Abstract
Preterm birth, defined as delivery before 37 weeks of gestation, is the leading cause of neonatal mortality and morbidity. Infection and inflammation are frequent antecedents of spontaneous preterm birth. Cathelicidin, an antimicrobial host defence peptide, is induced by infection and inflammation and although expressed in the reproductive tract and fetal tissues, its role in the pathogenesis of spontaneous preterm birth is unknown. Here we demonstrate that cathelicidin expression is increased at RNA and protein level in the mouse uterus in a model of inflammation-induced labour, where ultrasound guided intrauterine injection of lipopolysaccharide (LPS) at E17 stimulates preterm delivery within 24 hours. Cathelicidin-deficient (Camp−/−) mice are less susceptible to preterm delivery than wild type mice following intrauterine injection of 1 μg of LPS, and this is accompanied by a decrease in circulating IL-6, an inflammatory mediator implicated in the onset of labour. We also show that the proportion of cathelicidin expressing cells in the myometrium is higher in samples obtained from women in labour at term than pre-labour. Together, these data suggest that cathelicidin has roles in mediating pro-inflammatory responses in a murine model of inflammation-induced labour, and in human term labour.
Similar content being viewed by others
Introduction
Mechanisms controlling the onset of human labour are not well understood and disorders in the timing of labour onset are a major public healthcare burden. Spontaneous preterm labour is the most common cause of preterm birth (PTB) (delivery before 37 completed weeks of gestation). PTB is the leading cause of neonatal or infant death worldwide, and results in morbidities that can persist into adulthood1. The incidence of PTB ranges from 5% to 18% depending on the healthcare setting, with the incidence rising in many countries2. While approximately 30% of PTBs are indicated by maternal and/or fetal conditions, 70% of PTBs are spontaneous2.
Cathelicidins are a family of antimicrobial host defence peptides, a highly conserved component of the innate immune system. They are predominantly produced by epithelial cells, neutrophils and other inflammatory cells and are best known for their ability to act as broad-spectrum antimicrobial agents3,4. Cathelicidins also have functions in immunomodulation5, chemotaxis6, wound healing7 and angiogenesis8. This wide range of functions are believed to be mediated by an ability to activate multiple receptors, triggering different downstream pathways in a spatio-temporal manner that is dependent on the cell type and physiological context3. Humans and mice each express a single cathelicidin gene. In humans, CAMP encodes a precursor peptide (hCAP-18) that is either immediately released or stored intracellularly in neutrophil secondary granules. The precursor peptide is cleaved at the C-terminal by proteases to produce an active peptide called LL-373,9. LL-37 is abundant in the female reproductive tract10, fetal skin, vernix caseosa and in the amniotic fluid11. The murine orthologue, cathelicidin-related antimicrobial protein (mCRAMP) is encoded by the gene Camp. It is produced by the same cell types as in humans and is believed to have similar functions to that of LL-373,12.
Interestingly, the role of cathelicidin in pregnancy is unknown. We have recently shown that it can potentiate protective inflammation in response to infection in vivo13 suggesting that expression of this peptide could modulate inflammatory processes in the context of spontaneous PTB, which is often preceded by infection2,14.
Inflammation is recognized as a key feature of both normal term labour and PTB in humans2,14,15,16,17,18,19, characterised by infiltration of immune cells into gestational tissues and the release of inflammatory mediators that promote cervical ripening and myometrial activation20,21. Circulating progesterone levels remain high throughout parturition, and activation of inflammation in the uterus is thought to have a dominant role in promoting the change from uterine quiescence to a contractile phenotype. In most other mammals including mice, progesterone levels fall immediately prior to parturition. However, an intrauterine injection of lipopolysaccharide (LPS) is sufficient to activate inflammation and overcome the repressive effects of high circulating progesterone concentrations, inducing PTB19,22. This has become an established model for parturition research22,23.
In this study we used Camp−/− mice, which lack the murine cathelicidin peptide, mCRAMP, to determine whether cathelicidin plays a role in the onset of PTB using an established LPS-induced PTB mouse model22. To determine if human cathelicidin levels change in response to labour we investigated mRNA levels of CAMP in myometrial samples from women obtained at caesarean section either before labour onset, or during labour; at preterm or at term gestations.
Results
Intrauterine LPS injection induces mCRAMP expression in the mouse uterus at mRNA and protein level
We have previously shown that intrauterine administration of 20 μg LPS at gestation day 17 induced PTB in wild type C57Bl/6 mice, with an increase in pro-inflammatory cytokines and chemokines at the maternal-fetal interface22. To determine the minimal dosage required to induce PTB, an LPS dose response was performed in wild type mice ranging from 0.3–20 μg/dam (Supplementary Fig. S1). 1 μg LPS was the lowest dose found to induce PTB showing a similar time to delivery as 20 μg LPS. However, 1 μg LPS showed a significantly reduced expression of inflammatory genes Cxcl1 and IL-6 compared to 20 μg LPS (Supplementary Fig. S2).
