Abstract
The Azores, Madeira, Selvagens, Canary Islands and Cabo Verde are commonly united under the term “Macaronesia”. This study investigates the coherency and validity of Macaronesia as a biogeographic unit using six marine groups with very different dispersal abilities: coastal fishes, echinoderms, gastropod molluscs, brachyuran decapod crustaceans, polychaete annelids, and macroalgae. We found no support for the current concept of Macaronesia as a coherent marine biogeographic unit. All marine groups studied suggest the exclusion of Cabo Verde from the remaining Macaronesian archipelagos and thus, Cabo Verde should be given the status of a biogeographic subprovince within the West African Transition province. We propose to redefine the Lusitanian biogeographical province, in which we include four ecoregions: the South European Atlantic Shelf, the Saharan Upwelling, the Azores, and a new ecoregion herein named Webbnesia, which comprises the archipelagos of Madeira, Selvagens and the Canary Islands.
Similar content being viewed by others
Introduction
The Macaronesian region has been historically recognized as a biogeographically related group of oceanic archipelagos1. Located in the north-eastern Atlantic Ocean between 15 and 39°N in latitude, it includes, from north to south, the Azores, Madeira, Selvagens, Canary and Cabo Verde islands (Fig. 1). Macaronesia is renowned for its biodiversity, with extraordinary high levels of species diversity and endemism in both the terrestrial2,3,4 and marine realms5,6,7,8,9. Nowadays, these five volcanic archipelagos, with island ages ranging from 0.27 Ma (Pico, Azores)10 to 29.5 Ma (Selvagens)11, are assigned to a single Biodiversity Hotspot – the Mediterranean Basin12. Despite some criticism, such biodiversity hotspots have been used to set conservation priorities to preserve biodiversity in terrestrial and marine ecosystems13. The high number of archipelagos (5) and islands (31 in total), the varying degree of isolation, its latitudinal gradient and corresponding differences in climate and water temperatures, and the fact that these oceanic islands have never been connected with any mainland, make Macaronesia an ideal region in which to test biogeographic and evolutionary theories14,15,16,17,18.
Geographical areas used for the construction of the checklists (cf. Supplementary Material, Tables S1 to S5) and for the biogeographical analysis. AST – Asturias (north Spain); AZO – Azores Archipelago; BIS – Bay of Biscay sensu lato, from English Channel to Punta Estaca de Bares, Galicia, Spain; CAB – Cabo Verde Archipelago; CAD – Gulf of Cádiz; BRI – British Isles; CAN – Canary Archipelago; IBE – Iberian shores (from southern Bay of Biscay to Portugal and Gulf of Cádiz); MAD – Madeira Archipelago; MED – western Mediterranean Sea; NWA – northwest African shores (Atlantic Morocco, from Straits of Gibraltar south, Western Sahara to Cape Blanc (Mauritania); POR – Portugal [western Atlantic Iberian façade (from Cabo Vilán, western Galician shores, down to Cape São Vicente) and southern shores of Algarve]; SEL – Selvagens Islands; SEN – Senegal; STP – São Tomé and Príncipe Archipelago; TWAF – Tropical West Africa [from Cape Blanc (Mauritania) south to Cape Frio (Angola)].
The terrestrial point of view: state of the art
The word ‘Macaronesia’ was first coined by the British botanist Philip Barker-Webb (ca. 1845) to encompass the archipelagos of Madeira, Selvagens and Canary Islands19. Later, Engler included the Azores in the Macaronesian region20 and Dansereau broadened the concept even further to include the Cabo Verde Islands21. Some authors considered that other regions also have a significant number of common taxa with the Macaronesian Islands, namely certain areas in the Iberian Peninsula22 and some coastal areas of the adjoining north-western Africa23,24.
Although the term ‘Macaronesia’ has been used with different meanings, inclusion of Cabo Verde is a particularly controversial matter. Based on the analysis of the terrestrial flora, authors advocated the exclusion of this archipelago from Macaronesia23,24,25. Lobin went so far as to suggest that the term Macaronesia should be strictly used in a geographical sense and not to define a phytogeographical unit, proposing Cabo Verde to be included in the Saharo-Sindic floristic region24. In fact, early phytogeographical reviews26,27,28, were the first to point out the overall stronger affinity of the flora of Cabo Verde with that of adjoining Africa. In turn, White emphasized that the lowland flora of Cabo Verde was markedly Afrotropical, whereas the endemic mountain flora was mainly related to Madeira and the Canary Islands29. This biogeographical pattern is well illustrated, on the one hand, by native grass species growing in the arid lowlands of the archipelago, which share more affinities with Tropical Africa30; and, on the other hand, by the endemic plant species from mountain areas, which are closely related to species from the Canary and Madeira archipelagos31. Among the endemic plant species are some of the biggest plant radiations worldwide, which derive from recent colonization events from the Canary Islands: Aeonium32, Echium33 and Tolpis34. Lastly, Cabo Verde was included in the Paleotropics in the first geobotanical survey of the archipelago35.
A previous study36 proposed to restrict the use of the term Macaronesia to characterize the islands in the northeast Atlantic where laurel forests occur. This unique subtropical humid forest is characterized by a predominance of trees belonging to the family Lauraceae (e.g. Apollonias Nees; Ocotea Aubl.; Persea Mill.), and other species from genera such as Clethra L. (Clethraceae), and Picconia A.DC. (Oleaceae)37. These forests are mainly found in mountain areas from 400 to 1,200 metres elevation in the Azores, Madeira and Canary Islands, but are absent in Cabo Verde, where Afrotropical tree species (e.g. Ficus sycomorus L. and Faidherbia albida (Delile) A.Chev.) occur2. Looking at its cryptogamic flora, authors demonstrated that for all the taxonomic groups examined (mosses, liverworts and pteridophytes), the flora of Cabo Verde is more closely related to the flora of Tropical Africa than to the cryptogamic flora found in the Azores, Madeira and Canary Islands, thus rejecting a broad definition of Macaronesia38.
Cabo Verde’s native terrestrial fauna also denotes the singularity of the biogeography of this archipelago within Macaronesia, with different taxonomic groups presenting distinct biogeographic patterns. The affinities of Cabo Verde’s native bird species indicate that the origin of the extant terrestrial avifauna is predominantly closest to Palaearctic mainland areas, and not the adjoining Sahel39. The presence of the bat genus Plecotus2 and the Mediterranean/Canary Islands-Madeiran origin of a quarter of the native butterfly species40 also illustrate the Palearctic element of the Cabo Verde fauna. Conversely, the remaining butterfly fauna40, orthopterans41, some jumping spiders (Salticidae)42 and the endemic cockroach genus Caboverdea43 are Afrotropical in their affinities, setting Cabo Verde apart from the remaining Macaronesian archipelagos. Cabo Verde is also renowned for its endemic herpetofauna, which includes the outcomes of a radiation of the endemic skinks genus Chionina, most likely originating from adjoining mainland Africa44, and Tarentola geckos from Canary Islands45.
The marine point of view: state of the art
A number of studies based on marine coastal fishes and gastropods from Cabo Verde concluded that the community structure and biogeographic relationships of its marine biota differ significantly from the other Macaronesian archipelagos5,8,46,47,48,49,50,51,52,53,54,55.
Spalding et al.56 used the concept of “Marine Ecoregions” and classified the Azores, Madeira and Canary Islands (presumably also Selvagens) as a single ecoregion within the Lusitanian province, whereas Cabo Verde and the Sahelian Upwelling ecoregions were included in the West African Transition province. However, no quantitative data were provided to support this distinction of Cabo Verde in relation to the other Macaronesian archipelagos. In their analysis of the marine phytogeography of the Macaronesian archipelagos57, this biogeographical differentiation was also supported, with the Azores, Madeira, Selvagens and Canary Islands included in the Lusitanian province, a warm eastern Atlantic region with high tropicality, and thus biogeographically separated from the tropical Cabo Verde. Other authors also considered that “Macaronesia” sensu stricto (i.e., without Cabo Verde) was included in the Lusitanian province51.
Based on the fossil record and on the presence of thermophilic taxa in Pliocene fossiliferous sediments of Santa Maria Island (Azores), such as the large strombid gastropod Persististrombus coronatus (Defrance, 1827), the impact of the global climatic changes on the NE Atlantic Biogeographic Molluscan Provinces was revised, from the late Miocene (~6 Ma) to the present58. The conclusion was reached that the once widespread Miocene European-West African Province (all Macaronesian archipelagos from the Azores south to Cabo Verde then belonged to a single tropical Molluscan Biogeographical Province) changed over time to the present, distinct, tropical Mauritanian-Senegalese Province in the south (which includes Cabo Verde), and the subtropical Mediterranean-Moroccan Province in the north (which includes the Azores, Madeira, Selvagens and Canary Islands). In spite of this ongoing debate, there is no study that comprehensively evaluates this paradigm to date.
Framed by the premises of the Sea-Level Sensitive (SLS) dynamic model of marine island biogeography18 and grounded on the evolutionary insular biogeographic patterns and processes17,59, the present study offers, from a marine point of view, the first taxonomically diverse comparative analysis to reassess this debate and to seek an answer to the specific questions related to the singularities of the biodiversity of the Macaronesian archipelagos: (1) is Macaronesia a coherent biogeographic unit?; (2) is the Macaronesian marine distinctiveness taxon-dependent?; and (3) might some of the archipelagos be considered as distinct and separate biogeographic units? To do this, we used the six best-studied Macaronesian marine native groups (coastal fishes, echinoderms, gastropod molluscs, brachyuran decapod crustaceans, polychaete annelids, and macroalgae) as proxies of the biogeographical relationships within Macaronesia, and between Macaronesia and the nearest possible source regions, based on an exhaustive compilation of presence/absence data for the archipelagos of Macaronesia, and on a thorough revision of the biodiversity and endemism patterns across these six marine native groups. Taken together, they represent the breadth of taxonomic differentiation in the coastal marine biota of this region and support the most comprehensive study on marine Macaronesian biogeography taken to date.
