Abstract
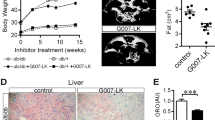
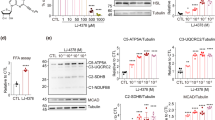
Obesity has become a worldwide epidemic. We have previously reported that systemic administration of pNaKtide which targets the Na/K-ATPase oxidant amplification loop (NKAL) was able to decrease systemic oxidative stress and adiposity in mice fed a high fat and fructose supplemented western diet (WD). As adipocytes are believed to play a central role in the development of obesity and its related comorbidities, we examined whether lentiviral-mediated adipocyte-specific expression of NaKtide, a peptide derived from the N domain of the alpha1 Na/K-ATPase subunit, could ameliorate the effects of the WD. C57BL6 mice were fed a WD, which activated Na/K-ATPase signaling in the adipocytes and induced an obese phenotype and caused an increase in plasma levels of leptin, IL-6 and TNFα. WD also decreased locomotor activity, expression of the D2 receptor and tyrosine hydroxylase in brain tissue, while markers of neurodegeneration and neuronal apoptosis were increased following the WD. Selective adipocyte expression of NaKtide in these mice fed a WD attenuated all of these changes including the brain biochemical alterations and behavioral adaptations. These data suggest that adipocyte derived cytokines play an essential role in the development of obesity induced by a WD and that targeting the adipocyte NKAL loop may serve as an effective therapeutic strategy.
Similar content being viewed by others
Article PDF
Change history
24 February 2021
Editor’s Note: The Editors are currently investigating concerns that have been raised relating to the reliability of data presented in this article. Appropriate editorial action will be taken once the investigation is complete.
30 May 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-022-13459-9
05 November 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41598-020-75948-z
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41598-022-13459-9
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pratt, R.D., Brickman, C., Nawab, A. et al. RETRACTED ARTICLE: The Adipocyte Na/K-ATPase Oxidant Amplification Loop is the Central Regulator of Western Diet-Induced Obesity and Associated Comorbidities. Sci Rep 9, 7927 (2019). https://doi.org/10.1038/s41598-019-44350-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44350-9
This article is cited by
-
The Na/K-ATPase Signaling and SGLT2 Inhibitor-Mediated Cardiorenal Protection: A Crossed Road?
The Journal of Membrane Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.