Abstract
Duchenne muscular dystrophy (DMD) is a uniformly fatal condition of striated muscle wasting resulting in premature death from respiratory and/or cardiac failure. Symptomatic therapy has prolonged survival by limiting deaths resulting from respiratory insufficiency, but there is currently no effective therapy for most patients with DMD. This grim prognosis has led patients and their families to seek unproven therapeutic approaches. One such approach is the use of hyperbaric therapies, which 14% of DMD patients self-report using. The primary goal of this study was to determine if intermittent hyperbaric exposure altered the muscle function of the mdx mouse, a genetic model of DMD. To do this, mdx mice were exposed to three daily 90-minute 1.3 atmosphere hyperbaric exposures for 4 weeks. Skeletal muscle, respiratory, and cardiac function were assessed in treated and untreated wild type and dystrophic mice. The results of these studies find that hyperbaric and hyperoxic approaches resulted in increased cardiac fibrosis in dystrophic mice and no beneficial effects on the functional parameters measured. These data suggest that these oxygen-based therapies are unlikely to provide therapeutic benefit to DMD patients.
Similar content being viewed by others
Introduction
Duchenne muscular dystrophy (DMD) is a devastating disease characterized by muscle wasting, respiratory insufficiency, and cardiomyopathy1,2,3. This disease is uniformly fatal and there are currently no therapies that are effective for all DMD patients. This has resulted in the pursuit of a wide variety of alternative therapies by patients and their families. Registry data of DMD patients reveals that 14% of patients are using hyperbaric therapies4, despite the lack of evidence supporting a therapeutic benefit. Given that patients and their families are already utilizing this approach, we undertook this study to assess the efficacy of hyperbaric exposure in a mouse model of DMD.
Hyperbaric oxygen therapy consists of placing patients into environments with elevations in barometric pressure, sometimes with additional supplemental oxygen. This increases the concentrations of oxygen available to the body. Hyperbaric therapy has FDA approval for a wide variety of disease conditions, most notably decompression syndrome5, carbon monoxide poisoning6, and poorly vascularized inflammatory conditions (e.g. crush injuries), especially when complicated by anaerobic bacterial infection7,8,9. Exposure to a hyperbaric environment increases the level of oxygen dissolved within the blood, but notably it does not have a dramatic effect on overall oxygen carrying capacity.
To our knowledge, there is no evidence supporting or refuting the efficacy of hyperbaric therapy in DMD. Despite this, hyperbaric therapy is being pursued by many DMD patients. In addition to questions regarding the efficacy of hyperbaric therapies, there remain significant questions about the overall safety of this approach. It is hypothesized that any beneficial effects of hyperbaric exposure will be the result of increased oxygen delivery. To control for this possibility, we have included an experimental group in which only the levels of ambient oxygen are changed. The study reported here assessed the effect of 4 weeks of hyperbaric or hyperoxic therapy on the skeletal muscle, respiratory muscle, and cardiac function in the mdx mouse.
Results
Hyperbaric and hyperoxia therapeutic approaches
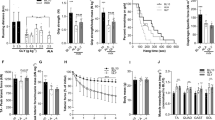
C57BL/10 and mdx mice were subjected to 4 weeks of hyperbaric, hyperoxia, or standard room conditions. All mice were housed in the same room to control for environmental factors that might contribute to the progression of the disease. The goal of this study was to test the hypothesis that increases in oxygen delivery, either by hyperbaric approaches or increasing the concentration of environmental oxygen, would improve the dystrophic phenotype. To achieve this, mice were subjected to three 90-minute periods of hyperoxia/hyperbaric conditions each day (Fig. 1). These periods occurred at set times during the day throughout the protocol. This protocol represents a high degree of hyperbaric exposure and is similar to protocols recommended to DMD patients. Hyperbaric chamber pressure and hyperoxic chamber oxygen levels were computer controlled with monitoring throughout the protocol to ensure that the degree of exposure was properly matched.