Results
Marine species richness and endemism
Our checklists comprise a total of 3,737 marine species reported for Macaronesian archipelagos: 465 coastal fishes, 151 echinoderms, 1,312 gastropods, 177 brachyurans, 683 polychaetes, and 949 macroalgae. The entire dataset also includes records from sites other than the Macaronesian archipelagos (e.g., Iberia, the western Mediterranean Sea), and reports a total of 7,492 species: 892 coastal fishes, 902 echinoderms, 2,359 gastropods, 198 brachyurans, 1,588 polychaetes, and 1,553 macroalgae (cf. Supplementary Tables S1–S6).
Some coastal fish families are extremely speciose, e.g., Gobiidae (92 spp., 6 of which are single archipelagic endemic species), Blenniidae (42 spp., 3 endemic), Sparidae (37 spp., 4 endemic), Carangidae (35 spp., 1 endemic) and Labridae (33 spp., 1 endemic) (Supplementary Table S3 and Fig. 2). For gastropods, the richest genera in the Macaronesian region are Conus (with 109 species, 85 of which are single archipelagic endemic (Macaronesian) species), Alvania (93 spp., 24 endemic), Odostomia (42 spp., 8 endemic), Chauvetia and Turbonilla (36 spp. each), Raphitoma (35 spp., 1 endemic), Rissoa (34 spp., 5 endemic) and Gibbula (33 spp., 5 endemic) (Supplementary Table S1). Other gastropod genera with a high number of single archipelagic endemic species are: Schwartziella (20 endemic species), Volvarina (18 spp.), Manzonia (17 spp.), Runcina (9 spp.) and Fissurella (8 spp.) (Supplementary Table S1 and Fig. 3). The most speciose polychaete families are Syllidae (145 species, 11 single archipelagic endemic species), Sabellidae (55 species, 3 endemic), Serpulidae (43 spp., 3 endemic), Spionidae (35 spp., 3 endemic), Nereididae (32 species, 2 endemic), Polynoidae (32 spp., 5 endemic) and Phyllodocidae (30 spp., 2 endemic) (Supplementary Table S6). The high number of endemic marine species of coastal fishes (25 endemic species out of a total of 465), gastropods (418 out of 1,312), brachyurans (10 out of 177) and polychaetes (30 out of 683) registered for the Macaronesian archipelagos contrast with the very low number of endemic species reported for the echinoderms (1 endemic species out of 151) and macroalgae (2 out of 949) (Table 1).
Of the 465 species of coastal fishes reported from the Macaronesian archipelagos, 39 (8.4%) occur in all archipelagos. Cabo Verde and the Canary Islands are the most diverse archipelagos in this regard, with a similar number of fishes (303 and 299 species, respectively), followed by Madeira (208) and the Azores (165). Selvagens is the least diverse archipelago, with only 76 species of fishes. Twenty-two species are endemic to Cabo Verde, two are endemic to the Canary Islands, and there is one endemic to the Azores (Table 1).
Nine (5.9%) out of the 152 species of shallow-water echinoderms reported from Macaronesia occur in all archipelagos. The Canary Islands is the archipelago with the highest number of species (85), followed by Cabo Verde (76), Madeira (69), the Azores (64) and Selvagens (18). There is a single probable endemic species of echinoderm, Ophiarachnella semicincta (Studer, 1882); however, this brittle-star has not been recorded again since it was first described for the shelf waters of Cabo Verde (Table 1).
Only 44 (3.4%) out of the 1,312 species of shallow marine gastropods reported from Macaronesia occur in all five archipelagos. Again, the Canary Islands is the archipelago with the highest overall number of species (811), followed by Cabo Verde (608), Madeira (397), the Azores (280) and Selvagens (207). Cabo Verde is the archipelago with highest numbers of endemic gastropods (268 species; 44.1%), followed by the Canary Islands (96; 11.8%), the Azores (37; 13.2%), Madeira (14; 3.5%) and Selvagens (3; 1.4%) (Table 1).
Of the 177 species of shallow brachyurans (Crustacea: Decapoda) registered from the Macaronesian archipelagos (no data for Selvagens Archipelago), 31 species (17.5%) occur in all archipelagos. The Canary Islands and Cabo Verde have similar numbers of brachyuran species (120 and 117, respectively), whereas Madeira has 75 and the Azores has 62 species. Ten species (8.5%) are endemic to Cabo Verde, with no examples of endemism in the remaining archipelagos (Table 1).
Regarding polychaetes, 18 of the 683 species reported for Macaronesia have been found in the five archipelagos (2.6%). The Canary Islands is the most diverse archipelago with 465 polychaete species, followed by Madeira (300 species), Cabo Verde (213), Azores (169), and Selvagens (86). A total of 30 species are considered endemic for one of the archipelagos, accounting for 10 species each for the Canary Islands and Madeira (2.2% and 3.3% of endemism, respectively), 9 species for Cabo Verde (4.2% endemism), and one species for the Azores (0.6%) (Table 1).
Of the 949 species of macroalgae reported from the Macaronesian region, 99 species (10.4%) occur in all archipelagos. The Canary Islands, with 689 species, are by far the most diverse archipelago, followed by the Azores (405), Madeira (396) and Cabo Verde (333). The Selvagens are the least diverse (295 species; cf. Table 1). With the probable exceptions of Osmundea silvae, which is only reported for Madeira, and Botryocladia canariensis for the Canary Islands, there are no other exclusive endemic species of macroalgae in any of the archipelagos under consideration.
Statistical analysis
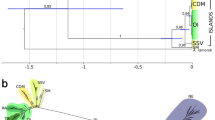
The main result from the cluster analysis is the clear separation between Cabo Verde and the remaining Macaronesian archipelagos across all groups except Polychaetes (Fig. 4). Cluster analysis also shows that: (1) Madeira and Canary Islands form the core of the Macaronesian region, always clustering together (and with the Selvagens); (2) The Selvagens and Canary Islands cluster together with regard to coastal fishes and gastropods, whereas with respect to macroalgae, the Selvagens cluster with the Madeira Archipelago; (3) The Selvagens and the Azores are biogeographically closer to Madeira/Canary Islands than to Cabo Verde (except for Polychaetes). In all dendrograms, the continental North Atlantic/Mediterranean areas cluster together, e.g., CAD + BIS + POR (Crustacea), IBE + BRI (Echinodermata), IBE/MED + BIS (coastal fishes), BIS + IBE + MED + BRI (Polychaetes) and AST + BIS + POR + CAD + BRI (macroalgae) (see Fig. 1 for acronyms).
Dendrograms depicting the biogeographic similarity between areas. Numbers correspond to the bootstrap values providing support for each tree node (100 repetitions of 100 trees). Coastal fishes (Simpson index/UPGMA; cophenetic correlation = 0.847), Echinodermata (Jaccard index/UPGMA; cophenetic correlation = 0.833), Gastropoda (Simpson index/UPGMA; cophenetic correlation = 0.936), Crustacea Brachyura (Jaccard index/UPGMA; cophenetic correlation = 0.915), macroalgae (Jaccard index/UPGMA; cophenetic correlation = 0.883), Polychaeta (Jaccard index/UPGMA; cophenetic correlation = 0.952). Mollusc gastropods and macroalgae from 0–50 m depth; coastal fishes, echinoderms, brachyuran crabs and polychaetes from 0–200 m. For acronyms of each geographical area, see legend of Fig. 1. Letters A, B, (…), Y, represent the optimal number of clusters which were validated by Mantel statistics (Pearson).
Molluscan provincial/subprovincial status of the Macaronesian archipelagos
The Provincial Combined Index is based on ten gastropod mollusc families and subfamilies that are common in tropical and subtropical shores, and its use allows for the classification of the biogeographic status of the area under consideration (see Methods section for a full description). Cabo Verde is the only archipelago with a significant Provincial Combined Index of 29.5%, equivalent to a molluscan subprovincial ranking of the archipelago (Table 2). The values of the Provincial Combined Index for the Canary Islands and Madeira are both 0.0%. It was not possible to calculate the Provincial Combined Index for both the Azores and Selvagens archipelagos, as none of these archipelagos had any species of the considered Provincial Index Taxa (Table 2).
Analysis of shared endemic Macaronesian marine species
In total, there are 150 shared endemic species, all of them with a geographical distribution restricted to two or more of the Macaronesian archipelagos. Of these, there are 104 shared endemic species of gastropods, 7 specific taxa of shared endemic brachyuran crabs, 13 shared endemic coastal fishes, 9 shared endemic annelids and 17 shared endemic macroalgae (Table 3; Supplementary Table S7).
