Abstract
Antarctic and Subantarctic lakes are unique ecosystems with relatively simple food webs, which are likely to be strongly affected by climate warming. While Antarctic freshwater invertebrates are adapted to extreme environmental conditions, little is known about the factors determining their current distribution and to what extent this is explained by biogeography or climate. We explored the distribution of freshwater crustaceans (one of the most abundant and diverse group of organisms in Antarctic and Subantarctic lakes) across four biogeographic provinces (Continental Antarctic, CA; Maritime Antarctic, MA; Subantarctic islands, SA; and Southern Cool Temperate, SCT) based on the literature, predicting that species distribution would be determined by biogeography, spatial autocorrelation among regions (in relation to dispersal) and climate. We found that variation in species composition was largely explained by the joint effect of spatial autocorrelation and climate, with little effect of biogeography – only regions within the SA province had a clearly distinct species composition. This highlights a plausible main influence of crustacean dispersal – mainly through migratory seabirds – and suggests that some regions will be more affected by climate warming than others, possibly in relation to the existence of nearby sources of colonists.
Similar content being viewed by others
Introduction
Antarctica is the Earth’s southernmost continent, almost entirely covered by an ice sheet. Remarkably, however, it holds a high variety of lake ecosystems, many located in ice-free coastal areas, and some in ice-free inland areas and in surrounding Antarctic and Subantarctic islands1. These lakes are characterized by their low metazoan diversity and low food-web complexity, with higher trophic levels such as fish being missing or largely absent1,2,3. Such low diversity and ecological complexity could make these ecosystems particularly vulnerable to ecological changes as a result of climate change-driven extinctions4. It is thus important to explore distribution patterns of their biota and the determinants of such patterns, which can shed light on future ecological changes5.
Crustaceans are the most diverse and well-documented freshwater invertebrates in Antarctic and Subantarctic lakes, where the eight major crustacean orders are represented6. Most taxa are common components of zooplankton, where they occupy a wide range of ecological niches, and can respond quickly to environmental change, including temperature increase7. Thus, they are considered sentinel organisms which can help understanding climate change effects8,9. The occurrence of crustacean species in Antarctic and Subantarctic lakes has been reported in many publications and compiled in two major reviews6,10, but no attempt has been made to explore whether their distribution is explained mostly by biogeography or whether climate is a main determinant. We explored this question using published information and tested the hypothesis that variation in crustacean species composition across regions within Antarctica is determined by (i) biogeography (i.e., regions from the same biogeographic province will have more similar species composition than regions from different provinces), (ii) spatial autocorrelation among regions (i.e., regions closer to each other will have more similar species composition than more distant regions due to higher dispersal among them) and (iii) climate (due to species-specific environmental constraints).
Results
We extracted a list of 66 crustacean taxa (59 species and 7 genera/mosphospecies; hereafter species for simplicity) representing 8 orders (Table 1). Species were distributed mainly across the Subantarctic islands (SA) and Southern Cool Temperate province (SCT) (46 and 26 species, respectively). SA showed at least 1 species from each of the 8 crustacean orders, and all SA islands except Prince Edward Island (Pe) contained species that were endemic of our study area. Iles Kerguelen (Kr) showed the greatest richness (19 species, 5 endemic), followed by South Georgia (Sg; 17 species, 5 endemic), Macquarie Island (Mc; 14 species, 7 endemic including Iais sp., the only isopod recorded at these latitudes) and Iles Crozet (Cr; 11 species, 2 endemic). For SCT, Falkland/Malvinas Islands (Fa) concentrated most of the species (25) allocated across 7 orders and a high number of endemic species (10). Campbell (Ca) and Auckland Islands (Ak) had one endemic species, Chiltonia mihiwaka (Amphipoda). The cladoceran Ovalona weinicki11 and the calanoid Boeckella poppei12 were present in most localities (8 and 6, respectively), but only B. poppei was present in all of them. In the Maritime Antarctic province (MA) there were 9 species across 4 orders. South Orkney Islands (So) was the richest region, with all 9 species and the only records of Podocopida and Cladocera within MA, with the exception of Macrothrix oviformis and O. weinicki, which were also found in South Shetland Islands (Ss) and Antarctic Peninsula (Pa). In the Continental Antarctic province (CA) there were 7 species of the orders Cladocera [Daphniopsis studeri at Enderby (En)], Calanoida [B. poppei at En and Gladioferens antarcticus at Wilkes (Wi)] and Cyclopoida (Diacyclop sp. in all 3 regions), with 3 species endemic of this province (D. joycei, D. kaupi and D. walkeri).