Effect of oxygen therapy on dystrophic skeletal muscle function
Whole body grip strength is an assay that provides a measure of global muscle strength in the mouse. This assay demonstrated a significant reduction in strength in mdx mice (Fig. 2A). The weakness observed in dystrophic mdx mouse muscle was not significantly altered by any of the oxygen based therapeutic approaches (Fig. 2A). Oxygen therapies also had no significant effect on quadricep fibrosis, central nucleation, or fiber cross-sectional area distribution in mdx or C10 mice (Fig. 2B,C), although highly significant genotype effects are present. Downhill running is another assay in which mdx mice perform significantly worse than wild type mice. Similar to the results from the hand grip assay, the performance of mdx mice on the treadmill was not significantly improved by hyperbaric or hyperoxic therapeutic modalities (Fig. 3).
Grip strength and histological assessments of C10 and mdx mice following 4 weeks of oxygen based treatments. (A) Mean whole body grip strength, (B) percentage of quadricep fibers with central nucleation, and (C) the distributions of quadricep cross-sectional area from wild type (C10) and dystrophic (mdx) mice after normobaric, hyperbaric, or hyperoxic exposure for 4 weeks. *P < 0.001, **P < 0.0001.
Effects of oxygen therapy on respiratory function
Respiratory failure is a leading cause of death in DMD and any therapy that improves respiratory function would be of great value. The use of whole body plethysmography provides a means to assess the respiratory function of conscious mice. Data were analyzed using a three-way ANOVA with genotype, time, and treatment as factoring groups. This analysis revealed a significant genotype effect for both minute volume and breathing frequency (P < 0.01). This effect was independent of any treatment protocol and is present in mice prior to beginning the experimental treatment, indicating that dystrophic mice have lower resting respiratory rates relative to wild type mice. Histopathological assessment of diaphragm fibrosis also found no effect of treatment on the degree of pathology present in the dystrophic diaphragm (Fig. 4).
Whole body plethysmography data from C10 and mdx mice following four weeks of oxygen based treatments. Measures of global respiratory function before (white) and after (grey) four weeks of hyperbaric or hyperoxic treatment. Breath rate (A), tidal volume (B), and minute volume (C) are shown. There is no significant treatment effect on the histopathological appearance of the diaphragm muscle (D,E). Group sizes range from 3–7 mice, no significant treatment effects are observed with a three-way ANOVA. Bar represents 100 µm.
Effects of oxygen therapy on cardiac function
Cardiac dysfunction is very common in patients with DMD and mdx mice have reductions in cardiac reserve10,11,12,13. To assess the effects of oxygen therapies on dystrophic cardiac function, cardiac catheterization was employed following 4 weeks of either hyperbaric therapy, hyperoxic therapy, or no therapy. The cardiac catheterization protocol consisted of three distinct treatments; baseline conditions, maximal contractile function caused by β-adrenergic receptor stimulation by dobutamine infusion, and measures of contractility in the presence of β-adrenergic receptor blockade with esmolol infusion. These data are provided in Tables 1–6. There are several parameters in which genotype effects are present, these include heart rate, systolic pressure, and minimum pressure derivative. As demonstrated in Tables 1–6, there are also parameters that have main effect differences based on the treatment protocol. Wild type hearts demonstrate significantly greater response to β-adrenergic stimulation by dobutamine in both systolic (Fig. 5A) and diastolic (Fig. 5B) parameters of cardiac function. None of these parameters were significantly altered by the oxygen therapy protocol. Similarly, the lack of a difference in the magnitude of response to the blockade of the β-adrenergic receptor by the antagonist esmolol, demonstrates that mdx mice have greater dependence on endogenous β-adrenergic receptor activation tone for maintenance of baseline systolic function (Fig. 5C). No differences were observed in measures of diastolic function. These studies demonstrate that the oxygen therapies implemented in these studies have no significant effects on baseline cardiac function, cardiac reserve, or the level of adrenergic receptor activation to support cardiac function.