A few shared endemic species restricted to Macaronesia are present in all of the archipelagos (see Supplementary Table S7): three species of gastropods [Columbella adansoni Menke, 1853; Pleurobranchus garciagomezi Cervera, Cattaneo-Vietti & Edmunds, 1996; and Tectarius striatus (King, 1832)], six species of fishes [Bodianus scrofa (Valenciennes, 1839); Canthigaster capistrata (Lowe, 1839); Muraena augusti (Kaup, 1856); Mycteroperca fusca (Lowe, 1838); Ophioblennius atlanticus (Valenciennes, 1836); and Similiparma lurida (Cuvier, 1830)], and one species of macroalgae [(Laurencia viridis Gil-Rodríguez & Haroun, 1992)] (Table 3). Most endemics are shared between two archipelagos (50.7%), the percentages decreasing with the increasing number of archipelagos where shared endemic species are present (31.3% in 3 archipelagos, 11.3% in 4 archipelagos, and only 6.7% in all archipelagos) (Table 3).
Fifty-six (53.8%) endemic species of shallow-water gastropods are shared between two archipelagos, 35 (33.7%) are shared between three archipelagos, and 10 (9.6%) are shared between four archipelagos (cf. Table 3 and Supplementary Table S7). Twenty-seven species (26.0%) out of the 104 shared endemic gastropods occur simultaneously in the archipelagos of Madeira, Selvagens and Canary Islands, followed by another 17 shared endemic species (16.3%) that occur simultaneously at Madeira and Canary Islands. In total, the three central Macaronesian archipelagos attain about 59% of all shared endemic gastropod species considering all possible combinations (MAD-SEL-CAN, MAD-SEL, MAD-CAN and SEL-CAN), a scenario also expressed by the cluster analysis (Fig. 5). Most of the shared endemic macroalgae are shared between two archipelagos (9 spp.; 52.9%), with decreasing numbers of shared endemic macroalgae for three archipelagos (4 spp.; 23.5%), four archipelagos (3 spp.; 17.7%) and five archipelagos (1 sp.; 5.9%) (Table 3 and Supplementary Table S7).
Dendrogram depicting the biogeographic similarity between areas for the shared endemic species (Jaccard index/UPGMA; cophenetic correlation = 0,935). Numbers correspond to the bootstrap values providing support for each tree node (100 repetitions of 100 trees). Letters A, B, C, represent the optimal number of clusters which were validated by Mantel statistics (Pearson).
Discussion
Species richness and endemism
Littoral area17, latitude and geological age of the islands largely explain the patterns of marine biodiversity and species geographical distribution in insular environments; moreover, they also affect speciation and extinction rates of marine species17,18,59. Littoral area is correlated with the geological age of the islands, older islands having larger insular shelfs60, and therefore a higher species richness, when compared to younger islands17. As littoral area peaks during interglacial episodes, the number of species increases, as do the speciation rates18. It is well known that successful colonization events in remote islands often produce high levels of endemicity61,62. It is also known that tropical species expand their geographical ranges towards higher latitudes during interglacial episodes, as clearly demonstrated by the fossil record of the Last Interglacial63,64,65,66. In contrast, the littoral area diminishes during glacial episodes, reducing the potential carrying capacity for species due to the loss of habitats, resulting in an increase of extinction rates18. Additionally, when sea level drops below the insular shelf edge, mobile substrates are exported to the deep sea, and species associated with this environment will locally disappear66,67. Furthermore, tropical species that, during the previous interglacial episode, had expanded their geographical ranges and reached higher latitudes will be extirpated during the subsequent glacial episode18,66. As a result, the overall archipelagic biodiversity will change throughout geological time with higher species richness during the interglacial intervals. Islands located in the tropical belt will be less affected by the drop in average sea-surface temperatures (SSTs) than islands located at higher latitudes, so the higher biodiversity and archipelagic endemics of Cabo Verde in all studied marine groups agree with the predictions of the Sea-Level Sensitive dynamic model of marine island biogeography18.
As all islands that comprise the Macaronesian region are volcanic in origin and were never connected to continental landmasses, most biogeographers assume (authors included) that they were mainly colonized by long-distance oceanic dispersal, a process where oceanic currents and the distance to the mainland or the nearest island/shallow seamount is known to play an important role57,68. The patterns of circulation of the most important sea-surface currents in the Macaronesian region (see Methods section below) make it possible to infer that during interglacial periods such as the present one, the Azores Current and the Madeira Current provide a plausible seaway for the dispersal of shallow water marine organisms from the Azores to the Canary Islands. Taken together with the present distances between archipelagos/islands (see Table 4), these currents help to elucidate their role as biogeographical filters, since gene flow depends on the dispersal capacity of each organism. Moreover, the Cabo Verde Front (located north of Cabo Verde Archipelago; Fig. 6) and its magnitude (4–6° of latitude) certainly function as an important biogeographical barrier for the dispersal of marine organisms, thus isolating Cabo Verdean islands from the remaining Macaronesian archipelagos. For the Azores, Selvagens and Cabo Verde, archipelagic isolation has varied little (0.5, 4.4 and 2.7%, respectively), when the present interglacial distances are compared with those estimated for the Last Glacial Maximum (Table 4). However, for Madeira and the Canary Islands, isolation decreases during glacial intervals by as much as 53.2% and 63.3% respectively, in relation to the present distances (Table 4). This factor, together with shallow seamounts that become islands and function as stepping-stones during periods of low sea levels, e.g. Ampère and Seine seamounts69 or Ormonde seamount70, must have facilitated both the dispersal of marine species between these three archipelagos, as well as the dispersal of mainland species towards the islands. This partially explains the similar values of endemic gastropods in the Azores (13.2%) and the Canary Islands (11.8%), as although the Canary Islands have almost three times the number of species than the Azores (811 vs. 280 shallow-water gastropod species; cf. Table 1), the reason for a lower-than-expected endemic component in the Canary Islands relates to the high number of shared endemics with Madeira (60 spp.) and with Selvagens (50 spp.) (see Supplementary Table S7).
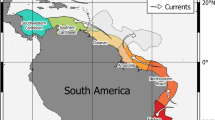
Scheme illustrating the circulation pattern of the main surface currents in the North and Central Atlantic Ocean. GS – Gulf Stream; NAC – North Atlantic Current; AC – Azores Current; SWEC – Southwest European Current; MADC – Madeira Current; CANC – Canary Current; NEC – North Equatorial Current; NECC – North Equatorial Counter Current; MC – Mauritania Current; GC – Guinea Current.
Cabo Verde Archipelago: a hotspot of marine biodiversity
Cabo Verde was the only island group within the Macaronesian archipelagos to show a significant marine Provincial Combined Index (see Methods section below) of 29.5%, which is equivalent to a molluscan subprovincial ranking (Table 2). Besides its rich marine biodiversity, which is comparable to that of the Canary Islands in fishes, echinoderms and brachyurans, the singularity of Cabo Verde in the context of the Macaronesian region is best represented by the high numbers of endemic species (7.3% of coastal fishes; 44.1% of endemic gastropods, 8.5% of brachyurans and 4.2% of polychaetes; Table 1). Cabo Verde is home to a unique marine gastropod fauna71,72, which has attracted major attention in recent years54,61,62,73,74,75. Moreover, single island marine endemics (SIME) are extremely rare in the marine realm, but the particular geological setting of Cabo Verde has favoured marine radiations in some genera62. For instance, the warm-water Conus marine gastropods experienced high speciation rates73,76, mainly during the Plio-Pleistocene74. All endemic Cabo Verdean Conus species have non-planktonic lecithotrophic larval stage73, although other species of this genus may present long-term planktotrophic larvae. These cone snails, with their direct development and low dispersal capability (also owing to microhabitat specificity), are the most notable marine fauna in Cabo Verde, represented by more than 70 SIME Conus species described to date (for a review see18, and references therein). The Cabo Verde archipelago is home to 8.9% of all Conus species in the world54,77, representing an exceptional endemism rate of 98.8%18. By contrast, only 3 non-endemic Conus species are present today in the Canary Islands72 and potentially one non-endemic species is reported from Madeira78, although the fossil record of the Last Interglacial testifies in favour of geographical range expansions of at least 8 Conus species to the Azores66,79. The diversity of Cabo Verde shallow-water keyhole limpets (fissurellids) consists of at least 11 Fissurella species (6 endemic), and 6 Diodora species (2 endemic)72. Currently, 6 shallow-water endemic gastropod species of the genus Euthria are known from Cabo Verde, but available data are largely insufficient and more new species are likely to be found along the southern islands75. Finally, this work lists 93 species of opisthobranchs from Cabo Verde, 20 species of which are endemic to the archipelago, indicating, once again, the uniqueness of these islands in the Macaronesian context. The results obtained for the different taxonomic groups indicate that the North West African Upwelling (NWAU) can explain the largest share of endemic species in Cabo Verde. The NWAU brings cold waters to the surface, which affects the coastal areas between Cape Blanc (Mauritania) in the north, and Cape Verde (Guinea) in the south80. This phenomenon results in an effective biogeographic barrier for marine species dispersal between Cabo Verde and the African mainland81,82.
Taxonomic revisions, description of new species, and new records of fish in Cabo Verde waters have increased significantly in recent decades [52 and references therein]. This work lists 303 coastal fish species from the Cabo Verde Islands (7.3% endemic; cf. Table 1) living in all habitats of the insular shelf down to 200 m depth. Among the endemic coastal fishes of Cabo Verde there are a few peculiarities: three sparids of which one, the white seabream Diplodus lineatus (Valenciennes, 1830), is considered a relic sister taxon of an originally more widespread ancestral species of the D. sargus (Linnaeus, 1758) clade83; the bulldog Virididentex acromegalus (Osório, 1911), the sole representative of this endemic monotypic genus; the black banded drummer Girella stuebeli Troschel, 1866, the only species of this Indo-Pacific genus in the Atlantic Ocean and considered a palaeo-endemic84; and the Cape damsel Similiparma hermani (Steindachner, 1887), another presumed palaeo-endemic, with a Macaronesian representative, Similiparma lurida (Cuvier, 1830)85, and whose nearest relatives are in the Eastern Pacific Ocean49,52,86. In addition, about half of the small crypto-benthic fishes are endemic to Cabo Verde waters8, with newly described endemic species, such as the labrisomid Malacoctenus carrowi87, or the gobies Gobius salamansa88 and Didogobius janetarum89. New species, including endemic ones, are being discovered regularly in all these groups.