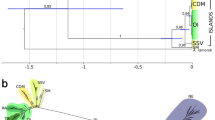
Cluster analysis showed 3 distinct groups of regions according to crustacean species composition: (1) Ca and Ak from SCT; (2) Sc from CA; and (3) all regions from SA, MA and CA (excluding En and Wi) and Fa from SCT. The latter group was further divided into 3 sub-groups: (3a) Cr, Pe, Kr, Heard Island (Hd) and Mc from SA, the latter without significant support; (3b) MA, Sg from SA and Fa from SCT, the latter without significant support; and (3c) En and Wi from CA (Fig. 1). The NMDS produced the same groups as cluster analysis (Fig. 2).
Results of hierarchical cluster analysis grouping the study regions based on crustacean species composition. Regions: see Table 1 footnote.
Results of NMDS ordination of study regions based on crustacean species composition (A), with inset of the main group (B). Regions: see Table 1 footnote.
ANOSIM showed significant differences among biogeographic provinces (Global R = 0.57, p = 0.001). Pairwise tests showed significant differences for CA vs. SA, MA vs. SA and SA vs. SCT and no significant differences among CA, MA and SCT (Table 2), thus revealing two groups (Group 1: SA; Group 2: CA, MA and SCT). Based on similarity percent analysis (SIMPER), the species that most contributed to Group 1 were Ovalona weinecki (19.5%), Epactophanes richardi (13.6%), Daphniopsis studeri (13.6%) and Tigriopus angulatus (11.1%); species that most contributed to Group 2 were Boeckella poppei (30.2%), Chiltonia mihiwaka (20.4%) and Branchinecta gaini (15.4%); dissimilarity between Group 1 and Group 2 was explained by a large number of species, all with lower contribution values (<5.2%) (Table 3).
The partial redundancy analysis (pRDA) showed that both spatial autocorrelation and climate explained a significant part of the variance (spatial autocorrelation: R2adj = 0.38, p = 0.003, variance explained = 26.67%; climate: R2adj = 0.05, p = 0.029, variance explained = 3.73%), but most variance was due to the shared contribution of both variables (R2adj = 0.60, p = 0.001, variance explained = 41.87). Residuals explained 27% of the variance (R2adj = 0.40) (Fig. 3).
Results of partial redundancy analysis (pRDA) showing the amount of variability in crustacean distribution attributable to spatial autocorrelation among regions, climate, and the shared contribution of both variables. The amount of variability explained by each factor or their shared contribution is based on R2adj; asterisks indicate significant results (at p < 0.05, based on 999 permutations).
Discussion
Our results showed that spatial autocorrelation among Antarctic and Subantarctic lakes and climate were key determinants of crustacean distribution, while biogeography had a secondary role. Multivariate analyses revealed that only the Subantarctic biogeographic province had a distinct crustacean fauna. This province contained 46 species belonging to the 8 crustacean orders described for Antarctica. The species that most contributed to the distinctness of the Subantarctic province was the cladoceran O. weinecki11, which is the only Antarctic representative of a genus of mainly tropical and subtropical distribution13. Van Damme and Dumont14 re-described this species from a complex of Alona sp. (principally, A. weinecki) described for Subantarctic islands6,10.
Geographic distance may explain the fact that Macquarie island, which is separated ~6,000 km from the other Subantarctic islands, shared only a few species with them; and could also explain the high incidence of endemic species of crustaceans and other freshwater organisms in Macquarie island15. The relevance of distance was also revealed by our partial redundancy analysis (which showed that spatial autocorrelation among regions explained a large amount of variance), and it is most likely related to patterns of dispersal. Dispersal among nearby islands occurs mainly via migratory seabirds, which can transport resistant eggs within the gut or in mud adhering to feet1,6,10,16,17. Distance among regions is often a key determinant in the distribution of freshwater fauna18, which may help explain some inconsistencies in the definition of biogeographic provinces.
