Abstract
Sponges are an important component of coral reef communities. The present study is the first devoted exclusively to coral reef sponges from Eastern Tropical Pacific (ETP). Eighty-seven species were found, with assemblages dominated by very small cryptic patches and boring sponges such as Cliona vermifera; the most common species in ETP reefs. We compared the sponge patterns from ETP reefs, Caribbean reefs (CR) and West Pacific reefs (WPR), and all have in common that very few species dominate the sponge assemblages. However, they are massive or large sun exposed sponges in CR and WPR, and small encrusting and boring cryptic species in ETP. At a similar depth, CR and WPR had seven times more individuals per m2, and between four (CR) and five times (WPR) more species per m2 than ETP. Perturbation, at local and large scale, rather than biological factors, seems to explain the low prevalence and characteristics of sponge assemblages in ETP reefs, which are very frequently located in shallow water where excessive turbulence, abrasion and high levels of damaging light occur. Other factors such as the recurrence of large-scale phenomena (mainly El Niño events), age of the reef (younger in ETP), isolation (higher in ETP), difficulty to gain recruits from distant areas (higher in ETP), are responsible for shaping ETP sponge communities. Such great differences in sponge fauna between the three basins might have consequences for coral reef structure and dynamics.
Similar content being viewed by others
Introduction
Coral reefs are among the most complex, and largest biological structures on earth1,2, and probably are the most diverse communities of the oceans and have an estimated of 1,330,000 species3,4. They are considered the ‘ocean’s rainforest by their high productivity5, and although they occupy less than one percent of the Earth’s marine environment6, provide important services to human communities, which represent a value of 352,249 US$ ha−1year−1 7.
In Eastern Tropical Pacific region (ETP), modern reef-building corals extend from the Gulf of California (Mexico) to Ecuador, with a distribution skewed toward the northern hemisphere8,9, since 46% of the coverage is located in Mexico10. These reefs are relatively recent, varying from 200 to 5600 years in age, with a thickness ranging from 0.2 to 13 m, 4.5 m in average11. They are mainly made up of interlocking, branching pocilloporids, constructed by very few species of the genus Pocillopora, or rarely built by massive corals of the genera Porites and Pavona11,12.
The general physical conditions of this vast region are not conducive to reef growth (see Discussion), and ETP reefs are typically small (a few hectares or less), patchily distributed, shallow and low in species diversity13,14,15. Comparing with Caribbean, ETP reefs are less vertically developed, less consolidated, smaller in extent and lacking biotic cementing and binding agents16,17,18.
In ETP there are also areas where corals do not form a continuous framework structure; rather, they form isolated patches of coral heads growing directly on bedrock called coral communities17,19. In both cases, whether forming true reefs or isolated patches, the interlocking, branching pocilloporids form a 3D framework with an extraordinary diversity of habitats.
The studies of coral reef organisms in ETP and their taxonomy are strong biased towards the most conspicuous organisms, such as fishes20,21, whereas most of the cryptic habitats that form the 3D framework remain largely ignored18,22,23. In fact, the coral reef cryptofauna community is understudied relative to surface reef fauna worldwide23,24,25.
Therefore, it is of great concern that our knowledge of coral associated invertebrates is so limited, especially in light of severe and ongoing degradation of coral reef habitats.
Sessile groups such as sponges usually dominate cryptofauna in coral reefs21,22. They constitute an abundant and functionally important component of coral reef systems that perform many important functional roles26,27. Sponges are important mediators of reef productivity28,29,30 and can take up dissolved organic matter (DOM) to generate an outflow of particulate organic matter (POM). Such outflow feeds other invertebrates at basal and intermediate levels of the reef trophic chain, therefore contributing to the energy requirements of these ecosystems via a pathway defined as the ‘sponge loop’30,31. Sponges also provide microhabitats for various invertebrate species as well as some fishes enhancing biodiversity26,32, and harbors microbial symbionts that can contribute to reef productivity33,34,35. Therefore, that changes in their abundance and diversity have the potential to affect overall reef ecosystem functioning. In addition, the importance of sponges on coral reefs worldwide is attracting more attention as the relative abundance of reef-building corals has declined36,37,38.
Unfortunately, despite of the important roles commented above, the sponge fauna of the ETP is probably the least known globally, with the exception of boring sponges39,40,41,42,43,44,45, and some conspicuous species46,47,48.
In this study, we examined patterns of sponge biodiversity in coral reefs across the entire Mexican Pacific Ocean. We also explore the possible causes that explain the differences between ETP and Caribbean (CR) and West Pacific Reefs (WPR).
Material and Methods
Study area
The present study was carried out on 20 coral reefs and coral communities distributed along the Mexican Pacific coast (Fig. 1). We consider coral reefs as coral-built structures elevated above the bottom and developed over an accumulation of dead coral framework49 (Fig. 2). On the other hand, we define, coral communities as areas with scattered colonies or isolated patches growing directly on bedrock. There is no continuous framework and these areas tend to have a low percentage of coral cover50.
In ETP we can find two different reef formations: Coral communities, defined as areas with scattered colonies or isolated patches growing directly on bedrock (A,B), and coral reefs, when a reef framework is developed (C). (D), Lower surface of B, showing different species of cryptic sponges (arrows). Images taken by JLC.
Owing to differences in the biological characteristics and composition of the coral communities, the reefs of the Pacific coast of México are naturally divided into three groups: those of the Gulf of California, the Revillagigedo Archipelago, and the tropical Mexican Pacific (Table 1).
The most important coral reefs in the ETP are in the southern region of Mexico (tropical Mexican Pacific), which harbors some of the most developed reefs such as La Entrega and San Agustín (Oaxaca state), and Caleta de Chon, Playa Manzanillo and Islote Zacatoso (Guerrero state)11,13,51. Nayarit state, in the center of the region, harbored some of the most important coral communities of the Mexican Pacific coast until the ENSO event of 1997/98, which caused a massive mortality and the loss of near 96% of coral cover52. This area harbors the archipelago islas Marías, that consists of four islands: María Madre, María Magdalena, María Cleofas and San Juanito; with María Cleofas having the largest coral cover14. In the north (Gulf of California) we can find coral reefs in Baja California Sur State, which harbors important coral reef formations in San Lorenzo, San Gabriel and Caleritas. Until the decade of the 90 s, the coral reef of Cabo Pulmo reached more than 150 ha, and it was acknowledged as the most important in the Mexican Pacific coast, but it lost most of its coverage after the El Niño 199753. Finally, the archipelago de Revillagigedo far away of the mainland, is a group of four volcanic Islands, with Socorro as the main one, which maintains a well-preserved reef in Playa Blanca, formed by branching corals of the genus Pocillopora, and massive corals of the genus Porites54.