The significant number of gastropods described in the literature as SIME illustrate the differences in species composition and community structure of the marine biota of Cabo Verde, when compared to other Macaronesian islands. This is well expressed by the biogeographical relationships of Cabo Verde for all studied groups, as well as by the analysis of the shared endemic Macaronesian marine species, which show that Cabo Verde consistently stands apart from the other Macaronesian archipelagos (Figs 4 and 5).
Macaronesia reappraised from a marine point of view
This contribution clearly demonstrates a congruent, taxon-independent, marine biogeographic pattern, supporting the exclusion of Cabo Verde from the Macaronesian biogeographic unit. Performance of cluster analyses indicate as well that the geographically contiguous archipelagos of Madeira, Selvagens (when high-quality data is available, e.g., gastropods, fishes and algae), and the Canary Islands are at the core of Macaronesia. In a later step, the Azores often clusters as a sister to this main group of archipelagos at different levels of confidence, according to the investigated taxon: very high for the coastal fishes and gastropods (100 and 95%, respectively), and high for the algae (75%), brachyurans (74%), echinoderms (70%) and polychaetes (70%; see Fig. 4).
The biogeographic patterns that separate Cabo Verde from the remaining Macaronesian archipelagos reflect the high tropical affinity and endemism of its fauna. The tropical affinity of Cabo Verde’s fauna is due to the lower latitude of the archipelago and its consequently higher SSTs. This affinity is particularly visible in the coastal fish and algae cluster analyses, in which Cabo Verde grouped with São Tomé and Príncipe/Tropical West Africa, and Senegal regions, respectively. In contrast, the remaining Macaronesian archipelagos nested within or next to the North-western Atlantic and Mediterranean regions in all the analyses except for those on polychaetes, a situation that we attribute to the current lack of knowledge (in comparison with the other marine groups) regarding the geographical distribution of the polychaetes in the archipelagos under study.
The processes underlying the higher endemism of Cabo Verde are more complex, but they can be partially explained by the combined effect of its tropical environment and its biogeographical isolation from the western African shores, mainly due to the presence of the NWAU. The tropical environment buffered most Cabo Verde’s marine species against Pleistocene climatic deterioration and its most extreme glacial events, preventing these islands from large SSTs variations and favouring the survival in Cabo Verde of relict lineages, thus explaining the presence of several palaeo-endemic species amongst coastal fishes. In contrast, isolation favoured by distance and NWAU reduces the rate in which African species arrive to the islands, promoting speciation, and potentially ecological radiation, following a set of processes in which the comparatively high littoral area of the archipelago, and its changes through time possibly played an important role. The dynamism of oceanic islands’ marine biota is expressed by the tropical Cabo Verdean marine fauna that saw their geographical ranges expanded towards northern latitudes, possibly during the final phase of glacial terminations or the initial phase of the interglacial18,90. The fossil record demonstrates this relationship for the Azores66,90, Madeira91, and the Canary Islands64,65,92. Therefore, Cabo Verde acts simultaneously as a cradle of species (mainly during interglacial periods) and a museum (with ancient species, buffered against the influence of glacial periods by the low latitude).
Taking into account the previous arguments, we recommend abandoning the use of “Macaronesia” in the sense of a biogeographical unit, accepting its use only to designate an informal geographical region (e.g., the NE Atlantic Macaronesian archipelagos). We improve on authors who used the term “Cabo Verde ecoregion”56 and further designate the Cabo Verde islands in the sense of a biogeographical subprovince, included in the tropical Mauritanian-Senegalese Province as defined by58, which is equivalent to the West African Transition Province56.
Finally, we coin the term “Webbnesia” ecoregion, which includes the Madeira, Selvagens and the Canary Islands, in honour to Philip Barker-Webb, the first to call attention to the biogeographical similarities between these three archipelagos in 184593. As indicated by our data, for some widely dispersing groups (e.g., coastal fishes, echinoderms and macroalgae), there is varying support for the inclusion of the Azores in the Webbnesia ecoregion, whereas other taxa (brachyurans, polychaetes and gastropods) suggest the Azores should be a biogeographical entity of its own, for which we thus propose the formal designation of “Azores ecoregion”. For this, and also because of the results from the shared endemics analysis, which indicate three different groups of islands (the Azores; the cluster Madeira-Selvagens-Canary Islands; and Cabo Verde), we propose the following biogeographical classification: the Azores ecoregion, the Webbnesia ecoregion, and the Cabo Verde subprovince (Fig. 7).
Biogeographical classification of the Macaronesian archipelagos. The Lusitania Province includes the Azores ecoregion, the Webbnesia ecoregion (which integrates the archipelagos of Madeira, Selvagens and Canary Islands), the South European Atlantic Shelf ecoregion and the Saharan Upwelling ecoregion. The West African Transition Province includes the Cabo Verde subprovince and the Sahelian Upwelling ecoregion. For acronyms of each geographical area, see legend of Fig. 1.
Conclusions
Despite the widespread acceptance of Macaronesia to include the Azores, Madeira, Selvagens, Canary Islands and Cabo Verde archipelagos, analyses of the biogeographical affinities of six marine groups with very different dispersal abilities consistently demonstrate that the central group of archipelagos (Madeira, Selvagens, Canary Islands) constitute a formal biogeographical unit – the Webbnesia ecoregion – with a higher number of shared restricted endemics in relation to both the Azores and Cabo Verde. In fact, there are only 10 out of 150 shared endemic marine species (6.7%) registered as occurring in all five archipelagos, whereas there are 37 shared endemic marine species (24.7%) reported for Webbnesia. In our opinion, and from a strictly marine biogeographical point of view, the three archipelagos that form the Webbnesia ecoregion are better seen as constituting a meta-archipelago94, i.e., between these archipelagos, genetic interchange (larvae, propagules, rafting adults, colonization events) occur much more frequently than with other areas, but much less than within each of the archipelagos.
Cabo Verde deserves the status of a biogeographical subprovince due to its high number of endemic species in several marine phyla, particularly SIME gastropod molluscs, which constitutes a very rare situation within the marine realm.
When checklist data are available from the tropical West African shores (e.g. for fishes and macroalgae), our results are partially similar to those of Spalding and collaborators56, with support for inclusion of the Cabo Verde Subprovince (previously classified as an independent marine ecoregion in the West African Transition province)58 and separated from the Lusitanian Province, which includes the Azores, Madeira, Selvagens and Canary Islands archipelagos. However, the inclusion of the Azores archipelago in a “Macaronesian” sensu stricto ecoregion (i.e., Azores-Madeira-Selvagens-Canary Islands) as suggested by Ávila and collaborators58 is not so clear, owing to the taxon-dependent biogeographic pattern. While widely dispersing fishes, echinoderms and algae suggest that the Azores are to be included in the “Macaronesian” sensu stricto ecoregion, the biogeographic distribution of gastropod molluscs, brachyuran decapods, and the shared endemic species argue for a separation of the Azores. Although not included in our analysis, Bryozoa also show a high rate of endemism in the Azores, with even some endemic genera95, thus supporting the separation of the Azores from Webbnesia. Therefore, we propose to redefine the Lusitanian biogeographical province of58, in which we now include the following ecoregions: the South European Atlantic Shelf, the Saharan Upwelling, the Azores and Webbnesia.
Finally, and in contrast to terrestrial patterns38, the degree of Cabo Verde distinctiveness does not depend on the chosen taxa, because a consistent pattern emerges for the six marine groups studied, all placing Cabo Verdean islands outside of Macaronesia. Therefore, from a strictly marine point of view, there exists no support for the current concept of Macaronesia as a coherent marine biogeographic unit.
Methods
Study area
The Azores is the northernmost archipelago of Macaronesia, currently comprising 9 islands and a few islets (e.g., Formigas) with ages ranging from 0.27 Ma (Pico) to 6.01 Ma (Santa Maria)58,96 (Fig. 1). It is one of the most isolated archipelagos in the Atlantic, located about 1,370 km west of mainland Portugal. The Azorean islands are under the major influence of the Gulf Stream and its southern branch, the Azores current/front, which transports warm water of Caribbean tropical origin to the north-eastern Atlantic97 (Fig. 6). Average monthly sea-surface temperatures range from 15–17 °C in the winter, to 22–24 °C in the summer98,99. Madeira archipelago is situated about 840 km SE of the Azores and about 630 km NE of the northwest African continent, and comprises 2 main islands and several islets (e.g., Desertas), with geological ages ranging from 7 Ma (Madeira Island) to 18.8 Ma (Porto Santo Island)100,101, and average SSTs ranging from 16 to 24 °C. In this area, the most important mesoscale oceanographic feature is the Madeira Current that flows southwards102 (Fig. 6). Selvagens archipelago is located about 285 km SSE of Madeira Island, surrounded by waters with SSTs similar to those of Madeira. Selvagens comprises two small, low-elevation islands (Selvagem Grande and Selvagem Pequena), the former with an age of 29.5 Ma11. About 180 km further to the south, the Canary archipelago includes 8 islands and 5 islets, their geological ages ranging from 1.1 Ma (El Hierro) to 23 Ma (Fuerteventura)103 (Fig. 1). SSTs around Canary Islands range from 17 to 25 °C, the archipelago being under the influence of the Canary Current, which results from the merging of one of the branches of the Azores Current (a southern branch of the Gulf Stream) with the Madeira Current, flowing southward between the Canary Islands and the Africa mainland102 (Fig. 6). The archipelago of Cabo Verde represents the southernmost island group, currently composed of 10 islands and 9 islets with ages ranging from ~3 to 15.8 Ma104. Santo Antão, São Vicente, Santa Luzia and São Nicolau constitute a north-western group; Santiago, Fogo and Brava form a southern cluster; and Sal, Boa Vista and Maio define an eastern group. Boa Vista is situated about 570 km offshore Senegal (West African coast). SSTs at Cabo Verde range from 20 to 25 °C. The islands are located at the eastern border of the North Atlantic Sub-Tropical Gyre (NASTG) and nearby the southern limit of the Canary Current, experiencing a tropical climate105. At about Cape Blanc (Mauritania), the Canary Current shifts westward, contributing to the North Equatorial Current106 (Fig. 6). North to Cabo Verde Archipelago, the Cabo Verde Front, a large thermohaline front separates two important water masses: the southern boundary of the NASTG, which is here formed by the North Equatorial Current, and the norther boundary of the North Atlantic Tropical Gyre107.