The Maritime Antarctic province had similar species composition to South Georgia island from the Subantarctic province and the South American Falkland/Malvinas islands from the Southern Cool Temperate province. All these regions are separated by less than 2,000 km, so geographic distance could again be important in their similarity. It has also been proposed that Antarctic and South American crustacean fauna could have a common origin, as both continents were separated ~30 Mya19,20, thus being vicariant faunas21,22. However, this is unlikely, because most crustacean species in Continental and Maritime Antarctic provinces are Holocene immigrants, having arrived within the last 11 ka10.
Campbell and Auckland Islands (New Zealand), from the Southern Cool Temperate province, had a distinct fauna and mainly shared the unique amphipod species C. mihiwaka23 and the widespread B. poppei. The separation of the Scott sector from the Continental Antarctic province from other regions was related to the Cyclopoida D. joycei24, which is the only species that inhabits this region. The other two sectors of the Continental Antarctic province (Enderby and Wilkes) have other species of this genus: D. mirnyi (present in both regions), D. walker (in Enderby) and D. kaupi (in Wilkes). This group of Diacyclops species is known as the “michaelseni group”25, a circum-Antarctic assemblage that shares some morphological characteristics and originated in Antarctic freshwater lakes in late Pliocene, prior to the onset of glaciation24. Lastly, the wide distribution of some species such as O. weinecki or B. poppei could be due to recent colonization events from northern latitudes, of anthropogenic origin in some cases10. Other authors have suggested an ancient origin for these species, which may have survived during Pleistocene glaciations in refugia26,27,28, such as Kerguelen Island14.
The lack of consistent climatic data for different Antarctic and Subantarctic regions precluded a more robust assessment of the influence of climate on freshwater crustacean distribution. However, our analyses using latitude as surrogate for climate suggested that climate affects distributional patterns, and its effect is variable among regions, depending on their location. Thus, some regions of Antarctica are likely to be more affected by climate warming than others, and this variation could be related to geographic distance to other sources of colonists. These differences may be further enhanced by the fact that some parts of Antarctica are experiencing greater temperature increases than others; the increase is particularly large in the Antarctic Peninsula, which has registered an increase of 0.67 °C per decade in the last 50 years29,30,31. Further studies are needed in order to improve our knowledge on biodiversity patterns and their main drivers in this continent that is experiencing some of the most rapid environmental changes on Earth32.
Methods
Study area
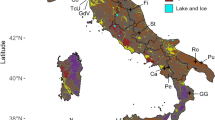
The Antarctic continent can be divided into 3 biogeographic provinces which differ considerably in climatic conditions3,32,33,34: the CA, which is the largest and coldest region with temperature rarely above freezing35, comprising the continent landmass south of 72°S and the Balleny Islands; the MA, which includes the western side of the Antarctic Peninsula north of 72°S and experiences seasonal snowmelt35; and the SA, which comprises a series of islands and small archipelagos in the Southern Ocean proximate to the zone of Antarctic Polar Front (APF), with temperatures that on average are above freezing point year-round36. Besides, we considered a fourth biogeographic province, north of the APF and influenced by low temperatures: the SCT province, which is formed by several islands from New Zealand and South America10, with cool to cold temperate climate37 (Fig. 4).
Data collection
We elaborated a presence/absence matrix of all freshwater crustacean species reported for Antarctic and Subantarctic lakes, based on two major literature reviews6,10, which contained all the available information to date. We divided each biogeographic province into regions following the above two reviews: CA comprised the En (30°E–90°E), Wi (90°E–150°E) and Sc (150°E–150°W) sectors; MA included the Pa, Ss and So; SA included Sg, Pe, Mc, Hd, Cr and Kr; and SCT included Ca and Ak from New Zealand and Fa from South Atlantic ocean (Table 1, Fig. 4). We excluded suspect records from the dataset, ruled out possible synonymies, and updated scientific names. We assumed that sampling effort of different taxa was similar across sites, although potential differences may have some influence on our results.