Qualitative sampling
All studied ETP reefs were between 1 and 6 m depth. In each one, the sampling was undertaken by a 2 h random dive55, during which three divers searched for sponges in different areas of the reef, both exposed and cryptic habitats, which included the lower surfaces of live or dead corals, interstices of coral framework, and loose heads which were overturned and examined.
Fragments of specimens that we were not able to identify “in situ” were collected, fixed and preserved in 70% ethanol. Spicules were cleaned in boiling nitric acid followed by water rinse and dehydration in alcohol, then dried on a microscope slide or circular cover slip for SEM (scanning electron microscopy). Spicule measurements (30 for each type) were made by light microscopy.
Sampling in cryptic spaces is difficult and time consuming, and the differentiation of individuals of sponges to estimate biomass is virtually impossible. However, the simple presence/absence is enough for species richness56,57,58 Therefore, similarity among reefs was established by means of a classification analysis, using species as variables. The similarity matrix for the classification was calculated by means of the Sørensen index based on presence/absence59. The results were then graphically described using dendrograms with the UPGMA (unweighted pair-group method using centroids) aggregation algorithm60.
Multidimensional scaling and ordination were used to detect community patterns, using the PRIMER (v 6.1.11) software program61, and a two-dimensional non-metric Multidimensional Scaling (MDS), based on the Sørensen similarity matrix, was used to visualize community patterns (Fig. 3). The adequacy of the MDS was assessed through the stress coefficient, which should be <0.15 in order to minimize misinterpretations61.
Quantitative sampling
A quantitative sampling (density and species per square meter) was undertaken only in two reefs, because is a very difficult and time-consuming process. Besides, it’s also necessary brakes large coral pieces to detect cryptic sponges. However, the data can be considered as representative for the whole Mexican Pacific reefs. A previous study in Panama (Pacific side) also reached the same conclusion62. For that, five transects 18 m long were set up, and six quadrats of 1 m2 were placed along each one, resulting in a sampling area of 6 m2 per transect (a total of 30 m2 per reef). Density (ind. m−2) was estimated by counting all patches found inside each quadrant and later average per square meter. Species richness was estimated in the total of the sampling area (30 m2), and later average by square meter. In the case of boring sponges, their appearance in the samples was quantified as a unique patch due to the difficulty to differentiate among individuals.
Data from Caribbean (CR) and West Pacific reefs (WPR)
In order to compare the information gained from this study with those from CR and WPR, an exhaustive research of the literature was done. All the papers about coral reef sponges with information about number of species, abundance (density), diversity (species per surface unit) were utilized to obtain mean values per basin and depth (see for example Fig. 4).
Box and whisker plot of the variation of the abundance of sponges per region (ETP, CR, and WPR), and depth (above), and species per square meter (below). Average is represented by X, median by the horizontal line inside the box. The maximum and minimum values are displayed with vertical lines (“whiskers”) connecting the points to the box. Points are outliers’ values.
Statistics analyses
Differences in abundance and species between different depths and regions were analyzed by two-way ANOVA after verifying normality (Kolmogorov-Smirnov test)63 and variance homogeneity (Levene’s test)64. If the results of the ANOVA’s revealed a significant difference, a post hoc analysis (Multiple Range Tests) was then performed to evaluate the differences observed. The level of significance was 5% (p < 0.05). When heteroscedasticity was detected64, even after transformation, a significance value of p < 0.01 was adopted to avoid Type I error. As mean values were used for each region and depth, there were no replicates that allowed for the estimation of region x depth interaction. For example, there is no data for ETP below 6 m depth, and comparison at deepest depth is not possible.
Results
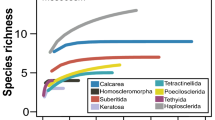
A total of 87 species belonged to 48 genera were identified. The order Clionaida had the highest number of species (23%), followed by Poecilosclerida (18%), Haplosclerida (13%) and Tetractinellida (10%). The rest of species are distributed in Agelasida, Axinellida, Bubarida, Dendroceratida, Dictyoceratida, Scopalinida, Suberitida, Tethyida and Verongida.
The most diverse family was Clionaidae, which contained four genera and 18 species, and the most common genus was Cliona with 13 species. In Haplosclerida the most common genus was Haliclona with five species, in Tetractinellida Thoosa with four species, and in Poecilosclerida it was Mycale with three species (Table 1). The species not identified are potential new species and are currently under study.
A high percentage of species (≈40%) (35 species) was recorded from one reef only, and only four species were common, co‐occurring in at least 10 different reefs (≥50% level of sites); these were Cliona vermifera (75% of the reefs), Thoosa mismalolli (63%), Pione carpenteri (55%) and Cliona tropicalis (52%). Other common species were Siphonodictyon crypticum (45%), Thoosa calpulli (45%), Callyspongia californica (41%) and Mycale cecilia (37%).
The number of species was highly variable among reefs (Table 1). The highest number was found at Playa Blanca (28 spp) (Isla Socorro, Revillagidedo archipelago), Isla Redonda (Marietas islands) (27), and Isla María Cleofas (Marías archipelago) (22); the lowest number of species was recorded at Roca Partida (4) and Clarion (5), both part of the Revillagigedo archipelago.
There were no clear groups because reefs were mixed on the cluster analysis (cluster not figured). In agreement with cluster, MDS did also not show a clear gradient and the reefs were arranged regionally only partially (stress 0.16) (Fig. 3); for example, the sponge community of Playa Blanca (Isla Socorro), is close to that of Punta Mita, although they are geographically separated from each other. On the right side of the ordination, some reefs that appeared together, as for example Islote Zacatoso, Playa las Gatas and Caleta de Chon, are spatially next to each other. In the left corner, appears some locations from Revillagigedo archipelago such as Clarion and Roca Partida, which are close to each other, and presented the lowest sponge diversity.
Regarding the quantitative sampling, the abundance varied from 0.57 to 4.3 (1.69 in average) ind. m−2. The number of species per m2 varied from 0.06 to 0.66 species per m2 (0.25 in average) (Fig. 4).