Measures of cardiac reserve and basal sympathetic tone in wild type and dystrophic hearts following 4 weeks of oxygen based therapy. The cardiac reserve (A,B) is determined during the infusion of 20 µg/kg/min of dobutamine and is expressed as the change above baseline levels. Sympathetic tone (C,D) is determined from the change from baseline levels to that during 250 µg/kg/min infusion of esmolol. The systolic (dP/dtMax) and diastolic (Tau) measures of cardiac reserve display a significant genotype effect, but there is no effect of the treatment protocol on cardiac reserve. The basal systolic function of the dystrophic heart is supported by a greater level of sympathetic tone relative to wild type hearts. The effect of removing sympathetic tone on diastolic function is not different between genotypes. The treatment protocol does not have an effect on basal sympathetic tone. Group sizes are listed in panel D. *P < 0.05.
Dystrophic heart disease is associated with the progressive accumulation of fibrosis. The area of cardiac fibrosis was measured in hearts of mice following oxygen-based therapies. Under normoxic conditions, the young dystrophic mice used in this study had normal levels of fibrosis. Importantly, dystrophic mice exposed to either hyperbaric or hyperoxic displayed significantly greater areas of fibrosis than non-dystrophic mice exposed to the same conditions (Fig. 6). Analysis of hydroxyproline content of these hearts show an increase in mdx hearts subjected to hyperbaric therapy (27.9 ± 2.2 vs. 21.6 ± 1.7 µg hydroxyproline per mg of protein for mdx and C10 hearts respectively; p = 0.04). Interestingly, this assay does not demonstrate significant changes between the genotypes of the other treatment groups; 33.3 ± 3.7 in mdx hearts vs. 25.1 ± 3.2 in C10 hearts with normoxia and 16.9 ± 5.0 in mdx hearts vs. 22.3 ± 4.2 in C10 hearts exposed to hyperoxic conditions.
Cardiac fibrosis in C10 and mdx mice following 4 weeks of oxygen based treatment. Sirius red/fast green stain reveals increased collagen staining (red) in mdx hearts with both hyperbaric and hyperoxic conditions. Each image is 1 mm2. ANOVA with Tukey posttest was used to determine statistical significance. *Denotes a significant (P < 0.05) difference from mdx under normoxic conditions; †Denotes a significant difference from corresponding C10 treatment group.
Discussion
The primary motivating factor for these studies was to determine if hyperbaric therapy would have an effect on the pathophysiology of Duchenne muscular dystrophy (DMD) as manifested in the mdx mouse. Patients are already using this therapy in the absence of convincing data that it is effective, or even safe, for DMD patients. The results of this study demonstrate an increase in cardiac fibrosis area in dystrophic mice exposed to either of the experimental protocols and increased collagen content in mdx mice exposed to hyperbaric conditions. Furthermore, there is no evidence that the hyperbaric or hyperoxic exposure has any significant effect on skeletal muscle function with no significant changes observed throughout the 4 weeks of the protocol. These data are consistent with the lack of an effect of elevated oxygen exposure in chickens with muscular dystrophy14.
The protocol of 90 minutes of 1.3 atmosphere hyperbaric therapy, three times a day, seven days a week was designed to mimic a protocol that might be feasible with a small in-home hyperbaric chamber. This is the most likely form of hyperbaric therapy to be used by DMD patients as it has relatively low cost and does not require a special trip to a hyperbaric clinic. All mice in this study also received daily doses of ascorbic acid, similar to what is recommended to DMD patients, to limit any potential oxidative damage resulting from prolonged periods of hyperbaric or hyperoxic exposures. Given the highly controlled nature of this experiment, compliance to the protocol was 100%, which is likely greater than what would be achieved by DMD patients.
The current study has some notable limitations. First is the relatively modest disease present in the mdx mouse used in these studies. The mdx mouse was chosen for these studies as it provides a genetic model of DMD with other compensatory pathways intact and the mild phenotype was hypothesized to provide the best opportunity to observe any beneficial effects of hyperbaric or hyperoxic therapies. It is possible that the duration of the protocol was not sufficient to achieve the maximal effects of hyperbaric or hyperoxic therapy. However, if these therapies, as proposed, limited muscle damage or improved muscle recovery these effects should have been evident after four weeks of therapy, as any damaged muscle fibers would have been expected to recover by the end of the study.