Data analysis
We explored the influence of biogeography on regional species composition using hierarchical cluster analysis integrated with similarity profile analysis in SIMPROF38 and metric multidimensional scaling, MDS39 based on a similarity matrix using the Jaccard index. We tested for significance of the different groups of regions generated by cluster analysis using one-way ANOSIM, with biogeographic province as factor40,41, followed by pairwise tests. Further, we identified the main species associated with each group through SIMPER based on the presence/absence matrix of crustacean species. These analyses were done using Primer v.6 software42.
We explored the separate and joint influence of spatial autocorrelation among regions and climate using pRDA. The amount of variation explained by each factor and by their shared contribution was calculated by variance partitioning analysis, which is based on adjusted R2 (R2adj), and their statistical significance tested through permutation tests (999 randomizations). Species composition data was Hellinger-transformed prior to analysis to provide an unbiased estimate of variance partitioning based on RDA. Spatial autocorrelation was obtained with the eigenfunction analysis known as Principal Coordinates of Neighbor Matrix PCNM43, which created 10 spatial variables (PCNM vectors) based on a matrix of Euclidean distances between regions calculated using the geographic coordinates. These vectors allow the representation of different spatial relationships among regions at different spatial scales and can be treated as independent variables44. As we were not able to obtain consistent climatic data for all the study regions – there are relatively few meteorological stations in Antarctica and any gross estimate based on different data sources could be misleading –, we used decimal latitude as surrogate for climate. To eliminate any effect caused by different elevations, we used the residuals of a linear regression with latitude (as a response variable) against elevation (as a predictor) in the analysis45,46. Elevation was obtained from www.gps-coordinates.net based on latitude and longitude. These analyses were performed on R v. 3.5.147, using the functions rda, varpart, anova.cca and pcnm from vegan package48.
Data Availability
Data will be available on the Open Science Framework online repository.
Change history
10 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-97702-9
30 January 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41598-020-58516-3
References
Laybourn-Parry, J. & Pearce, D. A. The biodiversity and ecology of Antarctic lakes: models for evolution. Philosophical Transactions of the Royal Society B: Biological Sciences 362, 2273–2289 (2007).
Butler, H., Atkinson, A. & Gordon, M. Omnivory and predation impact of the calanoid copepod Boeckella poppei in a maritime Antarctic lake. Polar Biology 28, 815–821, https://doi.org/10.1007/s00300-005-0014-4 (2005).
Gibson, J. A. E. et al. In Trends in Antarctic terrestrial and Limnetic Ecosystems. Antarctica as a Global Indicator. (Springer, 2006).
Dunne, J. A., Williams, R. J. & Martinez, N. D. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecology Letters 5, 558–567 (2002).
McDonald, M. E., Hershey, A. E. & Miller, M. C. Global warming impacts on lake trout in arctic lakes. Limnol Oceanogr 41, 1102–1108 (1996).
Dartnall, H. J. G. The freshwater fauna of the South Polar region: A 140-year review. Papers and Proceeding of the Royal Society of Tasmania 151, 19–58 (2017).
Richardson, A. J. In hot water: zooplankton and climate change. ICES J Mar Sci 65, 279–295 (2008).
Adrian, R. et al. Lakes as sentinels of climate change. Limnology and Oceanography 54, 2283–2297, https://doi.org/10.4319/lo.2009.54.6_part_2.2283 (2009).
Papa, R. D. S. & Briones, J. C. A. Climate and Human-induced changes to lake ecosystems: What we can learn from monitoring zooplankton ecology. Journal of Environmental Science and Managment 17, 60–67 (2014).
Pugh, P. J. A., Dartnall, H. J. G. & Mcinnes, S. J. The non-marine Crustacea of Antarctica and the Islands of the Southern Ocean: biodiversity and biogeography. Journal of Natural History 36, 1047–1103, https://doi.org/10.1080/00222930110039602 (2002).
Studer, T. Beitrige zur Naturgeschichte wirbelloser Thiere von Kerguelensland. Anatomie von (1878).
Mrázek, A. In Ergebnisse der Hamburger Magalhaenischen Sammelreise. Vol. 2 (ed. Hamburgisches Zoologisches Museum und Institut) Ch. I. Centropagidae, 1–29 (Hamburg, L. Friedrichsen,1896–1907, 1901).
Sinev, A. Y. Revision of the pulchella-group of Alona s. lato leads to its translocation to Ovalona Van Damme et Dumont, 2008 (Branchiopoda: Anomopoda: Chydoridae). Zootaxa 4044, 451–492, https://doi.org/10.11646/zootaxa.4044.4.1 (2015).