The overwhelming majority of the species was cryptic (Fig. 5), occurring as small encrusting patches underneath coral rubbles and dead corals, or boring, measuring in the order of centimeters, only six species were relatively large measuring in the order of decimeters. The latter have the capacity of overgrowing live coral: Callyspongia californica, Chalinula nematifera, Mycale cecilia, M. magnirhaphidifera, Haliclona caerulea, and Amphimedon texotli. The last two, are the only massive species in all the reefs (Fig. 6).
It’s also important to note the rare occurrence of keratose sponges. Except for four species of Aplysina and one of Ircinia, most of the reefs, and indeed the surrounded areas, are devoid of horny species or, if they are present, they are very small.
Discussion
Coral reefs are the largest structures created by any group of animals in the world. Their three-dimensional framework forms numerous habitats which are densely populated by an enormous variety of organisms65 such as sponges, which represent the major trophic link in organic matter transfer from the pelagic to the benthic compartment in these ecosystems30,31,66.
There are not many studies about diversity of sponges on coral reefs, most published papers focused on Caribbean (CR), and West Pacific (WPR)67, while ETP is practically unknown68. Previous to this study, we know only two works, one on coral reef sponges in Panama, which cited 22 species62, and other one from Colombia, which did not deal exclusively with coral reef sponges but other habitats as well, it recorded 21 species69. Thus, the present work is the first large-scale study devoted exclusively to coral reef sponges from ETP, which, despite of the high number of reefs studied, and the vast geographical area that they represent, showed a surprising low number of species (87 species). This difference is more evident if we compare the total diversity in the entire Mexican Pacific coast, with particular reefs from CR or WPR; e.g. in Thousands Islands reefs (North Western of Java), 118 species are reported70, in the Spermonde Archipelago (south western Sulawesi, Indonesia), 151 species are recorded71,72. Reefs in the Gulf of Mannar and Palk Bay region (India) harbor more than 319 species73 (see Fig. 7)74,75,76,77,78,79,80,81,82,83,84,85,86,87. A similar situation is found in the Caribbean. To quote some examples; 92 species in Bonaire reefs88, 124 species in Belize reefs -counting only cryptic species-22, which reach more than 300 species when included also exposed one26,89,90, 156 species in Curaçao (Saba Bank), 160 species in Cuba91 (Fig. 7). It is important to note that small cryptic, boring, and thinly encrusting (<4 cm in diameter) specimens were excluded from most of these studies, so, the inclusion of those, would increase dramatically the number of sponge species in CR and WPR.
By regions, the differences are more impressive yet: 420 species in coral reefs from Indonesia (830 in total in the country)92, 486 in coral reefs from Indian waters73,92,93, or 1500 species for the Great Barrier from Australia94. The WPR, particularly the “coral triangle” region, support the most diverse sponge assemblages in the world, probably including a high number of yet undescribed species.
When we compared standardized measures, such as abundance and species per square meter, the difference was also remarkable, with ETP drastically much lower than others regions (Fig. 4). In ETP we have 1.6 ind. per m2 and 0.25 species per m2, in average. At a similar depth (below 6 m), CR and WPR have seven times more ind. per m2 (≈11 ind. per m2), and between four (1.3 for CR) and five times (1.4 for WPR) more species per m2. These differences between ETP and the other two basins were significant (Tables 2 and 3).
Comparison at deepest depth is not possible because there is no data for ETP below 6 m depth. The difference between CR and WPR was only significant for abundance at the 6–10 m depth interval (highest values): 12 vs 22 ind. per m2; respectively. Previous studies also showed that diversity (per unit-area) is similar in CR and WPR, but sponge biomass is greater in CR95,96. The decrease of diversity at 16–20 m is better explained in terms of the smaller number of papers that report information at this depth, rather than an inherently poor fauna. This increase of abundance (density and cover) along a depth gradient with highest values at intermediate depths seems to be a general pattern of coral reef sponges previously observed in CR97,98.
Explaining the differences between CR and WPR is beyond the goal of this research. However, previous studies showed that factors such as food limitation99,100, chemical defense101, and nutritional strategies, with CR sponges more heterotrophic, and WPR more autotrophic95,96 could be responsible of the differences.
In ETP as in CR72,102,103 [among others] and WPR99,100 [among others] very few species dominate the sponge assemblages, with a high percentage of species recorded from only a single site. This seems to be a general pattern in coral reef sponges worldwide. However, in ETP, the species that dominate the assemblages are boring sponges such as Cliona vermifera, Thoosa mismalolli or Pione carpenteri, which have a wide ETP occurrence and very broad ecological distribution. The prevalence of boring sponges in Mexican reefs is very interesting and remarkable, since these sponges are highly resilient to anomalous temperature shifts104,105, especially when compared to tolerance thresholds found for corals106,107. Previous studies showed that high anomalous temperatures that were detrimental to corals, had no negative effect in abundance and reproduction of C. vermifera108. The resilience demonstrated by boring sponge species in the ETP to thermal shock supports ecological projections that sponges will become an increasing threat to coral and coral reef health. However, recently it has been shown that elevated temperature can disrupt the functionality of microbial symbionts of Cliona orientalis, which occurred at a lower temperature than the 32 °C threshold that induced sponge bleaching109.
In summary, ETP coral sponges are not only less diverse compares to CR and WPR, there are also striking differences in growth form and size, because they are mostly cryptic encrusting, and very small in size (generally less than a few square centimeters). No sponges can be seen, except by close inspection of the bases of corals or by breaking open the reef frame (Fig. 2). In contrast, in CR and WPR sponges are more diverse, also in morphology, more species are living on exposed surfaces, they reach larger sizes, to over 2 m in largest dimension, and they can constitute the most abundant animals on the reef. For example, in the Wakatobi Marine National Park (Sulawesi, Indonesia) more than 200 individuals per m2 have been reported which occupy more space than the corals110. Similar to CR, in WPR many large, conspicuous sponges are present, such as the giant barrel sponge Xestospongia testudinaria or species of Lamellodysidea, Phyllospongia, and Carteriospongia.
Explaining the interoceanic differences: the influence of local and large-scale factors
Different theories have arisen to explain the pattern observed in ETP, particularly considering the dominance of encrusting and cryptic species, and the low diversity of their assemblages111. Prevalence of small cryptic encrusting sponges has been traditionally explained by the predation pressure by spongivorous fishes112, since cavities provide some advantage to cryptobionts by excluding certain predators. However, the pressure from spongivores is a common factor in the three areas100 for WPR101,113,114 [among others for CR and115,116 for ETP]. There are also other sponge predators than fishes such as mollusks, echinoderms, and crustaceans, all of which have cryptobiontic representatives117,118,119.