We found that Camp mRNA expression and mCRAMP peptide levels were increased in the mouse uterus 6 hours after an intrauterine injection of both a 1 μg and 20 μg dose of LPS (Fig. 1). Immunofluorescence showed that mCRAMP is found in the uterine epithelium in PBS-treated control mice (Fig. 2a). Following a 1 μg LPS injection, mCRAMP was found in the uterine epithelium, stromal compartment and neutrophils as confirmed with dual staining with Ly6G (Fig. 2b).
Camp and mCRAMP expression is significantly increased in the mouse uterus after 1 µg and 20 µg intrauterine LPS injection. Real-time PCR analysis of relative Camp mRNA expression after 1 µg (a) or 20 µg (d) intrauterine LPS or PBS injection. Relative mCRAMP protein levels after 1 µg (b) or 20 µg (e) intrauterine LPS or PBS injection as quantified by Western Blot analysis (c, f). mCRAMP protein levels were normalised against housekeeping alpha-Tubulin (50 kDa). The mCRAMP protein band is indicated by a black arrow at approximately 18 kDa. Unpaired t-test (*p < 0.05, ****p = 0.0001). Data presented as mean ± SD. The full-length blots with mCRAMP expression are presented in Supplementary Figs S3 and S4.
Representative images of mCRAMP expression in the mouse uterus. (a) mCRAMP (green) is present in uterine epithelium (+) but absent in the stromal compartment (*) following a control PBS injection. No Ly6G positive cells (red) (neutrophils) can be found. (b) Following a 1 µg LPS injection, upregulated mCRAMP is present in the epithelium (+) and stromal compartment (*), with expression shown in neutrophils (red) as indicated by arrow and magnified in the inset panel. (c) Secondary antibody-only negative control. (d) Camp−/− uterus showing an absence of mCRAMP (green). mCRAMP negative neutrophil (red) is indicated by arrow and magnified in the inset panel.
Camp −/− mice are less susceptible to LPS – induced PTB
To assess whether LPS-induced cathelicidin could mediate PTB, Camp−/− and wild type mice were assessed after an intrauterine injection of either 1 μg or 20 μg of LPS, or PBS (negative control) (Fig. 3). Intrauterine injection of 1 μg LPS reduced time to delivery compared to PBS in wild type (25 ± 8.4 hrs following 1 μg LPS vs. 58 ± 20 hrs following PBS; p = 0.002) but not Camp−/− mice (50 ± 27.4 hrs following 1 μg LPS vs. 70 ± 11 hrs following PBS; p = 0.253), with less PTB (defined as delivery within 24 hours of intrauterine injection) in Camp−/− mice than wild type mice (82% vs. 40%; p = 0.015). In contrast, 20 μg LPS reduced the time to delivery similarly in wild type and Camp−/− mice, with no difference in PTB rates (83% and 100%, respectively, p > 0.999).
Time to delivery in Camp−/− and wild type mice after intra-uterine PBS or LPS injection. Time to delivery of first pup after intra-uterine injection with PBS, 1 µg or 20 µg of LPS in cathelicidin-deficient mice (Camp−/−) and wild type (C57BL/6J) mice. Significance representing LPS treated mice compared to PBS controls from same genotype (*p < 0.05, **p < 0.01, ***p = 0.001), #p < 0.05 difference between genotype in the same treatment group, Two-way ANOVA). No symbol signifies no significant difference between the groups (p > 0.05). Preterm Birth (PTB) rates, defined as delivery before 24 hours rates in percentage are shown below. Significance is calculated by using Fisher’s Exact test.
Camp −/− mice have significantly decreased circulating IL-6 following intrauterine LPS injection
Having shown that Camp−/− mice are less susceptible to LPS-induced PTB, we explored differences in maternal cytokine levels and uterine inflammatory gene expression following 1 μg LPS injection in wild type and Camp−/− mice. Maternal blood of Camp−/− mice had significantly lower circulating IL-6 compared to wild type following LPS injection (694 ± 1045 pg/ml vs. 2608 ± 1678 pg/ml; p = 0.045) (Fig. 4). No differences were seen in circulating CXCL10 or TNF levels (p = 0.996 and p > 0.999, respectively).
IL-6, CXCL10 and TNF production in the maternal circulation after LPS injection. 6 hours after intrauterine injection with 1 µg LPS, PBS or no treatment at the same gestation. (a) Circulating interleukin-6 (IL-6) was significantly greater in the wild type mice (C57Bl/6J) (N = 6) compared to Camp−/− mice (N = 5, *p = 0.045) upon LPS treatment. (b, c) No significant differences were seen in CXCL10 or TNF production. Data presented as mean ± SD, Two-way ANOVA, Tukey’s multiple comparison test.
The gene expression of prostaglandin-endoperoxide synthase 2 (PTGS2), chemokine (C-X-C motif) ligand 1 (CXCL1) and tumor necrosis factor (TNF) were unaltered in the uteri of Camp−/− mice compared to wild type mice, irrespective of whether 1 μg of LPS, PBS or no treatment was given (Fig. 5).