Van Damme, K. & Dumont, H. J. Further division of Alona Baird, 1843: separation and position of Coronatella Dybowski & Grochowski and Ovalona gen.n (Crustacea:Cladocera). Zootaxa 1960, 1–44 (2008).
Dartnall, H. J. G., Hollwedel, W. & de Paggi, J. C. The freshwater fauna of Macquarie Island, including a redescription of the endemic water-flea Daphnia gelida (Brady) (Anomopoda: Crustacea). Polar Biology 28, 922–939, https://doi.org/10.1007/s00300-005-0016-2 (2005).
Havel, J. E. & Shurin, J. B. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49, 1229–1238, https://doi.org/10.4319/lo.2004.49.4_part_2.1229 (2004).
Walton, D. W. H. Trends in Antarctic Terrestrial and Limnetic Ecosystems: Antarctica as a global indicator Edited by D.M. Bergstrom, P. Convey & A.H.L. Huiskes Springer, Dordrecht, 2006. ISBN 978-1-4020-5276-7, 369 pp, US$119. Antarctic Science 20, 611, https://doi.org/10.1017/s0954102008001624 (2008).
Hepp, L. U. & Melo, A. S. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia 703, 239–246, https://doi.org/10.1007/s10750-012-1367-7 (2012).
Barker, P. F. & Burrell, J. The opening of Drake passage. Marine geology 25, 15–34 (1977).
Barker, P. F. & Thomas, E. Origin, signature and palaeoclimatic influence of the Antarctic Circumpolar Current. Earth-Science Reviews 66, 143–162, https://doi.org/10.1016/j.earscirev.2003.10.003 (2004).
Chappuis, P. Copépodes de Tasmanie. Arch. Zool. Exp. Gen 87, 104–115 (1951).
Bowman, T. E. & Abele, L. G. In Systematic, the fossil record and biogeography. The biology of Crustacea Vol. 1 (ed. Abele, L. G.) 1–27 (Academic Press, 1982).
Chilton, C. LXV.—A new freshwater Amphipod from New Zealand. Journal of Natural History 1, 423–426 (1898).
Karanovic, T., Gibson, J. A. E., Hawes, I., Andersen, D. T. & Stevens, M. I. Diacyclops (Copepoda: Cyclopoida) in Continental Antarctica, including three new species. Antarctic Science 26, 250–260, https://doi.org/10.1017/s0954102013000643 (2013).
Morton, D. W. Revision of the Australian Cyclopidae (Copepoda: Cyclopoida). I Acanthocyclops Kiefer, Diacyclops Kiefer and Australocyclops, gen.nov. Australian Journal Marine and Freshwater Research 36, 615–634 (1985).
Bissett, A., Gibson, J. A. E., Jarman, S. N., Swadling, K. M. & Cromer, L. Isolation, amplification, and identification of ancient copepod DNA from lake sediments. Limnology and Oceanography: Methods 3, 533–542, https://doi.org/10.4319/lom.2005.3.533 (2005).
Convey, P. et al. Antarctic terrestrial life-challenging the history of the frozen continent? Biological reviews of the Cambridge Philosophical Society 83, 103–117, https://doi.org/10.1111/j.1469-185X.2008.00034.x (2008).
Gibson, J. A. E. & Bayly, I. A. E. New insights into the origins of crustaceans of Antarctic lakes. Antarctic Science 19, 157–163, https://doi.org/10.1017/S0954102007000235 (2007).
Vaughan, D. G. et al. Recent rapid regional climate warming on the Antarctic Peninsula. Climatic Change 60, 243–274, https://doi.org/10.1023/A:1026021217991 (2003).
Chapman, W. L. & Walsh, J. E. A synthesis of Antarctic temperatures. Journal of Climate 20, 4096–4117, https://doi.org/10.1175/Jcli4236.1 (2007).
Pritchard, H. D. et al. Antarctic ice-sheet loss driven by basal melting of ice shelves. Nature 484, 502–505, https://doi.org/10.1038/nature10968 (2012).