Perturbation, at local and large scale, rather than biologicals factors, seems to explain the low prevalence and characteristics of sponge assemblages in ETP reefs, which are very frequently located in shallow water, where turbulence is periodically very strong, which, together with abrasion provoked by particles in suspension, sedimentation41,120, and high levels of damaging light, limit sponge survival and shape ETP sponge assemblages. The low sponge abundance at shallow depth in the Caribbean too has been associated with turbulence and numerous studies97,98,99,100,112,121, among others concluded that three depth-related factors influence sponge community structure on most Caribbean reefs: turbulence, spatial competition and predation. The first two only influence sponge communities at shallow depths, mostly above 10 m, and competition mostly above 20 m. In WPR, the exclusion of sponges from shallow waters was also attributed to excessive turbulence and possibly by high levels of damaging light28,70,122 among others. Exposed reefs at Isla del Coco123, and Clipperton124, which present a similar pattern with a high proportion of thin encrustations, support the suggestion that the turbulence and abrasion shape shallow coral reef sponge communities.
Beside the influence of local abiotic variables that could explain the low diversity and the prevalence of small encrusting species in ETP reefs, and indeed in CR and WPR shallow reefs, it is important to highlight the recurrent large-scale phenomena in the ETP, such as frequent upwellings that bring cold water up onto the reefs12, and periods of high-water temperature during El Niño years, which cause death and destruction of corals that have not had the chance to reach the levels of development found in the CR15. Moreover, ocean conditions in CR are relatively constant providing an environment that is conducive to reef growth (the average age of Caribbean reefs is 5600 years old). ETP reefs are smaller, younger (varying from 200 to 5600 years old), and with variable conditions where disturbances are more pronounced125.
Explaining the interoceanic differences: the influence of evolutionary history
A hypothesis, which serves to explain the impoverished nature of the ETP coral fauna, is based on the unstable composition of faunas in remote marginal regions, and in the low resilience of these faunas. Due mainly to physical perturbations commented above, species already living near their tolerance limits become locally extinct and are not soon replenished after disturbances because of their isolation from source populations126.
The separation of ETP and the CR, occurred 3.5 million years ago, stopping the flow of species from the CR to the ETP, and since then, ETP has been highly isolated by cool currents from the north and south, and the Eastern Pacific Barrier (EPB) to the west; a vast expanse of deep water16,125. The isolation of the ETP sponge assemblages is supported by the fact that sponge fauna of Clipperton Island has stronger affinities with the Central and West Pacific regions than with the East Pacific region with which it shares only two or three species124. The majority of Clipperton species appears to have invaded from the west, evidenced by shared distributions or occurrence of close relatives in Hawaii, Tuvalu, Indonesia, New Caledonia and Australia. A study of the corals of Clipperton127 came to a similar conclusion.
High-diversity locations such as the Philippines, Indonesia, or the Great Barrier Reef show a greater resilience to recurrent disturbances128,129,130,131 that depauperate, marginal sites [e.g., the Galapagos Islands, Panama, Hawaii7,132, and indeed, a larger capacity to recovery after disturbances. They are also evidences that show that the cryptobenthic fauna of the Gulf of California is highly vulnerable to natural and anthropogenic disturbance as a result of the high specificity in habitat use of dominant species, and its low diversity, which limits the potential functional redundancy of the system, compromising the ecosystem’s functioning, resilience and stability133.
Unequal rates of speciation, extinction and migration have resulted in greater diversity in the Caribbean than in the Pacific since, ETP reefs are also impoverished with respect to coral diversity (130 species in ETP vs 240 in CR), gorgonians, zoanthids, calcareous green algae, and other sessile groups111,134,135.
All this, suggests that current patterns of biodiversity should be interpreted in light of both contemporary and historical processes, which have been hypothesized to be most important for taxonomic groups with poor dispersal abilities128.
In conclusion, factors such as isolation, difficulty to gain recruits from distant areas, perturbation, resilience, age of the reef, allowed processes like natural selection to change the species composition of each area12.
References
Veron, J. E. Corals of the World, Vol 1–3. Townsville: Australian Institute of Marine Science (2000).
Wood, R. Biodiversity and the history of reefs. Geol J. 36, 251–263 (2001).
Spalding, M., Spalding, M. D., Ravilious, C. & Greem, E. P. World atlas of coral reefs. Prepared at the UNEP World Conservation Monitoring Centre. Berkeley: University of California Press (2001).
Sorokin, Y. I. Coral Reef Ecology. Heidelberg: Springer-Verlag (1995).
Fisher, R. et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr Biol. 25(4), 500–505 (2015).
de Groot, R. et al. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst Serv. 1, 50–61 (2012).
Glynn, P. & Ault, J. A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs. 19, 1, https://doi.org/10.1007/s003380050220 (2000).
Cortés, J. Coral reefs of the Americas: an introduction to Latin American coral reefs. In: Cortés J, editor. Latin American coral reefs. Elsevier, Amsterdam; pp. 1–7 (2003).
López-Pérez, R. A. & Hernández-Ballesteros, L. M. Coral community structure and dynamics in the Huatulco area, western Mexico. Bull Mar Sci. 75(3), 453–472 (2004).
Cortés, J., Macintyre, I. G. & Glynn, P. W. Holocene growth history of an eastern Pacific fringing reef, Punta Islotes, Costa Rica. Coral Reefs. 13, 65–73 (1994).
Reyes-Bonilla, H. & López-Pérez, R. A. Biogeografía de los corales pétreos (Scleractinia) del Pacífico de México. Cien Mar. 24, 211–224 (1998).
Cortés, J. Biology and geology of eastern Pacific coral reefs. Coral Reefs. 16, 39–46 (1997).
Nava, H. & Ramírez-Herrera, M. T. Government conservation policies on Mexican coastal areas: is “top-down” management working? Rev Biol Trop. 59(4), 1487–501 (2011).
López‐Pérez, A. et al. The coral communities of the Islas Marias archipelago, Mexico: structure and biogeographic relevance to the Eastern Pacific. Mar Ecol (Berl). 37(3), 679–690 (2015).
Glynn, P. W. Some Physical and Biological Determinants of Coral Community Structure in the Eastern Pacific. Ecol Monogr. 46(4), 431–456 (1976).
Cortés, J. Comparison between Caribbean and eastern Pacific coral reefs. Rev Biol Trop. 41(1), 19–21 (1993).