PTGS2, CXCL1 and TNF gene expression in the mouse uterus after LPS injection. 6 hours after intra-uterine injection with 1 µg LPS, PBS or no treatment, at the same gestation. No significant differences were seen in PTGS2 (a) CXCL1 (b) or TNF (c) expression between wild type and Camp−/− mice. Data presented as mean ± SD, Two-way ANOVA, Tukey’s multiple comparison test.
CAMP gene expression and quantification of LL-37 positive cells in human myometrial samples
Myometrial samples were classified into term pre-labour caesarean section (Term No Labour [TNL]), term labour (TL), preterm pre-labour caesarean section (Preterm No Labour [PTNL]) preterm labour (PTL) (Table 1). Labour was defined as presence of regular uterine contractions leading to progressive cervical dilatation.
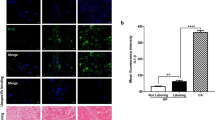
The myometrial samples were analysed for CAMP gene expression as well as the percentage of LL-37 positive cells (by immunofluorescence) in relation to total number of nuclei counted. Although no differences were found in CAMP gene expression between the groups (Fig. 6a), the percentage of LL-37 positive cells present in the human myometrium was higher in TL samples in comparison to TNL (35.5% vs 2.02% p < 0.0001), PTNL (35.5% vs 2.44% p < 0.0001) and PTL (35.5% vs 2.66% p < 0.0001) myometrial samples (Fig. 6b). Representative images of LL-37 immunofluorescent staining used to determine the percentage of LL-37 positive cells are shown in Fig. 7.
Relative CAMP expression and percentage of LL-37 positive cells in TNL, PTNL, TL, and PTL myometrium. (a) No difference in relative CAMP expression relative to β-Actin was found between TNL, PTNL, TL, and PTL myometrium. (b) The percentage of LL-37 positive cells was found to be significantly higher in TL myometrium in comparison to TNL, PTNL and PTL myometrium. Regular 2-way ANOVA with a Tukey’s multiple comparisons test was used to determine statistical significance. ****= p < 0.001. Data presented as mean ± SD.
Discussion
In this study, we demonstrate that mouse cathelicidin, mCRAMP, may play a role in the onset of inflammation associated PTB stimulated by LPS. We show that mCRAMP and IL-6 are upregulated in the uterus 6 hours following a labour-inducing intrauterine LPS injection stimulus. Camp−/− mice, which lack the mCRAMP protein, are less likely to go into labour following intrauterine LPS and show reduced levels of LPS-induced IL-6. We also show that human cathelicidin, LL-37, is highly expressed in myometrium from women who have had spontaneous labour at term, but we do not show this increase in myometrium from women who experienced preterm labour.
Cathelicidin is abundantly found in the reproductive tract10 and has been characterised as having both anti-inflammatory and pro-inflammatory roles3. Therefore, the role of this pleiotropic anti-microbial peptide in the context of PTB (well characterized as an inflammatory event)16,24, could be beneficial or detrimental. The direct microbicidal and anti-endotoxic properties of cathelicidins might be expected to be protective against PTB and the capacity of cathelicidin to bind to LPS and block activation of TLR4 might have been predicted to inhibit induction of PTB3,25,26. However, the level and timing of LPS and cathelicidin exposure is crucial to the response; cathelicidin is also capable of inducing inflammasome activation and IL-1β and IL-18 production in LPS-primed myeloid cells and suboptimally-stimulated, infected epithelial cells thereby contributing to an enhanced inflammatory response27,28. Furthermore, cathelicidin can act as a neutrophil chemoattractant29, thereby further enhancing inflammation13; highlighting the complexity and context-specificity of cathelicidin-mediated effects upon inflammation. Interestingly, it has been shown that endogenous mouse cathelicidin does not protect against LPS-induced shock, with similar or improved survival rates of Camp−/− compared to wild type mice30, suggesting that anti-endotoxic properties of the endogenous peptide may not be the dominant effect in an in vivo inflammatory setting31.
The pro-inflammatory effect of cathelicidin can be beneficial in certain circumstances. Indeed, we have shown that cathelicidin can promote pulmonary clearance of Pseudomonas aeruginosa in an in vivo murine model by enhancing the neutrophil response13. In our murine LPS-induced PTB model, intrauterine LPS injected Camp−/− mice, compared to wild type mice, showed significantly less circulating maternal IL-6; a pro-inflammatory cytokine shown to increase in myometrial cells after LPS stimulation32 and known to play an important role in the development of preterm and term labour33,34,35. This may suggest that mCRAMP influences the inflammatory profile to some extent and may have the potential to trigger the inflammatory cascade that ultimately leads to labour (Fig. 8). However, it may contribute to the onset of PTB through alternative pathways as CXCL10 and TNF, also known pro-inflammatory cytokines, were found to be similar in both wild type and Camp−/− mice following an intrauterine LPS injection. Transcription of PTGS2 (encoding COX-2 enzyme), CXCL1 (encodes the chemokine CXCL1 which regulates the recruitment of neutrophils and basophils during inflammation36,37) were also investigated in the mouse myometrium, however, these were found to be unaltered following 1 μg LPS injection at 6 hours.