The marine biota of the Canary Islands and, to a lesser degree, that of Madeira, are also influenced by the Canary Upwelling Current, one of the four major upwelling systems in the world, enhancing the arrival and persistence of diverse marine invertebrate larvae and juvenile fishes in those archipelagos, mainly through passive transport along its associated mesoscale filaments and eddies80. All archipelagos are separated from the closest mainland and from other plausible source regions, such as neighbouring archipelagos or shallow seamounts, by water depths exceeding 1,300–1,500 m.
Marine groups studied
The definition of biogeographic regions, as well as the study of biogeographic processes and patterns, depends on robust databases resulting from taxonomically rigorous faunistic/floristic checklists. We selected the best-known marine groups living in the Macaronesian islands (coastal fishes, echinoderms, gastropod molluscs, brachyuran decapod crustaceans, polychaete annelids and macroalgae), and compiled checklists of the littoral species. As these marine groups have different ecological and biological traits, we considered the bathymetric range zonation of each group as varying in accordance with their ecophysiological needs, a standard procedure in marine ecology and biogeography5,17,50,51,59. Thus, for gastropods and macroalgae, we used the 50-m isobath as the maximum depth for inclusion of species in the checklist, whereas for coastal fishes, echinoderms, decapod crustaceans and polychaetes, the maximum depth was 200 m. We consider in this study coastal fishes as teleost and chondrichthyan species (<200 m) that are benthic or demersal/benthopelagic, associated with both hard (i.e., coral, macroalgae or rocky “reefs”) or soft substrates.
Pelagic, bathypelagic, deep-water, exclusively anchialine, and introduced species were removed from the checklists and thus not considered in the subsequent analysis. All checklists were validated by reputed taxonomic experts, who also removed all dubious records and taxa inquirenda. We cite the brachyuran crab Calappa tuerkayana Pastore, 1995 (Crustacea: Decapoda) as an example. This species was reported from the Ionian Sea and the Balearic Islands108,109, and also from the Azores110. However, the validity of C. tuerkayana was questioned111, and new molecular evidence indicates that this species represents juvenile stages of Calappa granulata (Linnaeus, 1758) (Abelló & Palero, pers. comm.). Therefore, C. tuerkayana was excluded from the brachyuran checklist.
Taxonomic status was validated and synonymies were corrected using WoRMS – World Register of Marine Species (http://www.marinespecies.org/), last consulted 17 November, 2018; the Catalog of Fishes (https://www.calacademy.org/scientists/projects/catalog-of-fishes), last consulted 12 December, 2018; and AlgaeBase (http://www.algaebase.org/), last consulted 23 November, 2018. For a complete list of references used for each taxonomic group, see the Supplementary Tables S1 to S6.
Statistical analysis
Presence/absence matrices were compiled from a large number of published faunal lists of Macaronesia (including unpublished results from the authors) and “grey literature”, and tables were constructed with the geographical distribution of each species (cf. Supplementary Information, Tables S1 to S6). All analyses were performed using the software R version 3.3.3112, namely the R packages vegan113, ade4114, cluster115, gclus116, and recluster117. Species richness and percentage of endemism were calculated for each archipelago. For each marine group, dendrograms depicting the relationships among areas were constructed, using dissimilarity indices and cluster analysis. We applied several classical distance metrics for presence/absence data, namely Jaccard, Sørensen, Ochiai and Simpson dissimilarities118,119,120,121. Also, for each dissimilarity coefficient, we tested several agglomeration methods122, namely complete linkage, centroid distance, unweighted pair group method with arithmetic mean (UPGMA), and Ward’s minimum variance clustering123,124,125. To determine the best combination of dissimilarity measure and agglomeration method, we calculated the cophenetic correlation value between the region’s distance matrix and the dendrogram representation126. We followed the guidelines defined in127, and also the hierarchical clustering approach reported by128. For each dendrogram, the putative number of groups formed by the target regions was estimated using both the Rousseeuw quality index, that determines the optimal number of clusters according to silhouette widths129 and the Mantel statistic, that determines the optimal number of clusters according to Mantel statistic (Pearson)122. We followed the guidelines of127,128 for dendrogram implementation. This was further supported by a bootstrap validation procedure, implemented using the Recluster package, which provides robust techniques to analyse patterns of similarity in species composition117,130,131,132. Each dendrogram was targeted by a resampling procedure with 100 trees per iteration and a total of 1,000 iterations. We retested all the dissimilarity coefficients using this approach, to ensure consistency in the number of groups formed by the target regions, for each taxonomic group.
Molluscan provincial/subprovincial status of the Macaronesian archipelagos
Molluscs were also used to test the Molluscan Provincial/Subprovincial status of the Macaronesian archipelagos. A table was constructed, containing the “Provincial Index Taxa”, i.e., all species of the following families and subfamilies of gastropods: Modulidae, Turbinellidae, Conidae, Conorbidae (=Conilithidae), Muricinae, Fasciolariinae, Volutinae (=Lyriinae), Olivinae, Cancellariinae and Plesiotritoninae. Each “Provincial Index Taxon” was calculated as the percentage of endemism for each family/subfamily:
where N is the total number of species in the considered family/subfamily, and n is the number of endemic species133. The “Provincial Combined Taxa” were then calculated as:
where PIT1 is the percentage of endemism in the Modulidae, PIT2 is the percentage of endemism in the Turbinellidae, and so on. If the percentage of Provincial Combined Taxa is greater than 50%, that area is attributed a biogeographic provincial status; if the percentage of Provincial Combined Taxa is between 25% and 50%, that area is attributed a biogeographic subprovincial status133.
Analysis of shared endemic Macaronesian marine species
Endemics have been used as the primary biogeographic dataset in many previous terrestrial studies aiming to establish “natural biogeographic areas” (e.g.134,135 and references therein). Areas sharing unique taxa are more related to each other than to areas lacking these taxa, therefore shared endemic taxa are considered equivalent to synapomorphies in a cladistic study (see Table 3 and Supplementary Table S. The method does, however, assume perfect knowledge of the distribution patterns, i.e. that the absence of a taxon is not due to insufficient sampling. Although we have used the best-studied Macaronesian marine groups, this assumption is not met for echinoderms and polychaetes, as well as for some sites (e.g., Selvagens). The method also rests on the assumption that extinction has not significantly modified the distribution pattern of each species136. For this analysis, we have listed all endemic species restricted to the archipelagos of Macaronesia (see Supplementary Table S7).
References
Sjögren, E. Aspects on the biogeography of Macaronesia from a botanical point of view. Arquipélago. Life and Marine Sciences Supplement 2 (Part A), 1–9 (2000).
Arechavaleta, M., Zurita, N., Marrero, M. C. & Martín, J. L. Lista preliminar de especies silvestres de Cabo Verde (hongos, plantas y animales terrestres). Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias, Santa Cruz de Tenerife (2005).
Arechavaleta, M., Rodríguez, S., Zurita, N. & García, A. (coord.) Lista de especies silvestres de Canarias. Hongos, plantas y animales terrestres. Gobierno de Canarias, Santa Cruz de Tenerife, 579 pp (2010).
Borges, P. A. V. et al. A list of the terrestrial fungi, flora and fauna of Madeira and Selvagens archipelagos. Direcção Regional do Ambiente da Madeira and Universidade dos Açores, Funchal and Angra do Heroísmo, 440 pp. (2008).
Ávila, S. P. Shallow-water marine molluscs of the Azores: biogeographical relationships. Arquipélago. Life and Marine Sciences Supplement 2 (Part A), 99–131 (2000).
Ávila, S. P. Processos e Padrões de Dispersão e Colonização nos Rissoidae (Mollusca: Gastropoda) dos Açores, x + 329 pp. PhD thesis on Biology/Palaeontology, Universidade dos Açores, Ponta Delgada (2005).
Borges, P. A. V. et al. A list of the terrestrial and marine biota from the Azores. Princípia, Cascais, 429 pp (2010).
Wirtz, P. et al. The coastal fishes of the Cape Verde Islands – new records and an annotated check-list (Pisces). Spixiana 36, 113–142 (2013).