Cortés, J. et al. Marine Biodiversity of Eastern Tropical Pacific Coral Reefs. In: Glynn, P., Manzello, D., Enochs, I., editors. Coral Reefs of the Eastern Tropical Pacific. Springer, Dordrecht; pp. 203–250 (2017).
Carricart-Ganivet, J. P. & Horta-Puga, G. Arrecifes de coral en México. In: Salazar-Vallejo S. I., González, N. E., editors. Biodiversidad Marina y Costera de México. CONABIO y CIQRO. Chetumal; pp. 80–90 (1993).
Glynn, P. W., Enochs, I. C., Afflerbach, J. A., Brandtneris, V. W. & Serafy, J. E. Eastern Pacific reef fish responses to coral recovery following El Niño disturbances. Mar Ecol Prog Ser. 495, 233–247 (2014).
Ramírez‐Ortiz, G. et al. Functional diversity of fish and invertebrates in coral and rocky reefs of the Eastern Tropical Pacific. Mar Ecol. 38, e12447, https://doi.org/10.1111/maec.12447 (2017).
Richter, C., Wunsch, M., Rasheed, M., KoÈtter, I. & Badran, M. I. Endoscopic exploration of Red Sea coral reefs reveals dense populations of cavity-dwelling sponges. Nature. 413(6857), 726–730 (2001).
Rützler, K., Piantoni, C., Van Soest, R. W. & Díaz, M. C. Diversity of sponges (Porifera) from cryptic habitats on the Belize barrier reef near Carrie Bow Cay. Zootaxa. 3805, 1–129 (2014).
Reaka-Kudla, M. L. The global biodiversity of coral reefs: a comparison with rain forests. In: Reaka-Kudla, M. L., Wilson, D. E., Wilson, E. O., editors. Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press, Washington, D.C. pp. 83–108 (1997).
Liversage, K., Cole, V., Coleman, R. & McQuaid, C. Availability of microhabitats explains a widespread pattern and informs theory on ecological engineering of boulder reefs. J Exp Mar Bio Ecol. 489, 36–42 (2017).
Pearman, J. K. et al. Cross-shelf investigation of coral reef cryptic benthic organisms reveals diversity patterns of the hidden majority. Sci Rep. 8, 8090 (2018).
Díaz, M. C. & Rützler, K. Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci. 69(2), 535–546 (2001).
Bell, J. J. The functional roles of marine sponges. Estuar Coast Shelf Sci. 79, 341–353 (2008).
Wilkinson, C. R. & Cheshire, A. C. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs. 8, 127–134 (1989).
Wulff, J. Ecological interactions of marine sponges. Can J Zool. 84, 146–166 (2006).
de Goeij, J. M., van Oevelen, D., Vermeij, M. J. A., Osinga, R. & Middelburg, J. J. de Goeij AFPM, Admiraal W. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science. 342, 108–110 (2013).
Rix, L. et al. Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar Ecol Prog Ser. 589, 85–96 (2018).
Fiore, C. L. & Cox-Jutte, P. Characterization of macrofaunal assemblages associated with sponges and tunicates collected off the southeastern United States. Invertebr Biol. 129(2), 105–120 (2010).
Wilkinson, C. R. Microbial associations in sponges. I. Ecology, physiology and microbial populations of coral reef sponges. Mar Biol. 49(2), 161–167 (1978).
Wilkinson, C. R. Net primary productivity in coral reef sponges. Science. 219(4583), 410–412 (1983).
Webster, N. S. & Taylor, M. W. Marine sponges and their microbial symbionts: love and other relationships. Environ Microbiol. 14(2), 335–346 (2012).
Maliao, R. J., Turingan, R. G. & Lin, J. Phase-shift in coral reef communities in the Florida Keys National Marine Sanctuary (FKNMS), USA. Mar Biol. 154(5), 841–853 (2008).
Norström, A. V., Nyström, M., Lokrantz, J. & Folke, C. Alternative states on coral reefs: beyond coral–macroalgal phase shifts. Mar Ecol Prog Ser. 376, 295–306 (2009).
Bell, J. J., Davy, S. K., Jones, T., Taylor, M. W. & Webster, N. S. Could some coral reefs become sponge reefs as our climate changes? Glob Chang. Biol. 19, 2613–262 (2013).
Carballo, J. L., Cruz-Barraza, J. A. & Gómez, P. Taxonomy and description of clionaid sponges (Hadromerida, Clionaidae) from the Pacific Ocean of Mexico. Zool J Linn Soc. 141(3), 353–397 (2004).
Carballo, J. L. & Cruz-Barraza, J. A. Cliona microstrongylata, a new species of boring sponge from the Sea of Cortés (Pacific Ocean, México). Cah Biol Mar. 46(4), 379 (2005).
Bautista-Guerrero, E., Carballo, J. L., Cruz-Barraza, J. A. & Nava, H. New coral reef boring sponges (Hadromerida: Clionaidae) from the Mexican Pacific Ocean. J Mar Biol Assoc UK. 86(5), 963–970 (2006).
Carballo, J. L., Hepburn, L., Nava, H., Cruz-Barraza, J. A. & Bautista-Guerrero, E. Coral boring Aka-species (Porifera: Phloeodictyidae) from Mexico with description of Aka cryptica sp. nov. J Mar Biol Assoc UK. 87(6), 1477–1484 (2007).
Cruz-Barraza, J. A., Carballo, J. L., Bautista-Guerrero, E. & Nava, H. New excavating sponges (Porifera: Demospongiae) from coral reefs from Mexican Pacific Coast. J Mar Biol Assoc U.K. 91, 999–1013 (2011).
Nava, H. & Carballo, J. L. Environmental factors shaping boring sponge assemblages at Mexican Pacific coral reefs. Mar Ecol. 34(3), 269–279 (2013).
Nava, H., Ramírez-Herrera, M. T., Figueroa-Camacho, A. G. & Villegas-Sanchez, B. M. Habitat characteristics and environmental factors related to boring sponge assemblages on coral reefs near populated coastal areas on the Mexican Eastern Pacific coast. Mar Biodivers. 44(1), 45–54 (2014).
Cruz-Barraza, J. A. & Carballo, J. L. Taxonomy of sponges (Porifera) associated with corals from the Mexican Pacific Ocean. Zool Stud. 47, 741–758 (2008).