The role of cathelicidin in the onset of preterm labour triggered by infection and inflammation as modelled by an intrauterine LPS injection. mCRAMP is increased in the wild type mouse uterus following exposure to LPS. This increase mediates a pro-inflammatory response that leads to an enhanced inflammatory cascade, influx of inflammatory cells and an increase in IL-6 (left hand panel). This in turn leads to preterm parturition. In Camp−/− mice, in the absence of mCRAMP, LPS fails to trigger the inflammatory cascade and an increase in IL-6 production. Consequently, Camp−/− mice have a decreased rate of preterm labour in comparison to wild type mice following a low dose intrauterine LPS injection (right hand panel).
Similar delivery rates were seen in wild type and Camp−/− mice when a high concentration of LPS (20 μg) was administered, suggesting that a sufficiently strong inflammatory stimulus can override the protective effect of mCRAMP deficiency.
It has been previously shown that cathelicidin is increased in a variety of tissues in various inflammatory conditions, and upon LPS exposure3,38,39,40. This is consistent with our observations in the mouse uterus with mCRAMP significantly increased 6 hours after LPS injection. Although mouse pregnancy is considerably different from human pregnancy, studies have indicated that the mouse model can potentially be useful for preclinical studies of human labour19,22,41. In our study we used human myometrium as an initial insight into the expression of LL-37 in context of labour as it forms the majority of the uterine wall and is central to labour as it is responsible for uterine contractions. We observed an increase in LL-37 protein levels during human term labour, compared to non-labour. These findings are in line with those of Lim et al. who found an increase in LL-37 protein levels in human myometrium from term women in labour compared to term women undergoing a pre-labour caesarean section42. They found LL-37 localised to myometrial muscle fibres in both labouring and pre-labouring samples, and in labouring samples LL-37 was also found in leukocytes42. LL-37 is known to be predominantly produced by neutrophils and epithelial cells3 and as there is an influx of neutrophils in term and pre-term labouring myometrium17,43, we hypothesise that the LL-37 increase is at least in part due to this infiltration20,21. However, we were unable to confirm whether neutrophils were a source of the LL-37 increase in our human myometrial samples, due to technical failure of dual immunofluorescent staining on paraffin embedded tissues. To overcome this limitation in future experiments, collected samples could be frozen for staining on frozen tissue, which may work better in this type of analyses. Furthermore, flow cytometry could be utilised to identify the LL-37 positive infiltrate cells. Importantly, future investigations should also include the study of other reproductive tissues, such as the endometrium and the amnion/chorion, as inflammatory mechanisms vary in different tissues.
Despite a greater proportion of LL-37 positive cells present in labouring human myometrium, we did not observe an equivalent increase in myometrial CAMP gene expression, suggesting that the LL-37 we observe in these human samples may primarily be stored and released from granules, as opposed to being synthesized de novo. Indeed, LL-37 in neutrophils is known to be predominantly pre-synthesised and stored as pre-peptides in granules9,44. This may potentially explain the discrepancy between the levels of mRNA and of protein seen in the human labouring myometrium. This contrasts our findings in the mouse, where LPS stimulated the production of mCRAMP at both mRNA and protein level. We did not detect a difference in LL-37 levels in the human myometrium when we compared samples from women in preterm labour and those having pre-labour preterm delivery. As all preterm deliveries are abnormal, finding an appropriate control group for the study of gene and protein expression changes during preterm labour is notoriously difficult. Many of the conditions that indicated iatrogenic PTB in our control, pre-labour caesarean section preterm group, are themselves associated with inflammation (e.g. pre-eclampsia, placental insufficiency and abruption). Thus, the lack of a detectable difference may reflect increased inflammation in both groups. In the present study, we cannot exclude that the regulation of LL-37 in the myometrium is different in preterm labour compared to preterm pre-labour, future research is needed for this, using appropriate control groups which are difficult to obtain. However, there was less LL-37 protein expression in preterm labour samples compared to term labour samples which may indicate differences in the pathophysiology of preterm and term labour, or may reflect other differences between the groups (e.g. duration of labour at time of sampling). The lack of an LL-37 upregulation in myometrium of the PTNL women might be considered inconsistent with our findings in the mouse model where we see an upregulation of mCRAMP 6 hours post-LPS injection. However, a previous study has shown that changes in the transcriptome in mouse myometrium induced by intrauterine LPS injection mirror those in the human myometrium seen during term parturition more closely than those seen during preterm labour19. Our data supports this finding as we also see a parallel between human TL myometrium and mouse uteri exposed to LPS, in that both show an upregulation of cathelicidin. In the mouse term labour is initiated through progesterone withdrawal and is associated with much less inflammation45. This corresponds with our observation in preliminary experiments examining mCRAMP expression throughout mouse gestation, where we found no evidence of upregulation in term labour.