The observed increase in cardiac fibrosis in dystrophic mice exposed to hyperbaric and hyperoxic conditions raises important questions regarding the safety of these approaches. One possible mechanism of this cardiac damage may result from stress the dystrophic mice feel in response to the raising of pressure or increases in oxygen. Perhaps the noise within the chambers causes an increase in sympathetic nervous system activity in the heart, increasing heart rate and cardiac contractility. The resulting increased work load may either damage the dystrophic heart or activate profibrotic signaling pathways resulting in the accumulation of fibrosis in the myocardium. Another potential mechanism is the effect of increased oxygen delivery on the respiratory drive of the dystrophic mice. Patients with chronic hypoventilation begin to rely on a degree of hypoxia to drive respiration, such that the sudden introduction of supplemental oxygen can decrease their respiratory efforts15. The result is that additional oxygen decreases ventilation resulting the development of respiratory acidosis, which may contribute to cardiac injury leading to the development of cardiac fibrosis. Based on the results of this study, DMD patients should be careful when using hyperbaric therapies to ensure that respiratory function is maintained during the course of the treatment period.
In summary, our findings suggest that 4 weeks of intermittent hyperbaric or hyperoxic exposure may result in an acceleration of cardiac disease and has no significant benefit on the skeletal muscle or respiratory disease process in mdx mice. The poor efficacy of these oxygen delivery based therapeutic approaches indicate that the levels of dissolved oxygen are not a significant contributor to the pathophysiology of the dystrophic mouse. The inability of these therapeutic approaches to provide any benefit to the mdx mouse raises important questions about the potential efficacy in DMD patients. The current lack of an effective therapy for many DMD patients results in patients and their families to seek alternative therapies, our data suggests that hyperbaric approaches, are unlikely to slow the course of disease progression and there is some potential that they may accelerate cardiac injury.
Methods
Animals
The mice used in these studies were single-housed males ranging from 4–6 months of age and taken from colonies of C57BL/10-SnJ (C10) and C57BL/10ScSn-DMD〈mdx〉/J mice maintained at the University of Minnesota. Genetic stability of the colony is maintained through the introduction of purchased breeders from Jackson Laboratories (Bar Harbor, ME) every 5 generations. While in the protocol of this study, all mice were given 12 mg/kg ascorbic acid in a small cookie (0.25 of a miniNilla wafer, Mondelez Global LLC), to mirror the antioxidant load in many patients using hyperbaric therapy. The mice readily consumed the cookie under the observation of study staff. All procedures were performed according to protocols reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Hyperbaric chamber
The hyperbaric chamber used was a Vitaeris 320 (OxyHealth LLC, Santa Fe Springs, CA), mice in standard mouse cages were placed within the chamber. The pressure within the chamber was controlled by custom designed computer-controlled valves and switches that allowed precise control of the timing of chamber pressure. Pressure within the chamber was determined by the pressure valve that is standard with the chamber and monitored using a barometric pressure sensor (BMP280 Pressure Sensor, Adafruit, New York, NY). Chamber carbon dioxide levels were monitored by using a K-30 CO2 sensor (SE-0018 CO2Meter.com). These signals were digitized using an Arduino R3 microcontroller (SainSmart, Lenexa, KS) connected via Bluetooth serial connection to a computer outside of the chamber. All electronic elements within the chamber were battery powered. During periods of normobaric pressures, the chamber air was exchanged using an air-pump (EcoPlus Commercial Air 7, Amazon, Seattle, WA) that maintained CO2 levels below 600 ppm.
Hyperoxic chamber
Mice were placed in standard mouse cages; these cages were placed into a sealed cabinet. Oxygen levels were monitored using oxygen sensors (R17S, OxyCheq, Marianna, FL) digitized with Ardunino R3 microcontroller with 12-bit amplifier (ADS1015, Adafruit, New York, NY). Oxygen flow into the chamber was controlled by the same computer controlling the hyperbaric chambers, such that timing of experimental manipulations was synced. During periods of normoxia the chamber was flushed with room air to maintain a CO2 level below 600 ppm. Normoxic mice were housed in the same room as the HBOT and Hyperoxic treated mice, but were allowed to breathe room air.