Cruz-Barraza, J. A., Carballo, J. L., Rocha-Olivares, A., Ehrlich, H. & Hog, M. Integrative taxonomy and molecular phylogeny of genus Aplysina (Demospongiae: Verongida) from Mexican Pacific. PLoS ONE. 7, e42049 (2012).
Cruz-Barraza, J. A., Vega, C. & Carballo, J. L. Taxonomy of family Plakinidae (Porifera: Homoscleromorpha) from eastern Pacific coral reefs, through morphology and cox1 and cob mtDNA data. Zool J Linn Soc 171(2), 254–276 (2014).
Reyes-Bonilla, H. Biogeografía y ecología de los corales hermatípicos (Anthozoa: Scleractinia) del Pacífico de México. In: Salazar-Vallejo, S. I., González, N. E., editors. Biodiversidad Marina y Costera de México. CONABIO y CIQRO. Chetumal; pp. 207–222 (1993).
Fagerstrom, J. A. The Evolution of Reef Communities. New York: Wiley (1987).
López‐Pérez, R. A. et al. Coral communities and reefs from Guerrero, southern Mexican Pacific. Mar Ecol. 33(4), 407–416 (2012).
Carriquiry, J. D., Cupul-Magaña, A. L., Rodríguez-Zaragoza, F. & Medina-Rosas, P. Coral bleaching and mortality in the Mexican Pacific during the 1997–98 El Niño and prediction from a remote sensing approach. Bull Mar Sci. 69(1), 237–249 (2001).
Reyes-Bonilla, H. Effects of the 1997–1998 El Niño–Southern Oscillation on coral communities of the Gulf of California, Mexico. Bull Mar Sci. 69(1), 251–266 (2001).
Ketchum, J. T. & Reyes-Bonilla, H. Taxonomía y distribución de los corales hermatípicos (Scleractinia) del Archipiélago de Revillagigedo, México. Rev Biol Trop. 49(3-4), 803–848 (2001).
McClanahan, T. R. Kenyan coral reef-associated gastropod fauna: a comparison between protected and unprotected reefs. Mar Ecol Prog Ser. 53, 11–20 (1989).
Alcolado, P. M. & Herrera, A. Efectos de la contaminación sobre las comunidades de esponjas en el litoral de la Habana. Cuba. Instituto de Oceanología. Academia de Ciencias de Cuba. 68, 1–23 (1987).
Carballo, J. L., Naranjo, S. A. & García-Gómez, J. C. Use of marine sponges as stress indicators in marine ecosystems at Algeciras Bay (Southern Iberian Peninsula). Mar Ecol Prog Ser. 135, 109–122, https://doi.org/10.3354/meps135109 (1996).
Carballo, J. L. & Naranjo, S. Environmental assessment of a large industrial marine complex based on a community of benthic filter-feeders. Mar Pollut Bull. 44(7), 605–610, https://doi.org/10.1016/S0025-326X(01)00295-8 (2002).
Magurran, A. E. Measuring Biological Diversity. Oxford: Blackwell (2004).
Sneath, P. H & Sokal, R. R. Numerical taxonomy. San Francisco: Freeman (1973).
Clarke, K. R. & Warwick, R. M. Changes in Marine Communities: An Approach to Statistical Analyses and Interpretation. Plymouth: Natural Environment Research Council (1994).
Berman, J. Sponge diversity in coral frameworks and coral communities within the Las Perlas archipelago, Panama. M. Sci. Thesis, Heriot-Watt University (2004).
Sokal, R. R. & Rohlf, F. J. Biometry. 2nd Edition. New York: W. H. Freeman and Company (1981).
Levene, H. A robust approximate confidence-interval for components of variance. In Ann Math Stat. 31(2), 534–535 (1960).
Burke, L, Reytar, K, Spalding, M & Perry, A. Reefs at Risk Revisited. Washington, D. C.: World Resources Institute (2011).
Gili, J. M. & Coma, R. Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol. 13(8), 316–321 (1998).
Soest, R. W. M. van, van Kempen, T. M. G. & Braeckman, J. C. Sponges in time and space. Rotterdam: Balkema (1994).
Soest, R. W. M. et al. Global Diversity of Sponges (Porifera). PLoS One. 7(4), e35105, https://doi.org/10.1371/journal.pone.0035105 (2012).
Escobar-Zorrilla, T. Inventario y estudio taxonómico de las esponjas (Phylum porifera) de algunas áreas del Pacífico colombiano. Thesis, Universidad del Valle (sede Pacífico) (2000).
de Voogd, N. J. & Cleary, D. F. R. Relating species traits to environmental variables in Indonesian coral reef assemblages. Mar Freshw Res. 58(3), 240–249 (2007).
de Voogd, N. J., Cleary, D. F., Hoeksema, B. W., Noor, A. & van Soest, R. W. Sponge beta diversity in the Spermonde Archipelago, SW Sulawesi, Indonesia. Mar Ecol Prog Ser. 309, 131–142 (2006).
de Voogd, N. J. & Cleary, D. F. An analysis of sponge diversity and distribution at three taxonomic levels in the Thousand Islands/Jakarta Bay reef complex, West‐Java, Indonesia. Mar Ecol. 29(2), 205–215 (2008).
Vinod, K., Rani, M. G., Thomas, P. A., Manisseri, M. K. & Shylaja, G. Diversity and distribution of shallow water sponges (Porifera) in the coastal waters from Enayam to Kollam, south-west coast of India. Indian J Fish. 6(3), 52–57 (2014).
Meesters, E. et al. Sub-rubble communities of Curaçao and Bonaire coral reefs. Coral Reefs 10, 189–197 (1991).
Zea, S. Patterns of sponge (Porifera, Demospongiae) distribution in remote, oceanic reef complexes of the Southwestern Caribbean. Rev. Acad. Colomb. Cienc. 25(97), 579–592 (2001).
Valderrama, D. & Zea, S. Annotated checklist of sponges (Porifera) from the southernmost Caribbean reefs (north-west Gulf of Urabá), with description of new records for the Colombian Caribbean. Rev. Acad. Colomb. Cienc. 37(144), 379–404 (2013).
Schmahl, G. P. Community structure and ecology of sponges associated with four southern Florida coral reefs. En K. Rützler (ed.): New perspectives in sponge biology. Smithsonian Institution Press, Washington, D.C.: 376–383 (1990).
Thacker, R. W. et al. Preliminary Assessment of Sponge Biodiversity on Saba Bank, Netherlands Antilles. PLOS ONE 5(5), e9622 (2010).