In conclusion, we show that LPS increases the expression of cathelicidin in wild type mouse uterus and an absence of cathelicidin in Camp−/− mice reduces the incidence of PTB triggered by a low dose of LPS. In humans, cathelicidin protein levels are increased in myometrium undergoing term labour. These data suggest that cathelicidin plays a role in mediating some of the effects which lead to inflammatory PTB in mice, and may also be relevant in the onset of human labour.
Methods
In vivo experiments
In vivo studies were conducted under a UK Home Office license to J.E.N. (60/4241) in accordance with the Animals Scientific Procedures Act (1986). C57Bl/6 virgin female mice were purchased from Charles River Laboratories (Margate, UK). Camptm1Rig mice46 were backcrossed to congenicity onto a C57Bl/6J OlaHsd strain, and bred from homozygous matings in house in specific pathogen free facilities, in individually ventilated cages at the University of Edinburgh. Following matings, mice were housed in grouped cages of up to 6 mice per cage under specific pathogen free conditions at the same facility. Mice were permitted food and water as required and maintained in a 12-hour cycle of light and dark. All mice were acclimatized for a minimum of 1 week before use. Female mice were mated with stud males from the same genotype. The presence of a vaginal copulatory plug indicated day 1 of gestation. Pregnant mice with no appearance of a plug were not used in experiments, as gestational age was uncertain.
In vivo preterm birth model
The ultrasound guided intrauterine injection mouse model of PTB previously optimised in our group was used22. A dose response curve using wild type mice was performed to determine the minimal dosage needed to induce PTB (1 μg/dam), which was used parallel with the highest dose previously used (20 μg/dam) to determine the effects on PTB rates of the different dosages, (Fig. S1). Differences in the inflammatory response were also investigated (Fig. S2). Space between two intra-amniotic sacs was located with ultrasound where the injection was administered. 25 μl of sterile DPBS or LPS (20 μg/dam or 1 μg/dam) was injected at gestational age day 17. After recovery, mice were monitored using closed circuit television cameras and a video recorder. Time of delivery was the time between injection and delivery of first pup. PTB was defined as delivery within 24 hours of intrauterine injection.
In vivo timed collection experiments
A separate cohort of mice was used to assess the inflammatory effect of the stimuli used on maternal tissues. Mice were sacrificed by lethal exposure to carbon dioxide 6 hours after the intrauterine injection. A group of “no treatment” mice were also included. These mice were 17 days of gestation and received no treatment, and were culled in the same manner described above. Maternal serum was acquired by collecting whole blood through puncture of the aorta using 21G needles in Brand Tubes Microtainers tubes and spun for 5 minutes at 5.9 × g for ELISA analysis. Uterine samples were dissected and snap frozen in RNA-free tubes for protein and RNA analysis and separate samples were fixed in 4% neutral buffered formalin and embedded in paraffin blocks for immunofluorescence analysis.
Enzyme-linked Immunosorbent Assay (ELISA)
EBiosceince duosets for IL-6 and TNF-α and an R&D Duoset for CXCL-10 was used for cytokine analysis. Duosets were used according to the manufacturer’s protocols.
Protein extraction
Uterine samples were homogenised in RIPA Buffer (R0278, Sigma Life Sciences, Sigma-Alrich, UK) with a protease inhibitor (Protease Inhibitor Cocktail Tablets, Roche Diagnostics, IN, US) in a tissue lyser (Qiagen, Crawley, UK). Lysates were spun down at 4 °C for 10 minutes at 10,000 g. Protein was quantified using a Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories Ltd, Hertfordshire, UK).
Western blot
The protein from the uteri samples was run at 40 µg on NuPAGE Novex 4–12% and Bis-Tris Gels (Invitrogen, Life Technologies Ltd, Paisley, UK). Protein was added to 2.5 µl of LDS Sample Buffer and 1 µl of Reducing Agent (Invitrogen, Life Technologies Ltd, Paisley, UK). Samples were denatured at 70 °C for 10 minutes on a G-Storm Thermocycler. A positive control of 0.1 µg of mCRAMP peptide was used. A Wet Blotting System (Bio-Rad Laboratories Ltd, Hertfordshire, UK) was used for the wet transfer of the protein in the gel to the Immobilon Fl PVDF membrane (Millipore, UK). The membrane was blocked for 1 hr in 5% Milk (Marvel, UK) in TBST (0.1%Tween in 1 L TBS). Rabbit-Anti-CRAMP antibody (Innovagen, Lund, Sweden) was used at a 1:100 dilution. Mouse-Anti-α-Tubulin (Sigma, T9026) at a 1:5000 dilution was included as a loading control. Fluorescent secondary antibodies (Polyclonal Donkey Anti-Rabbit 800CW (926-32213) and Polyclonal Donkey Anti-Mouse 680RD (926-68072), LICOR Biosciences) were used in 10 ml of 5% Milk in TBST. Signal detection was through the use of LI-COR Odyssey Infrared Imaging System. The intensity of fluorescence in each lane was calculated using Image Studio Version 4.