Cordeiro, R. & Ávila, S. P. New species of Rissoidae (Mollusca: Gastropoda) from the Archipelago of the Azores (northeast Atlantic) and a checklist of the family for the region. Zookeys 480, 1–19 (2015).
Demand, J., Fabriol, R., Gerard, F., Lundt, F. & Chovelon, P. Prospection géothermique, îles de Faial et de Pico (Açores). Rapport géologique, geochimique et gravimétrique. Technical report, BRGM 82 SGN 003 GTH (1982).
Geldmacher, J., Hoernle, K., van den Bogaard, P., Zankl, G. & Garbe-Schönberg, D. Earlier history of the>70-Ma-old Canary hotspot based on temporal and geochemical evolution of the Selvagens Archipelago and neighbouring seamounts in the eastern North Atlantic. Journal of Volcanology and Geothermal Research 111, 55–87 (2001).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Marchese, C. Biodiversity hotspots: A shortcut for a more complicated concept. Global Ecology and Conservation 3, 297–309 (2015).
Fernández-Palacios, J. M. et al. A reconstruction of Palaeo–Macaronesia, with particular reference to the long–term biogeography of the Atlantic island laurel forests. Journal of Biogeography 38, 226–246 (2011).
Fernández-Palacios, J. M. et al. Towards a glacial-sensitive model of island biogeography. Global Ecology and Biogeography 25, 817–830 (2016).
Rijsdijk, K. F. et al. Quantifying surface area changes of volcanic islands driven by Pleistocene sea level cycles: biogeographic implications for Macaronesian archipelagos, Atlantic Ocean. Journal of Biogeography 41, 1242–1254 (2014).
Ávila, S. P. et al. Global change impacts on large-scale biogeographic patterns of marine organisms on Atlantic oceanic islands. Marine Pollution Bulletin 126, 101–112 (2018).
Ávila, S. P. et al. Towards a “Sea-Level Sensitive” dynamic model: impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biological Reviews 94, 1116–1142 (2019).
Stearn, W. T. Philip Barker Webb and Canarian Botany. Monographiae Biologicae Canariensis 4, 15–29 (1973).
Engler, A. Versuch einer Eintwicklungsgeschichte, insbesondere der Florengebiete seit der Tertiärperiode. I. Die extra-tropischen Gebiete der nördlischen Hemisphäre. Engelmann, Leipzig, Germany (1879).
Dansereau, P. Études macaronésiennes I: géographie des Cryptogames vasculaires. Agronomica Lusitanica 23, 151–181 (1961).
Sunding, P. Endemism in the Flora of the Cabo Verde islands, with special emphasis on the Macaronesian flora element. Monographs on the Biology of the Canary Islands 4, 112–117 (1973).
Sunding, P. Origins of the Macaronesian flora; pp. 13–40 In Bramwell D. (Ed.), Plants and Islands. Academic Press, London (1979).
Lobin, W. Untersuchung über Flora, Vegetation und biogeographische Beziehungen der Kapverdischen Inseln. Courier Forschungsinstitut Senckenberg 53, 1–112 (1982).
Nicolás, J. P., Fernández-Palacios, J. M., Ferrer, F. J. & Nieto, E. Inter-island floristic similarities in the Macaronesian region. Vegetatio 84, 117–125 (1989).
Schmidt, J. A. Beitrage zur Flora der Cap Verdischen inseln. Akademische Buchhandlung von Ernst Mohr, Heidelberg, 356 pp. (1852).
Hooker, J. D. Insular floras. Gardeners’ Chronicle 1867, 6–7; 27; 50–51; 75–76 (1867).
Hooker, J. D. On insular floras: a lecture. Journal of Botany 5, 23–31 (1867).
White, F. The vegetation of Africa. A descriptive memoir to accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa. Natural Resources Research, XX. UNESCO, 356 pp. (1983).
Duarte, M. C., Romeiras, M. M. Cabo Verde Islands; pp.: 143–148 in: R. Gillespie, D. Clague (Eds.), Encyclopedia of Islands. University of California Press, USA (2009).
Romeiras, M. M., Monteiro, F., Duarte, M. C., Schaefer, H. & Carine, M. Patterns of genetic diversity in three plant lineages end to the Cabo Verde Islands. Special Issue: Island Plant Biology—Celebrating Carlquist’s Legacy. AoB PLANTS 7, plv051, https://doi.org/10.1093/aobpla/plv051 (2015).
Kim, S. C. et al. Timing and tempo of early and successive adaptive radiations in Macaronesia. PLoS One 3, e2139 (2008).
Romeiras, M. M. et al. Origin and diversification of the genus Echium (Boraginaceae) in the Cabo Verde archipelago. Taxon 60, 1375–1385 (2011).
Mort, M. E. et al. Multiplexed-shotgun-genotyping data resolve phylogeny within a very recently derived insular lineage. American Journal of Botany 102, 634–641 (2015).
Rivas-Martínez, S., Lousã, M., Costa, J. C. & Duarte, M. C. Geobotanical survey of Cabo Verde Islands (West Africa). International Journal of Geobotanical Research 7, 1–103 (2017).
Beyhl, F. E., Mies, B. & Ohm, P. Macaronesia – a biogeographical puzzle. Boletim do Museu Municipal do Funchal (História Natural) Supl. 4, 107–113 (1995).
Kondraskov, P. et al. Biogeography of Mediterranean Hotspot Biodiversity: Re-Evaluating the ‘Tertiary Relict’ Hypothesis of Macaronesian Laurel Forests. PLoS One 10(7), e0132091 (2015).
Vanderpoorten, A., Rumsey, F. J. & Carine, M. A. Does Macaronesia exist? Conflicting signal in the bryophyte and pteridophyte floras. American Journal of Botany 94, 625–639 (2007).
Hazevoet, C. J. The Birds of the Cabo Verde Islands. B.O.U, Check-list N 13. Tring, 192 pp (1995).
Tennent, J. W. & Russell, P. J. C. Butterflies of the Cabo Verde Islands (Insecta, Lepidoptera). Zoologia Caboverdiana 5, 64–104 (2015).
Duranton, J. F., Launois, M., Launois-Luong, M. H. & Lecoq, M. Contribution à l’inventaire faunistique des Acridiens de l’Archipel du Cap-Vert (Orthoptera). Bulletin de la Société entomologique de France 88, 197–224 (1983).
Wesołowska, W. Taxonomic notes on jumping spiders from the Cabo Verde Islands (Araneae: Salticidae). Boletim do Museu Municipal do Funchal 50, 125–135 (1998).
Princis, K. On the Blattariae of the Azores and Madeira. Boletim do Museu Municipal do Funchal 17, 19–24 (1963).
Miralles, A., Vasconcelos, R., Perera, A., Harris, D. J. & Carranza, S. An integrative taxonomic revision of the Cabo Verdean skinks (Squamata, Scincidae). Zoologica Scripta 40, 16–44 (2011).
Vasconcelos, R., Perera, A., Geniez, P., Harris, D. J. & Carranza, S. An integrative taxonomic revision of the Tarentola geckos (Squamata, Phyllodactylidae) of the Cabo Verde Islands. Zoological Journal of the Linnean Society 164, 328–360 (2012).
van der Land, J. Marine biota of the Cape Verde Islands. Courier Forschungsinstitut Senckenberg 159, 39–44 (1993).
Wirtz, P. Unterwasserführer Underwater Guide Fische Fish. Naglschmid Verlag, Stuttgart, 160 pp. (1994).
Santos, R. S., Hawkins, S. J., Monteiro, L. R., Alves, M. & Isidro, E. J. Marine research, resources and conservation in the Azores. Aquatic Conservation Marine and Freshwater Ecosystems 5, 311–354 (1995).
Brito, A., Falcón, J. M. & Herrera, R. Características zoogeográficas de la ictiofauna litoral de las islas de Cabo Verde y comparación con los archipiélagos macaronésicos. Revista de la Academia Canaria de Ciencias 18, 93–109 (2007).
Floeter, S. R. et al. Atlantic reef fish biogeography and evolution. Journal of Biogeography 35, 22–47 (2008).
Almada, V. C. et al. Complex origins of the Lusitania biogeographic province and northeastern Atlantic fishes. Frontiers of Biogeography 5, 1 (2013).
Freitas, R. The coastal ichthyofauna of the Cape Verde Islands: a summary and remarks on endemism. Zoologia Caboverdiana 5, 1–13 (2014).
Falcón, J. M. Ictiofauna de las Islas Canarias. Análisis biogeográfico. Tesis Doctoral (no publicada). Universidad de La Laguna, 310 pp (2015).
Peters, H., O’Leary, B. C., Hawkins, J. P. & Roberts, C. M. The cone snails of Cape Verde: Marine endemism at a terrestrial scale. Global Ecology and Conservation 7, 201–213 (2016).
Machín-Sánchez, M., Gil-Rodríguez, M. C. & Haroun, R. Phylogeography of the red algal Laurencia complex in the Macaronesia region and nearby coastal areas: recent advances and future perspectives. Diversity 10, 10 (2018).
Spalding, M. D. et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583 (2007).
Tuya, F. & Haroun, R. Phytogeography of Lusitanian Macaronesia: biogeographic affinities in species richness and assemblage composition. European Journal of Phycology 44, 405–413 (2009).
Ávila, S. P. et al. Persististrombus coronatus (Mollusca: Strombidae) in the early Pliocene of Santa Maria Island (Azores: NE Atlantic): palaeoecology, palaeoclimatology and palaeobiogeographic implications on the NE Atlantic Molluscan Biogeographical Provinces. Palaeogeography, Palaeoclimatology, Palaeoecology 441, 912–923 (2016).