Valderrama, D. & Zea, S. Esquemas de distribución de esponjas arrecifales (Porifera) del noroccidente del golfo de Urabá, Caribe sur, Colombia. Bol. Invest. Mar. Cost. 32(1), 37–56 (2003).
Gochfeld, D. J., Schlöder, C. & Thacker, R. W. Sponge community structure and disease prevalence on coral reefs in Bocas del Toro, Panama. In: Custódio, M. R., Lõbo-Hajdu, G., Hajdu, E., Muricy G (eds) Porifera research: biodiversity, innovation, and sustainability. Série Livros 28. Museu Nacional, Rio de Janeiro, p 335–343 (2007).
Chiappone, M. & Sullivan, K. M. Ecological Structure and Dynamics of Nearshore Hard-Bottom Communities in the Florida Keys. Bulletin of Marine Science 54(3), 747–756 (1994).
Engel, S. & Pawlik, J. R. Interactions among Florida sponges. I. Reef habitats. Mar Ecol Prog Ser 303, 133–144 (2005).
Cedro, V. R., Hajdu, E., Sovierzoski, H. H. & Correia, M. D. Demospongiae of the shallow coral reefs of Maceió, Alagoas state, Brasil.In Porifera research: biodiversity, innovation and sustainability (M. R. Custódio, G. Lôbo-Hajdu, E. Hajdu & G. Muricy, eds). Museu Nacional, Rio de Janeiro, p.223–237 (2007).
Thomas, P. A. Sponge fauna of Lakshadweep. CMFRI Bull. 43, 150–161 (1989).
Venkataraman, K. & Wafar, M. Coastal and marine biodiversity of India. Indian J. Mar. Sci. 34(1), 57–75 (2005).
Hooper, J. N. A. & Kennedy, J. A. Small-scale patterns of sponge biodiversity (Porifera) on Sunshine Coast reefs, eastern Australia. Invertebrate Systematics. 16, 637–653 (2002).
de Voogd, N. J., Becking, L. E. & Cleary, D. F. R. Sponge community composition in the Derawan Islands, NE Kalimantan, Indonesia Marine Ecology Progress Series, 169–180 (2009).
Kobluk David, R. & van Soest, R. W. M. Cavity-dwelling sponges in a southern caribbean coral reef and their paleontological Implications. Bull Mar Sci. 44(3), 1207–1235 (1989).
Cairns, S. D. Stony Corals (Cnidaria: Hydrozoa, Scleractinea) of Carrie Bow Cay, Belize. In: Rützler, K., Macintyre, I. G., editors. The Atlantic Barrier Reef ecosystem at Carrie Bow Cay, Belize, I. Structure and communities. Smithson. Inst. Press. Washington, D.C.; pp. 271–302 (1982).
Muzik K. Octocorallia (Cnidaria) from Carrie Bow Cay, Belize. In: Rützler, K., Macintyre, I. G, editors. The Atlantic Barrier Reef ecosystem at Carrie Bow Cay, Belize, I. Structure and communities. Smithsonian Contributions to the Marine Sciences. Smithson Inst Press Washington, D. C; pp. 303–310 (1982).
Alcolado, P. M. General trends in coral reef communities of Cuba. In: Soest, R. W. M. Van, Kempen, T. M. Van, Braekman, J., editors. Sponges in time and space. Balkema; pp. 251–255 (1994).
Van Soest, R. W. M. The Indonesian sponge fauna: a status report. Netherlands. J Sea Res. 23(2), 223–230 (1989).
Thomas, P. A. Sponge fauna of Lakshadweep. CMFRI Bulletin Marine living resources of the union territory of Lakshadweep An Indicative Survey With Suggestions For. Development. 43, 150–161 (1989).
Hooper, J. N. A., Kennedy, J. A., List-Armitage, S. E. & Cook, S. D. Quinn R. Biodiversity, species composition and distribution of marine sponges in northeastern Australia. Proc 5th Int Sponge Symp. Mem Queensl Mus. 44, 263–271 (1999).
Wilkinson, C. R. Interocean differences in size and nutrition of coral reef sponge populations. Science. 236(4809), 1654–1657 (1987).
Wilkinson, C. R. & Cheshire, A. C. Comparisons of sponge populations across the Barrier Reefs of Australia and Belize: evidence for higher productivity in the Caribbean. Mar Ecol Prog Ser. 67, 285–294 (1990).
Schmahl, G. P. Community structure and ecology of sponges associated with four southern Florida coral reefs. In Rützler, K., editor. New Perspectives in Sponge Biology. pp, 376–383 (1990).
Suchanek, T. H., Carpenter, R. C., Witman, J. D. & Harvell, C. D. Sponges as important competitors in deep Caribbean coral reef communities. The ecology of deep and shallow coral reefs. 1, 55–60 (1983).
Pawlik, J. R., McMurray, S. E., Erwin, P. & Zea, S. No evidence for food limitation of Caribbean reef sponges: Reply to Slattery & Lesser. Mar Ecol Prog Ser. 527, 281–284 (2015a).
Pawlik, J. R., McMurray, S. E., Erwin, P. & Zea, S. A review of evidence for food limitation of sponges on Caribbean reefs. Mar Ecol Prog Ser. 519: 265–283 (2015b).
Pawlik, J. R., Loh, T. L., McMurray, S. E. & Finelli, C. M. Sponge communities on Caribbean coral reefs are structured by factors that are top-down, not bottom-up. PLoS One. 8(5), e62573 (2013).
Hooper, J. N. A., Kennedy, J. A. & Quinn, R. J. Biodiversity ‘hotspots’, patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera). Biodivers Conserv. 11, 851–885 (2002).
Fromont, J., Vanderklift, M. A. & Kendrick, G. A. Marine sponges of the Dampier Archipelago, Western Australia: patterns of species distributions, abundance and diversity. Biodivers Conserv. 15, 3731–3750 (2006).
Miller, A. N., Strychar, K. B., Shirley, T. C. & Rützler, K. Effects of heat and salinity stress on the sponge Cliona celata. Int J Biol. 2(2), 3–16 (2010).
Duckworth, A. R. & Peterson, B. J. Effects of seawater temperature and pH on the boring rates of the sponge Cliona celata in scallop shells. Mar Biol. 160(1), 27–35 (2013).
Glynn, P. W., Maté, J. L., Baker, A. C. & Calderón, M. O. Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño–Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci. 69(1), 79–109 (2001).