Collection of human myometrial samples
Myometrium was collected from the inferior margin of the incision site of women delivering by caesarean section, at the Royal Infirmary, Edinburgh. Written and informed consent was obtained according to the ethical approval and governance granted to the Edinburgh Reproductive Tissues BioBank by the West of Scotland Research Ethics Committee 4 (REC reference: 09/S0704/3) until 30/09/2014; and consequently by the East of Scotland Research Ethics Service Tayside Committee on Medical Research Ethics B (REC reference:13/ES/0126). Upon collection, samples were placed into RNAlater solution (R0901 Sigma-Aldrich) for 24 hours at 4 °C, then taken out and stored at −80 °C prior to use. Inclusion criteria was singleton pregnancies that were term (>37 weeks of gestation) or preterm (<37 weeks of gestation) whilst exclusion criteria were age under 16 and any blood borne infections. Women either underwent an elective pre-labour caesarean section (no labour group) or an emergency CS in labour due to maternal and/or fetal indications (e.g. delay in labour, pre-eclampsia, fetal distress). Labour was defined as regular uterine contractions with cervical dilation.
qRT-PCR
Total RNA was extracted using the Rneasy Mini Kit (Qiagen, Crawley, UK) as per the manufacturer’s guidelines and converted into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies Ltd, Paisley, UK) in a G-Storm GS1 Thermocycler (G-storm, Somerton, UK). Taqman Gene Expression Assay was used to determine gene expression, with a TaqMan Universal Mastermix (Applied Biosystems, Life Technologies, Paisley, UK). CAMP (Hs00189038_m1), ACTB (431088E), Camp (Mm00438285_m1), Ptgs-2 (Mm00478374_m1), Cxcl1 (Mm04207460_m1), Tnf (Mm00443258_m1), Actb (4352341E) probes Applied Biosystems, Life Technologies, Paisley, UK) were used. The qRT-PCR was performed on the ABI 7900Ht (Applied Biosystems, Carlsbad, CA). Gene expression was determined using the ΔΔCt method, relative to housekeeping gene ACTB.
Immunofluorescence
Paraffin-embedded uterine sections on slides were dewaxed in xylene and rehydrated in decreasing alcohols to 70%. For LL-37 and mCRAMP detection, antigen retrieval was undertaken (0.1 M Citrate Buffer; decloaking chamber). Peroxidase blocking was carried out for 30 minutes (300 mls of 0.1% H2O2, in methanol) followed by a 30 minute serum block (Normal Goat Serum (NGS) in TBS with 5% BSA). Primary antibodies 1:10 LL-37 (Cayman; CAY 15637), 1:2500 mCRAMP (Abcam -ab74868) and 1:4000 Ly6G (Biolegend; 127602) was incubated on slides in a hydrated slide tray overnight at 4 °C. Negative controls were performed using NGS in place of the primary antibody. After two TBS washes, secondary antibodies were incubated on the slides for 30 minutes with goat anti-Rabbit AF555 (A-21429, Thermo Fisher Scientific, 10 µg/ml) Immpress Anti-Rabbit and Immpress Anti-Rat (Vector Laboratories Ltd, Peterborough, UK) for LL-37, mCRAMP and Ly6G respectively. Tyramide Signal Amplification (TSA; Tyramide Blue (TSA™-Plus Cyanine 5 System, NEL745B00KT, Perkin Elmer, MA, US), 1:50 for mCRAMP and Tyramide Red (TSA™-Plus Cyanine 3 System, NEL744B00KT, Perkin Elmer, MA, US), 1:50 for Ly6G and counterstaining for nuclei (Sytox Green (S7020, Invitrogen Molecular Probes, Life Technologies Ltd, Paisley, UK), 1:1000). Mouse uteri slides were mounted with permafluor (Thermo Scientific, UK) and human myometrium slides were mounted with Vectashield containing DAPI (Vector Laboratories Ltd, Peterborough, UK) with for confocal analysis.
Counting % of LL-37 positive cells
Images at Magnification of x40 were captured on the LSM710 confocal microscope. At least five areas were imaged from each sample. Four individual patient samples were analysed from the TL group and 5 patient samples were analysed from the TNL group. Image files were then imported into ImageJ and analysed using the Cell Counter plug-in. LL-37 positive cells were counted in each section and expressed as a percentage of total cells in section which was counted as total number of DAPI nuclei in the section.
Statistical analysis
Statistical analysis was carried out on GraphPad Prism version 7 (GraphPad Software, San Diego, California, US), with data presented as mean ± SD. Time to delivery data, mouse/human gene expression, cell count and ELISA data were analysed using a two-way ANOVA followed by Tukey’s post-hoc analysis. PTB rate was calculated using Fisher’s exact test, where PTB was defined as delivery less than 24 hours after intrauterine injection. Mouse data in which mCRAMP expression was quantified, was analysed using a one-way ANOVA. p < 0.05 was considered as statistically significant in all analyses.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269, https://doi.org/10.1016/S0140-6736(08)60136-1 (2008).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84, https://doi.org/10.1016/S0140-6736(08)60074-4 (2008).