Whole-body plethysmography
Assessments of respiratory function were performed using the commercially available Buxco plethysmography system from Data Sciences International (Harvard Bioscience, Inc., Holliston, MA). Briefly, mice were placed in the plethysmography chamber following the first hyperbaric/hyperoxic period of the day. Mice were left in the chamber for 90 minutes during which time they were monitored by closed-circuit camera. Quality plethysmography data is obtained once the mice have fallen asleep, thus maintaining an undisturbed state was particularly important. Following completion of the measurement period, mice were returned to their home cages and returned to their treatment chambers.
Treadmill protocol
On days for which treadmill protocols were scheduled, mice were removed from their treatment chambers after the first hyperbaric/hyperoxic period and placed in one of the lanes of a Columbus Instruments rodent treadmill (Columbus, OH). Prior to initiation of the studies mice were trained by being placed on the flat treadmill running a 5 m/min for 10–15 minutes. During the study, the treadmill was oriented such that mice ran down a 15° slope. Initial speed was set to 5 m/min for 5 minutes, then increased 1 m/min every minute until a maximum speed of 20 m/min was obtained. Mice were continued until exhaustion such that mice could no longer be encouraged to get on the treadmill and did not attempt to escape when removed from the treadmill lane.
Grip strength
Grip strength was assessed using the grip strength meter (Columbus Instruments, Columbus, OH). Overall muscle strength was assessed by taking the maximum tension produced from ten repeated pulls on the grip bar. This tension was then corrected for the body weight of the mouse.
Muscle morphology measurements
Quadricep cryosections were stained with WGA, goat-anti-mouse IgG, and DAPI to assess fiber morphology, acute muscle fiber injury, and nuclear localization. Image montages at 10x magnification were analyzed using a custom script in FIJI. Briefly, this script used the WGA channel to identify the boarders of fibers. A filter was applied to remove fibers that were not oriented in a cross-sectional plane. Central nucleation was determined by examining the DAPI signal within the central core of each fiber. For each mouse between 960 and 6049 fibers were assessed. Fiber cross sectional area distribution was calculated and plotted using R with ggplot16,17,18.
Hemodynamic measurements
Cardiac function was assessed by cardiac catheterization using pressure-volume conductance catheter (Transonic Systems, Ithaca, NY) and previously described12,13,19,20,21. Briefly, mice were anesthetized using isoflurane, ventilated, and the heart was exposed via thoracotomy. The catheter was inserted through an apical stab incision and positioned within the ventricle. A jugular catheter was placed, and the mouse allowed to stabilize following infusion of 10% albumin. Cardiac contractile reserve was assessed through the intravenous infusion of 20 µg/kg/min dobutamine and basal adrenergic tone was determined by the infusion of 250 µg/kg/min esmolol.
Fibrosis determination
Histological: At the time of euthanasia, hearts and muscles were collected and immediately frozen in liquid nitrogen cooled isopentane. These hearts were cryo-sectioned and stained with Sirius red/fast green as described previously10,22. Fibrosis was quantified in a blinded manner using ImageJ23. Briefly, 4x montages were made from short-axis cross sections of sirius red/fast green stained heart, diaphragm, and quadricep sections. The red staining collagen areas and total muscle area were manually determined using the color thresholding tool in ImageJ. The percentage of fibrosis was determined by calculating the number of positive red pixels divided by the total number of muscle pixels. Biochemical: Portion of hearts were homogenized in buffer (Tris-HCl: 20 mM, NaCl: 150 mM, Na-Pyrophosphate: 2.5 mM, EDTA: 1 mM, SDS: 1%, Leupeptin: 0.5 µg/ml, Pepstatin: 0.6 µg/ml, and PMSF: 0.1 mM). Protein concentration determined using the BCA assay from Thermo-Fisher. Hydroxyproline content was determined using previously described methods24.
Statistics and analysis
All collected data was analyzed using R25. All comparisons were analyzed by ANOVA with a Tukey post-hoc test to control for multiple comparisons.
References
Bushby, K. et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 9, 177–189 (2010).
Bushby, K. et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9, 77–93 (2010).
