in a large multigenerational family with asthma. Sci Rep 9, 19029 (2019). https://doi.org/10.1038/s41598-019-54718-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54718-6
This article is cited by
-
Bitter Taste and Olfactory Receptors: Beyond Chemical Sensing in the Tongue and the Nose
The Journal of Membrane Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.