Kahlenberg, J. M. & Kaplan, M. J. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191, 4895–4901, https://doi.org/10.4049/jimmunol.1302005 (2013).
Beaumont, P. E., Li, H.-N. & Davidson, D. J. LL-37: an immunomodulatory antimicrobial host defence peptide. In Antimicrobial peptides and Innate Immunity. Progress in Inflammation Research (eds Hiemstra, P. S. & Zaat, S. A. J.) 97–122 (Springer Basel, 2013).
Bowdish, D. M. et al. Impact of LL-37 on anti-infective immunity. J Leukoc Biol 77, 451–459, https://doi.org/10.1189/jlb.0704380 (2005).
Yang, D. et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192, 1069–1074 (2000).
Heilborn, J. D. et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 120, 379–389, https://doi.org/10.1046/j.1523-1747.2003.12069.x (2003).
Koczulla, R. et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest 111, 1665–1672 (2003).
Sørensen, O. E. et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 (2001).
Frew, L. & Stock, S. J. Antimicrobial peptides and pregnancy. Reproduction 141, 725–735, https://doi.org/10.1530/REP-10-0537 (2011).
Yarbrough, V. L., Winkle, S. & Herbst-Kralovetz, M. M. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update 21, 353–377, https://doi.org/10.1093/humupd/dmu065 (2015).
Gallo, R. L. et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272, 13088–13093 (1997).
Beaumont, P. E. et al. Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS One 9, e99029, https://doi.org/10.1371/journal.pone.0099029 (2014).
Agrawal, V. & Hirsch, E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 17, 12–19, https://doi.org/10.1016/j.siny.2011.09.001 (2012).
Cappelletti, M., Della Bella, S., Ferrazzi, E., Mavilio, D. & Divanovic, S. Inflammation and preterm birth. J Leukoc Biol 99, 67–78, https://doi.org/10.1189/jlb.3MR0615-272RR (2016).
Christiaens, I. et al. Inflammatory processes in preterm and term parturition. J Reprod Immunol 79, 50–57, https://doi.org/10.1016/j.jri.2008.04.002 (2008).
Gomez-Lopez, N., StLouis, D., Lehr, M. A., Sanchez-Rodriguez, E. N. & Arenas-Hernandez, M. Immune cells in term and preterm labor. Cell Mol Immunol 11, 571–581, https://doi.org/10.1038/cmi.2014.46 (2014).
Gotsch, F. et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med 21, 529–547, https://doi.org/10.1080/14767050802127349 (2008).
Migale, R. et al. Modeling hormonal and inflammatory contributions to preterm and term labor using uterine temporal transcriptomics. BMC Med 14, 86, https://doi.org/10.1186/s12916-016-0632-4 (2016).
Osman, I. et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9, 41–45 (2003).
Young, A. et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 66, 445–449 (2002).
Rinaldi, S. F. et al. Ultrasound-guided intrauterine injection of lipopolysaccharide as a novel model of preterm birth in the mouse. Am J Pathol 185, 1201–1206, https://doi.org/10.1016/j.ajpath.2015.01.009 (2015).
Elovitz, M. A., Wang, Z., Chien, E. K., Rychlik, D. F. & Phillippe, M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 163, 2103–2111, https://doi.org/10.1016/S0002-9440(10)63567-5 (2003).
Bollapragada, S. et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 200, 104.e101–111, https://doi.org/10.1016/j.ajog.2008.08.032 (2009).
Larrick, J. W. et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun 63, 1291–1297 (1995).
Rosenfeld, Y., Papo, N. & Shai, Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem 281, 1636–1643, https://doi.org/10.1074/jbc.M504327200 (2006).
McHugh, B. J. et al. Cathelicidin is a “fire alarm”, generating protective NLRP3-dependent airway epithelial cell inflammatory responses during infection with Pseudomonas aeruginosa. PLoS Pathog 15, e1007694, https://doi.org/10.1371/journal.ppat.1007694 (2019).
Elssner, A., Duncan, M., Gavrilin, M. & Wewers, M. D. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol 172, 4987–4994 (2004).
Tjabringa, G. S., Ninaber, D. K., Drijfhout, J. W., Rabe, K. F. & Hiemstra, P. S. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol 140, 103–112, https://doi.org/10.1159/000092305 (2006).
Pinheiro da Silva, F., Gallo, R. L. & Nizet, V. Differing effects of exogenous or endogenous cathelicidin on macrophage toll-like receptor signaling. Immunol Cell Biol 87, 496–500, https://doi.org/10.1038/icb.2009.19 (2009).
Severino, P. et al. Cathelicidin-deficient mice exhibit increased survival and upregulation of key inflammatory response genes following cecal ligation and puncture. J Mol Med (Berl) 95, 995–1003, https://doi.org/10.1007/s00109-017-1555-z (2017).