McNally, E. M. et al. Contemporary cardiac issues in duchenne muscular dystrophy. Circulation 131, 1590–8 (2015).
Wang, R. T. et al. Online Self-Report Data for Duchenne Muscular Dystrophy Confirms Natural History and Can Be Used to Assess for Therapeutic Benefits. PLoS Currents, https://doi.org/10.1371/currents.md.e1e8f2be7c949f9ffe81ec6fca1cce6a (2014).
Vann, R. D., Butler, F. K., Mitchell, S. J. & Moon, R. E. Decompression illness. The Lancet 377, 153–164 (2011).
Clower, J. H., Hampson, N. B., Iqbal, S. & Yip, F. Y. Recipients of hyperbaric oxygen treatment for carbon monoxide poisoning and exposure circumstances. American Journal of Emergency Medicine 30, 846–851 (2012).
Gandhi, J. et al. Clinical utility of hyperbaric oxygen therapy in genitourinary medicine. Medical Gas Research 8, 29 (2018).
Skyhar, M. J. et al. Hyperbaric oxygen reduces edema and necrosis of skeletal muscle in compartment syndromes associated with hemorrhagic hypotension. Journal of Bone and Joint Surgery - Series A 68, 1218–1224 (1986).
Strauss, M. B. et al. Reduction of skeletal muscle necrosis using intermittent hyperbaric oxygen in a model compartment syndrome. The Journal of bone and joint surgery. American volume 65, 656–62 (1983).
Meyers, T. A. & Townsend, D. Early right ventricular fibrosis and reduction in biventricular cardiac reserve in the dystrophin-deficient mdx heart. American Journal of Physiology - Heart and Circulatory Physiology 308, H303–H315 (2015).
Yasuda, S. et al. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature 436, 1025–1029 (2005).
Townsend, D., Yasuda, S., McNally, E. & Metzger, J. M. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. The FASEB journal 25, 3106–3114 (2011).
Townsend, D. et al. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther 15, 1086–1092 (2007).
Hirotani, H. & Kuyama, T. Hyperbaric oxygen therapy for muscular dystrophy. Nihon geka hokan. Archiv fur japanische Chirurgie 43, 161–7 (1974).
Gay, P. C. & Edmonds, L. C. Severe Hypercapnia After Low-Flow Oxygen Therapy in Patients With Neuromuscular Disease and Diaphragmatic Dysfunction. Mayo Clinic Proceedings 70, 327–330 (1995).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 (2012).
Team, R. C. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2017).
Hadley Wickham. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag New York, 2016).
Townsend, D., Daly, M., Chamberlain, J. S. & Metzger, J. M. Age-dependent dystrophin loss and genetic reconstitution establish a molecular link between dystrophin and heart performance during aging. Molecular Therapy 19, 1821–1825 (2011).
Townsend, D. Measuring Pressure Volume Loops in the Mouse. Journal of Visualized Experiments 1–9, https://doi.org/10.3791/53810 (2016).
Townsend, D., Yasuda, S., Li, S., Chamberlain, J. S. & Metzger, J. M. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Molecular Therapy 16, 832–835 (2008).
Stelter, Z. et al. Hypoxia-induced cardiac injury in dystrophic mice. American journal of physiology. Heart and circulatory physiology 310, H938–48 (2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 9, 676–682 (2012).
Reddy, G. K. & Enwemeka, C. S. A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry 29, 225–229 (1996).
Team, R. C. R: A language and environment for statistical computing, http://www.r-project.org/ (2017).
Acknowledgements
The authors would like to acknowledge OxyHealth for providing the hyperbaric chamber used in these studies and Tatyana Meyers for technical assistance.
Author information
Authors and Affiliations
Contributions
K.F. and J.H. performed experiments. K.F. and D.T. designed experiments and analyzed data. D.T. wrote the paper and prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, K.D., Heitzman, J.A. & Townsend, D. Hyperbaric therapy provides no benefit for skeletal muscle and respiratory function and accelerates cardiac injury in mdx mice. Sci Rep 9, 12306 (2019). https://doi.org/10.1038/s41598-019-48744-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-48744-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.