Hachich, N. F. et al. Island biogeography: patterns of marine shallow-water organisms in the Atlantic Ocean. Journal of Biogeography 42, 1871–1882 (2015).
Quartau, R. et al. The morphology of insular shelves as a key for understanding the geological evolution of volcanic islands: insights from Terceira Island (Azores). Geochemistry, Geophysics, Geosystems 15, 1801–1826 (2014).
Abalde, S. et al. Phylogenetic relationships of cone snails endemic to Cabo Verde based on mitochondrial genomes. BMC Evolutionary Biology 17, 231 (2017).
Cunha, R. L. et al. Drivers of Cape Verde archipelagic endemism in keyhole limpets. Scientific Reports 7, 41817 (2017).
Meco, J. Los Strombus neógenos y cuaternarios del Atlántico euroafricano, taxonomía, bioestratigrafía y paleoecología. Ph.D. Thesis, Universidad Complutense de Madrid, 1976, Cabildo Gran Canaria, 207 pp (1977).
Meco, J., Petit-Maire, N., Fontugne, M., Shimmield, G. & Ramos, A. J. G. The Quaternary deposits in Lanzarote and Fuerteventura (eastern Canary Islands, Spain): an overview; pp. 123–136 In: J. Meco, N. Petit-Maire, (Eds.), Climates of the Past. Universidad de Las Palmas de Gran Canaria, Unesco, (International Union of Geological Sciences) (1997).
Montesinos, M. et al. Extralimital Senegalese species during Marine Isotope Stages 5.5 and 11 in the Canary Islands (29° N): Sea surface temperature estimates. Palaeogeography, Palaeoclimatology, Palaeoecology 410, 153–163 (2014).
Ávila, S. P. et al. A review of the MIS 5e highstand deposits from Santa Maria Island (Azores, NE Atlantic): palaeobiodiversity, palaeoecology and palaeobiogeography. Quaternary Science Reviews 114, 126–148 (2015).
Ávila, S. P. et al. Local disappearance of bivalves in the Azores during the last glaciation. Journal of Quaternary Science 23, 777–785 (2008).
MacArthur, R. H. & Wilson, E. O. The Theory of Island Biogeography. Princeton University Press, Princeton (1967).
García-Talavera, F. L. Macaronesia. Consideraciones geológicas, biogeográficas y paleoecológicas; pp. 41–63 in: J. M. Fernández-Palacios, J. J. Bacallado, J. A. Belmonte (Eds), Ecología y cultura en Canarias. Museo de las Ciencias y el Cosmos: (1999).
Ávila, S. P. & Malaquias, M. A. E. Biogeographical relationships of the molluscan fauna of the Ormonde seamount (Gorringe bank, Northeast-Atlantic Ocean). Journal of Molluscan Studies 69, 145–150 (2003).
Rolán, E. Malacological fauna from the Cape Verde archipelago - Part I - Polyplacophora and Gastropoda. ConchBooks, Vigo, 455 pp (2005).
Rolán, E. Moluscos y conchas marinas de Canarias. ConchBooks, Hackenheim & Emilio Rolán, Vigo, 716 pp (2011).
Cunha, R. L., Castilho, R., Rüber, L. & Zardoya, R. Patterns of cladogenesis in the venomous marine gastropod genus Conus from the Cape Verde Islands. Systematic Biology 54, 634–650 (2005).
Cunha, R. L. et al. Evolution at a different pace: distinctive phylogenetic patterns of cone snails from two ancient oceanic archipelagos. Systematic Biology 63, 971–987 (2014).
Fraussen, K. & Swinnen, F. A review of the genus Euthria Gray, 1839 (Gastropoda: Buccinidae) from the Cape Verde Archipelago. Xenophora Taxonomy 11, 9–31 (2016).
Duda, T. F. J. & Rolán, E. Explosive radiation of Cape Verde Conus, a marine species flock. Molecular Ecology 14, 267–272 (2005).
Peters, H., O’Leary, B. C., Hawkins, J. P., Carpenter, K. E. & Roberts, C. M. Conus: First Comprehensive Conservation Red List Assessment of a Marine Gastropod Mollusc Genus. PLoS One 8, e83353 (2013).
Segers, W., Swinnen, F., de Prins, R. Marine molluscs of Madeira. Snoeck Publishers, Zwijndrecht, 612 pp (2009).
Ávila, S. P. et al. Palaeoecology of the Pleistocene (MIS 5.5) outcrops of Santa Maria Island (Azores) in a complex oceanic tectonic setting. Palaeogeography, Palaeoclimatology, Palaeoecology 274, 18–31 (2009).
Valdés, L. & Déniz‐González I. Oceanographic and biological features in the Canary Current Large Marine Ecosystem. IOC‐UNESCO, Paris. IOC Technical Series, No. 115: 383 pp. (2015).
Wirtz, P. Thirteen new records of marine invertebrates and fishes from the Cape Verde Islands. Arquipélago. Life and Marine Sciences 26, 51–56 (2009).
Wirtz, P. Seven new records of fish from Ngor Island, Senegal. Arquipélago. Life and Marine Sciences 29, 77–81 (2012).
Summerer, M., Hanel, R. & Sturmbauer, C. Mitochondrial phylogeny and biogeographic affinities of sea breams of the genus Diplodus (Sparidae). Journal of Fish Biology 59, 1638–1652 (2001).
Rocha, L. et al. Recent invasion of the tropical Atlantic by an Indo-Pacific coral reef fish. Molecular Ecology 14, 3921–3928 (2005).
Cooper, W. J., Albertson, R. C., Jacob, R. E. & Westneat, M. W. Re-description and reassignment of the damselfish Abudefduf luridus (Cuvier, 1830) using both traditional and geometric morphometric approaches. Copeia 2014, 473–480 (2014).
Cooper, W. J., Smith, L. L. & Westneat, M. W. Exploring the radiation of a diverse reef fish family: phylogenetics of the damselfishes (Pomacentridae), with new classifications based on molecular analyses of all genera. Molecular Phylogenetics and Evolution 52, 1–16 (2009).
Wirtz, P. A new species of Malacoctenus from the Cape Verde Islands, eastern Atlantic (Pisces Teleostei, Labrisomidae). Arquipélago. Life and Marine Sciences 31, 15–20 (2014).
Iglésias, S. P., Frotté, L. & Sellos, D. Y. Gobius salamansa, a new species of goby (Gobiidae) from the Cape Verde Islands supported by a unique cephalic lateral line system and DNA barcoding. Ichthyological Research 63, 356–369 (2016).
Schliewen, U. K., Wirtz, P. & Kovačić, M. Didogobius janetarum sp. nov., a new cryptobenthic goby species from the Cape Verde Islands (Teleostei: Gobiidae). Zootaxa 4438, 381–393 (2018).
Ávila, S. P. et al. How did they get here? Palaeobiogeography of the Pleistocene marine molluscs of the Azores. Bulletin of the Geological Society of France 180, 201–213 (2009).
Gerber, J., Hemmen, J. & Groh, K. Eine pleistozäne marine molluskenfauna von Porto Santo (Madeira-Archipel). Mitteilungen der Deutschen Malakozoologischen Gesellschaft 44–45, 19–30 (1989).
Cabero, A. et al. The role of Cape Verde and the Canary Islands in the Atlantic Mediterranean molluscan migrations (interglacial stages); pp. 163–189 in: J. M. Fernández-Palacios, L. Nascimento, J. C. Hernández, S. Clemente, A. González, J. P. Díaz-González (Eds), Climate Change perspectives from the Atlantic: past, present and future. Universidad de La Laguna, Tenerife (2013).
Whittaker, R. J. & Fernández-Palacios, J. M. Island Biogeography. Ecology, evolution, and conservation, 2nd Edition. Oxford University Press, Oxford, UK (2007).
Whittaker, R. J., Fernández-Palacios, J. M., Matthews, T. J., Rigal, F. & Triantis, K. A. Archipelagos and meta-archipelagos. Frontiers of Biogeography, https://doi.org/10.21425/F5FBG41470 (2019).
Micael, J. et al. Shallow-water bryozoans from the Azores (central North Atlantic): native vs. non-indigenous species, and a method to evaluate taxonomic uncertainty. Marine Biodiversity 49, 469–480 (2019).
Ramalho, R. S. et al. The emergence and evolution of Santa Maria Island (Azores) – the conundrum of uplifted islands revisited. Geological Society of America Bulletin 129, 372–390 (2017).
Kleine, B. & Siedler, G. On the origin of the Azores current. Journal of Geophysical Research 94(C5), 6159–6168 (1989).
Bashmachnikov, I., Lafon, V. & Martins, A. Sea surface temperature distribution in the Azores region. Part II: space-time variability and underlying mechanisms. Arquipélago. Life and Marine Sciences 21A, 19–32 (2004).
Caldeira, R. M. A. & Reis, J. C. The Azores confluence zone. Frontiers in Marine Science 4, 37 (2017).
Mata, J. et al. O arquipélago da Madeira. Geologia de Portugal. Escolar Editora vol. 2, pp 691–746 (2013).
Ramalho, R. et al. The emergence of volcanic oceanic islands on a slow-moving plate: the example of Madeira Island, NE Atlantic. Geochemistry, Geophysics, Geosystems 16, 522–537 (2015).
Hernández-Guerra, A., López-Laatzen, F., Machín, F., Armas, D. & Pelegrí, J. L. Water masses, circulation and transport in the eastern boundary current of the North Atlantic subtropical gyre. Scientia Marina 65, 177–186 (2001).