Boyle, A. K., Rinaldi, S. F., Rossi, A. G., Saunders, P. T. K. & Norman, J. E. Repurposing simvastatin as a therapy for preterm labor: evidence from preclinical models. FASEB J, fj201801104R, https://doi.org/10.1096/fj.201801104R (2018).
Herrera, C. A. et al. Cell-Free DNA, Inflammation, and the Initiation of Spontaneous Term Labor. Am J Obstet Gynecol, https://doi.org/10.1016/j.ajog.2017.05.027 (2017).
Rinaldi, S. F., Makieva, S., Saunders, P. T., Rossi, A. G. & Norman, J. E. Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol Hum Reprod 23, 708–724, https://doi.org/10.1093/molehr/gax038 (2017).
Robertson, S. A. et al. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 151, 3996–4006, https://doi.org/10.1210/en.2010-0063 (2010).
Geiser, T., Dewald, B., Ehrengruber, M. U., Clark-Lewis, I. & Baggiolini, M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem 268, 15419–15424 (1993).
Moser, B., Schumacher, C., von Tscharner, V., Clark-Lewis, I. & Baggiolini, M. Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem 266, 10666–10671 (1991).
Ong, P. Y. et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347, 1151–1160, https://doi.org/10.1056/NEJMoa021481 (2002).
Nakamichi, Y., Horibe, K., Takahashi, N. & Udagawa, N. Roles of cathelicidins in inflammation and bone loss. Odontology 102, 137–146, https://doi.org/10.1007/s10266-014-0167-0 (2014).
Horibe, K. et al. Roles of cathelicidin-related antimicrobial peptide in murine osteoclastogenesis. Immunology 140, 344–351, https://doi.org/10.1111/imm.12146 (2013).
Kemp, M. W. et al. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci 17, 619–628, https://doi.org/10.1177/1933719110373148 (2010).
Lim, R., Barker, G. & Lappas, M. Human cathelicidin antimicrobial protein 18 (hCAP18/LL-37) is increased in foetal membranes and myometrium after spontaneous labour and delivery. J Reprod Immunol 107, 31–42, https://doi.org/10.1016/j.jri.2014.10.002 (2015).
Singh, N. et al. Is myometrial inflammation a cause or a consequence of term human labour? J Endocrinol 235, 69–83, https://doi.org/10.1530/JOE-17-0318 (2017).
Sørensen, O., Cowland, J. B., Askaa, J. & Borregaard, N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods 206, 53–59 (1997).
Gonzalez, J. M., Romero, R. & Girardi, G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. J Reprod Immunol 97, 112–119, https://doi.org/10.1016/j.jri.2012.07.008 (2013).
Nizet, V. et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457, https://doi.org/10.1038/35106587 (2001).
Acknowledgements
This study was supported by funding from Tommy’s Charity within the Medical Research Council Centre for Reproductive Health (MR/N022556/1). Sarah J. Stock was funded by a Wellcome Trust Clinical Research Career Development Fellowship (209560/Z/17/Z). Donald J. Davidson was supported by a Medical Research Council Senior Non-clinical Fellowship (G1002046). Julia R. Dorin was supported by a Medical Research Council Human Genetics Unit (MRC) University Unit core grant and a Medical Research Council grant (MR/P02338X/1). Tinan L. Baker was supported by an MRC studentship. The authors would like to acknowledge Lauren Melrose for technical Assistance, and Robert Gray and Emily Gwyer Findlay for valuable discussions and Sara F. Rinaldi for contribution to supplementary data. The findings of this research article were presented in the Society for Reproductive Investigation Reproduction Meeting in 2017.
Author information
Authors and Affiliations
Contributions
Sara R. van Boeckel and Lenka Hrabalkova conducted the experiments, interpreted the data and wrote the manuscript. Tina Baker conducted the experiments, interpreted the data and contributed to the manuscript. Heather MacPherson assisted in the conduction of in vivo experiments, contributed to the experimental design, interpretation of data and writing of the manuscript. Lorraine Frew conducted and designed the experiments, interpreted the data and contributed to the manuscript. Ashley K. Boyle conducted and designed the experiments, interpreted the data and contributed to the manuscript. Brian McHugh assisted in the conduction of in vitro experiments, contributed to the experimental design, interpretation of data and writing of the manuscript. Kirsten Wilson conducted and designed the experiments, interpreted the data and contributed to the manuscript. Jane E. Norman contributed to the writing of the manuscript. Julia Dorin contributed to experimental design, interpretation of data and writing of the manuscript. Donald J. Davidson contributed to experimental design, interpretation of data and writing of the manuscript. Sarah J. Stock designed the experiments, contributed to the interpretation of data and writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boeckel, S.R.v., Hrabalkova, L., Baker, T.L. et al. Cathelicidins and the Onset of Labour. Sci Rep 9, 7356 (2019). https://doi.org/10.1038/s41598-019-43766-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43766-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.