Hueerkamp, C., Glynn, P. W., D’Croz, L., Maté, J. L. & Colley, S. B. Bleaching and recovery of five eastern Pacific corals in an El Niño-related temperature experiment. Bull Mar Sci. 69(1), 215–236 (2001).
Bautista-Guerrero, E., Carballo, J. L. & Maldonado, M. Abundance and reproductive patterns of the excavating sponge Cliona vermifera: a threat to Pacific coral reefs? Coral Reefs. 33(1), 259–266 (2014).
Ramsby, B. D., Hoogenboom, M. O., Whalan, S. & Webster, N. S. Elevated seawater temperature disrupts the microbiome of an ecologically important bioeroding sponge. Mol Ecol. 27(8), 2124–2137, https://doi.org/10.1111/mec.14544 (2018).
Bell, J. J. & Smith, D. Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Ass U.K. 84, 581–591 (2004).
Wulff, J. L. Causes and consequences of differences in sponge diversity and abundance between the Caribbean and eastern pacific of Panama. Proc 8th lnt Coral Reef Symp. 2, 1377–1382 (1997a).
Pawlik, J. R. Coral reef sponges: Do predatory fishes affect their distribution? Limnol Oceanogr. 43(6), 1396–1399 (1998).
Bakus, G. The effects of fish‐grazing on invertebrate evolution in shallow tropical waters. Allan Hancock Foundation Occasional Paper. 27, 1–29 (1964).
Dunlap, M. & Pawlick, J. Video‐monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges. Mar Biol. 126, 117–123 (1996).
Wulff, J. L. Parrotfish predation on cryptic sponges of Caribbean coral reefs. Mar Biol. 129, 41–52 (1997b).
Wulff, J. L. Do the same sponge species live on both the Caribbean and eastern Pacific sides of the Isthmus of Panama? Bull. Inst. r. sci. nat. Belg. Biologie 66, 165–173 (1996).
Graham, A. Molluscan diets. Proc. Malac. Soc. London. 31, 145–159 (1955).
Kobluk, D. R. & Lysenko, M. A. Reef-dwelling molluscs in open framework cavities, Bonaire N.A., and their potential for preservation in a fossil reef. Bull Mar Sci. 39, 657–672 (1985).
Guida, V. G. Sponge predation in the oyster reef community as demonstrated with Cliona celata Grant. J Exp Mar Biol Ecol. 25(2), 109–122 (1976).
Powell, A. et al. Reduced Diversity and High Sponge Abundance on a Sedimented Indo-Pacific Reef System: Implications for Future Changes in Environmental Quality. PLoS One. 9(1), e85253, https://doi.org/10.1371/journal.pone.0085253 (2014).
Loh, T. L. & Pawlik, J. R. Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proc Nat Acad Sci. 201321626, https://doi.org/10.1073/pnas.1321626111 (2014).
Wilkinson, C. R. & Evans, E. Sponge distribution across Davies Reef, Great Barrier Reef, relative to location, depth, and water movement. Coral Reefs. 8, 1–7 (1989).
Bakus, G. J. Marine zonation and ecology of Cocos Island, off Central America. Rev Biol Trop. 33(2), 197–202. (1975).
Soest, R. W. M., van, Kaiser, K. L. & Van Syoc, R. Sponges from Clipperton Island, East Pacific. Zootaxa. 2839(1), 1–46 (2011).
Grigg, R. W. & Hey, R. Paleoceanography of the Tropical Eastern Pacific. Ocean. Science. 255(5041), 172–178 (1992).
Veron, J. E. N. Corals in Space & Time. The Biogeography & Evolution of the Scleractinia. xiii + 321 pp (1995).
Glynn, P. W., Veron, J. E. N. & Wellington, G. M. Clipperton Atoll (eastern Pacific): oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs. 15(2), 71–99 (1996).
Graham, C. H., Moritz, C. & Williams, S. E. Habitat history improves prediction of biodiversity in rainforest fauna. PNAS. 103(3), 632–636 (2006).
Brown, B. E. Suharsono. Damage and Recovery of Coral Reefs Affected by El Nino Related Seawater Warming in the Thousand Islands, Indonesia. Coral Reefs. 8, 163–170, https://doi.org/10.1007/BF00265007 (1990).
Russ, G. & Alcala, A. Natural fishing experiments in marine reserves 1983–1993: roles of life history and fishing intensity in family responses. Coral Reefs. 17, 399–416, https://doi.org/10.1007/s003380050146 (1998).
Connell, J. Disturbance and recovery of coral assemblages. Coral Reefs. 16(Suppl 1), S101–113, https://doi.org/10.1007/s003380050246 (1997).
Dollar, S. J. & Tribble, G. W. Recurrent storm disturbance and recovery: a long-term study of coral communities in Hawaii. Coral Reefs. 12, 223–233, https://doi.org/10.1007/BF00334481 (1993).
González‐Cabello, A. & Bellwood, D. R. Local ecological impacts of regional biodiversity on reef fish assemblages. Journal of biogeography 36(6), 1129–1137 (2009).
Glynn, P. W. Observations on the ecology of the Caribbean and Pacific coasts of Panama. Bull Biol Soc Wash. 2, 13–30 (1972).
Glynn, P. W., Stewart, R. H. & McCosker, J. E. Pacific coral reefs of Panama: structure, distribution and predators. Geol Rundsch. 61(2), 483–519 (1972).
Acknowledgements
Authors are grateful to the following sources of funding: CONACYT SEP CB-2015-01 (254806), CONACYT SEP CB-177537, and to the Research Council of Scientific Research of the Universidad Michoacana de San Nicolás de Hidalgo. We also thank CONANP and PROZONA AC for their logistic support in some field samplings, and SAGARPA for collecting permits (PPF/DGOP-019/16 and PPF/DGOPA-296/17).
Author information
Authors and Affiliations
Contributions
J.L.C. conceived the presented idea, collected the data, performed the analysis and wrote the paper. J.A.C.B., H.N., C.V. & M.C.C.F. collected the data, participated in the analysis of the results and drafted the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carballo, J.L., Cruz-Barraza, J.A., Vega, C. et al. Sponge diversity in Eastern Tropical Pacific coral reefs: an interoceanic comparison. Sci Rep 9, 9409 (2019). https://doi.org/10.1038/s41598-019-45834-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-45834-4
This article is cited by
-
Coral reef ecological pump for gathering and retaining nutrients and exporting carbon: a review and perspectives
Acta Oceanologica Sinica (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.