Bogaard, Pvanden The origin of the Canary Island Seamount Province — new ages of old seamounts. Scientific Reports 3, 2107 (2013).
Ramalho, R. S. Building the Cape Verde Islands (1st ed.): Berlin, Springer, 207 pp, https://doi.org/10.1007/978-3-642-19103-9 (2011).
Peña-Izquierdo, J. et al. The continental slope current system between Cape Verde and the Canary Islands. Scientia Marina 76, 65–78 (2012).
Mason, E., Coombs, S. H. & Oliveira, P. B. An overview of the literature concerning the oceanography of the eastern North Atlantic region. Relatórios Científicos e Técnicos IPIMAR Serie Digital 33, 1–57 (2005).
Pelegrí, J. L. & Peña-Izquierdo, J. Eastern boundary currents off North-West Africa; pp. 80–92. In: JL. Valdés, I. Déniz-González (Eds.), Oceanographic and biological features in the Canary Current Large Marine Ecosystem. Intergovernmental Oceanographic Commission Technical Series 115 (2015).
García, L. Presencia de Calappa tuerkayana Pastore, 1995 (Decapoda: Brachyura: Calappidae) en el Mediterráneo Occidental. Bolletí de la Societat d’Història Natural de les Balears 45, 217–223 (2002).
García, L. & Corbera, J. Els crancs de les Balears. Inca: Edicions Documenta Balear, 104 pp (2007).
d’Udekem d’Acoz, C. Remarks on the genera Balssia Kemp, 1922 and Acanthonyx Latreille, 1828 in the Azores, and first record of Calappa tuerkayana Pastore, 1995 (Crustacea, Decapoda) in the Atlantic Ocean. Arquipélago. Life and Marine Sciences 18-A, 53–59 (2001).
Holthuis, L. B. Nomenclatural notes on Mediterranean species of Calappa Weber, 1795 (Crustacea: Decapoda: Brachyura). Zoologische Verhandelingen, Leiden 334, 99–102 (2001).
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (2017).
Oksanen, J. et al. vegan: Community Ecology Package. R package version 2, 4–2 (2017).
Dray, S. & Dufour, A. B. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22, 1–20 (2007).
Maechler, M., Rousseeuw, P., Struyf, A., Hubert, M. & Hornik, K. cluster: Cluster Analysis Basics and Extensions. R package version 2.0.7–1 (2018).
Hurley, C. gclus: Clustering Graphics. R package version 1.3.1 (2012).
Dapporto, L. et al. L. recluster: Ordination Methods for the Analysis of Beta-Diversity Indices. R package version 2, 8 (2015).
Jaccard, P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bulletin de la Société vaudoise des sciences naturelles 37, 547–579 (1901).
Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskab 5, 1–34 (1948).
Ochiai, A. Zoogeographical studies on the soleoid fishes found in Japan and its neighbouring regions. Bulletin of the Japanese Society of Scientific Fisheries 22, 526–530 (1957).
Simpson, G. G. Notes on the measurement of faunal resemblance. American Journal of Science 258-A, 300–311 (1960).
Legendre, P. & Legendre, L. Numerical ecology. 2nd English Edition, Elsevier, Amsterdam, 852 pp. (1998).
Sokal, R. R. & Michener, C. A statistical method for evaluating systematic relationships. University of Kansas Science Bulletin 38, 1409–1438 (1958).
Sokal, R. R. & Sneath, P. H. A. Principles of Numerical Taxonomy. W.H. Freeman, San Francisco (1963).
Ward, J. H. Jr. Hierarchical Grouping to Optimize an Objective Function. Journal of the American Statistical Association 58, 236–244 (1963).
Sokal, R. R. & Rohlf, F. J. The comparison of dendrograms by objective methods. Taxon 11, 33–40 (1962).
Borcard, D., Gillet, F. & Legendre, P. Numerical Ecology with R. 1st ed. Springer-Verlag. New York, US., 306 pp (2011).
Pavão, D. C., Elias, R. B. & Silva, L. Comparison of discrete and continuum community models: Insights from numerical ecology and Bayesian methods applied to Azorean plant communities. Ecological Modelling 24, 93–106 (2019).
Rousseeuw, P. J. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. Journal of Computational and Applied Mathematics 20, 53–65 (1987).
Kreft, H. & Jetz, W. A framework for delineating biogeographic regions based on species distributions. Journal of Biogeography 37, 2029–2053 (2010).
Dapporto, L., Fattorini, S., Voda, R., Dinca, V. & Vila, R. Biogeography of western Mediterranean butterflies: combining turnover and nestedness components of faunal dissimilarity. Journal of Biogeography 41, 1639–1650 (2014).
Dapporto, L. et al. recluster: an unbiased clustering procedure for beta-diversity turnover. Ecography 36, 1070–1075 (2013).
Petuch, E. J. Biogeography and biodiversity of Western Atlantic mollusks, xvii+234 pp. Taylor & Francis, Boca Raton, Florida (2013).
Morrone, J. J. Homology, biogeography and areas of endemism. Diversity and Distributions 7, 297–300 (2001).
Morrone, J. J. Presentación sintética de un nuevo esquema biogeográfico de América Latina y el Caribe. Red Iberoamericana de Biogeografía y Entomología Sistemática 2, 267–275 (2002).
Myers, A. A. & de Grave, S. Endemism: origins and implications. Vie et Milieu 50, 195–204 (2000).
Acknowledgements
PW was funded by FCT UID/Multi/04326/2019. This study was also funded by FCT UID/AGR/04129/2019 to Linking Landscape, Environment, Agriculture and Food (LEAF). We benefited from helpful discussions with many colleagues including Ricardo Ramalho and José Madeira (University of Lisbon) and Rui Quartau (Instituto Hidrográfico, Portugal). As is the case with all independent peer-reviewed research, the views and conclusions in the paper are those of the authors alone. The funding organizations did not have any role in writing the paper and did not review it. This work was supported by FEDER funds through the Operational Programme for Competitiveness Factors – COMPETE, by National Funds through FCT – Foundation for Science and Technology under the UID/Multi/04326/2013, UID/BIA/50027/2013, UID/BIA/00329/2013, 2015–2018, UID/BIA/00329/2019, and POCI-01-0145-FEDER-006821; and by regional funds through DRCT-M1.1.a/005/Funcionamento-C-/2016 (CIBIO-A). This work was also financed by FEDER in 85% and by Azorean Public funds by 15% through Operational Program Azores 2020, under the project AZORESBIOPORTAL –PORBIOTA (ACORES-01-0145-FEDER-000072). RF is supported by Campus do Mar International Campus of Excellence PhD program (DO*MAR) from University of Vigo, Spain. MR was funded by MACDIV–FCT-PTDC/BIABIC/0054/2014, and CVAgrobiodiversity/333111699—Aga Khan Development Network (AKDN) and “Fundação para a Ciência e a Tecnologia” (FCT). RC, PM and LB benefited from PhD grants SFRH/BD/60366/2009, SFRH/BD/61146/2009, and SFRH/BD/135918/2018, respectively, funded by FCT. J.A.G has benefited from research funds provided by the EU ERDF projects MACAROFOOD (MAC/2.3d/015) and MARISCOMAC (MAC/2.3d/097). RH has benefited from research funds provided by the EU ERA-Chair project EcoAqua (Grant # 621341). CSM is benefiting from a PhD grant M3.1.a/F/100/2015 from FRCT/Açores 2020 by Fundo Regional para a Ciência e Tecnologia (FRCT). SPA acknowledges his IF/00465/2015 research contract funded by the Portuguese Science Foundation (FCT).
Author information
Authors and Affiliations
Contributions
Initial framework by S.P.A., M.R., R.F. and P.W. The writing was led by S.P.A., M.R., B.B. and M.J. and all co-authors equally contributed to its final design. L.S. performed all the statistical analyses. S.P.A., L.S. and M.R. interpreted the results with input from all authors. Checklists were compiled by: coastal fishes (R.F., J.F., A.B., P.W., S.F., P.A., F.P. and S.P.A.); echinoderms (PM); gastropods (R.C., S.P.A.); brachyuran decapods (J.A.G., O.O., S.P.A.); annelids (A.M., J.N.); and algae (M.A.V.-R., A.I.N., R.H., A.C.R. and S.P.A.). Biogeographic-related bibliography review was made by S.P.A., M.R. and L.B. Figures were structured by S.P.A. and C.S.M. and drafted by C.S.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Freitas, R., Romeiras, M., Silva, L. et al. Restructuring of the ‘Macaronesia’ biogeographic unit: A marine multi-taxon biogeographical approach. Sci Rep 9, 15792 (2019). https://doi.org/10.1038/s41598-019-51786-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51786-6
This article is cited by
-
The zoogeographic regionalization for cephalopoda linked to the canary current upwelling system
Marine Biology (2024)
-
Morphospecies and molecular diversity of ‘lace corals’: the genus Reteporella (Bryozoa: Cheilostomatida) in the central North Atlantic Azores Archipelago
BMC Ecology and Evolution (2022)
-
Molecular evidence for extensive discontinuity between peracarid (Crustacea) fauna of Macaronesian islands and nearby continental coasts: over fifty candidate endemic species
Marine Biology (2022)
-
Biogeography of endosymbionts (Symbiodiniaceae) associated with zoantharian species (Hexacorallia: Anthozoa) from the Macaronesia and Cape Verde ecoregions
Coral Reefs (2022)
-
The geography of body size in cuttlefishes (Cephalopoda, Sepiidae)
Swiss Journal of Palaeontology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.