Convey, P. Antarctic terrestrial biodiversity in a changing world. Polar Biology 34, 1629–1641, https://doi.org/10.1007/s00300-011-1068-0 (2011).
Holdgate, M. W. Terrestrial Ecosystems in the Antarctic. Philosophical Transactions of the Royal Society B: Biological Sciences 279, 5–25, https://doi.org/10.1098/rstb.1977.0068 (1977).
Convey, P. In Encyclopedia of Biodiversity Vol. 1 (ed. Levin, S. A.) 171–184 (Academic Press, 2001).
Walton, D. W. H. In Antarctic Ecology Volume 1 (ed. Laws, R. M.) 1–60 (Academic Press, 1984).
Gremmen, N. J. M. The vegetation of the Subantarctic islands Marion and Prince Edward. (Junk Publishers, 1981).
McDowall, R. M. Falkland Islands biogeography: converging trajectories in the South Atlantic Ocean. Glob Ecol Biogeogr 32, 49–62 (2005).
Clarke, K. R., Somerfield, P. J. & Gorley, R. N. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366, 56–69, https://doi.org/10.1016/j.jembe.2008.07.009 (2008).
Clarke, K. & Green, R. Statistical design and analysis for a ‘biological effects’ study. Marine Ecology Progress Series 46, 213–226, https://doi.org/10.3354/meps046213 (1988).
Clarke, K. R. & Warwick, R. M. A further biodiversity index applicable to species lists: variation in taxonomic distinctness. Marine Ecology Progress Series 216, 265–278, https://doi.org/10.3354/meps216265 (2001).
Warton, D. I., Wright, S. T. & Wang, Y. Distance‐based multivariate analyses confound location and dispersion effects. Methods in Ecology and Evolution 3, 89–101 (2012).
Clarke, K. & Gorley, R. Primer. Primer-E, Plymouth (2006).
Borcard, D. & Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Modell 153, 51–68 (2002).
Borcard, D., LEgendre, P., Avois-Jacquet, C. & Tuomisto, H. Dissecting the spatial structures of ecological data alt all scales. Ecology 85, 1826–1832, https://doi.org/10.1890/03-3111 (2004).
Boyero, L. et al. Global patterns of stream detritivore distribution: implications for biodiversity loss in changing climates. Glob Ecol Biogeogr 21, 134–141, https://doi.org/10.1111/j.1466-8238.2011.00673.x (2012).
Graham, M. H. Confronting multicollinearity in ecological multiple regression. Ecology 84, 2809–2815, https://doi.org/10.1890/02-3114 (2003).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing v. 3.2.5. (2018).
Oksanen, J. et al. Vegan: Community Ecology PackageR package version 2.5-2, https://CRAN.R-project.org/package=vegan (2018).
Acknowledgements
Funding was obtained from PIA CONICYT ACT172065 to AD and CM, CONICYT Ph.D. Grant 21150317, P05-002 ICM, CONICYT PIA APOYO CCTE AFB170008 from Institute of Ecology and Biodiversity (IEB), INACH DT_04-16 to CM, FONDECYT INICIACIÓN 11170390, and Basque Government funds (IT951-16) to the Stream Ecology Group at UPV/EHU led by J. Pozo. We thank the English language editing provided by Journal Prep Services.
Author information
Authors and Affiliations
Contributions
A.D. y F.C.A. conceived the study; A.D. led the research; F.C.A., P.D.R.E., A.M.T. and L.B. carried out data analysis; A.D. and L.B. wrote the paper, with substantial contribution to writing and analysis interpretation from C.M.; all authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Díaz, A., Maturana, C.S., Boyero, L. et al. Spatial distribution of freshwater crustaceans in Antarctic and Subantarctic lakes. Sci Rep 9, 7928 (2019). https://doi.org/10.1038/s41598-019-44290-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44290-4
This article is cited by
-
Distribution of Boeckella poppei (Copepoda: Centropagidae) in the region of the Wilhelm Archipelago (Kyiv Peninsula, Maritime Antarctica)
Polar Biology (2024)
-
Bdelloid rotifers (Bdelloidea, Rotifera) in shallow freshwater ecosystems of Thala Hills, East Antarctica
Polar Biology (2023)
-
Phytoplankton in Antarctic lakes: biodiversity and main ecological features
Hydrobiologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.