Abstract
Coenzyme Q10 (CoQ10) deficiencies are a group of heterogeneous conditions that respond to ubiquinone administration if treated soon after the onset of symptoms. However, this treatment is only partially effective due to its poor bioavailability. We tested whether vitamin K2, which was reported to act as a mitochondrial electron carrier in D. melanogaster, could mimic ubiquinone function in human CoQ10 deficient cell lines, and in yeast carrying mutations in genes required for coenzyme Q6 (CoQ6) biosynthesis. We found that vitamin K2, despite entering into mitochondria, restored neither electron flow in the respiratory chain, nor ATP synthesis. Conversely, coenzyme Q4 (CoQ4), an analog of CoQ10 with a shorter isoprenoid side chain, could efficiently substitute its function. Given its better solubility, CoQ4 could represent an alternative to CoQ10 in patients with both primary and secondary CoQ10 deficiencies.
Similar content being viewed by others
Introduction
The mitochondrial respiratory chain (MRC) is composed of four multienzymatic complexes embedded in the mitochondrial inner membrane (MIM) of eukaryotes and of two electron carriers, cytochrome c (cyt c) and Coenzyme Q10 (CoQ10) or ubiquinone.
CoQ is a lipophilic molecule present in all cell membranes; its structure consists of a benzoquinone ring bound to a hydrophobic polyisoprenic tail of variable length, depending on the different species (ten units in humans, nine in mice, six in yeast)1. Beyond its fundamental role as electron carrier, CoQ has many other functions, including its involvement in pyrimidine biosynthesis, cell growth and differentiation, counteraction of apoptosis, mitophagy, functional modification of mitochondrial uncoupling proteins2,3 and regulation of sulfide metabolism4,5; moreover, in its reduced form it is the only lipid-soluble antioxidant synthesized endogenously6.
CoQ biosynthesis requires a series of proteins encoded by COQ genes and has been extensively studied in bacteria and yeast7,8. Currently, the complete metabolic biosynthetic pathway remains to be elucidated9,10 and in humans thirteen genes required for CoQ10 biosynthesis have been identified so far11.
Defects of ubiquinone biosynthesis cause CoQ10 deficiencies, a group of clinically and genetically heterogeneous conditions12,13,14. Mutations in ten COQ genes have been associated with primary CoQ10 deficiencies11,15. Depending on the localization and extent of the defect, the clinical symptoms vary greatly: the brain, the cerebellum, the muscle and/or the kidney may be involved, usually resulting in complex disease patterns16. Fibroblasts and muscle of affected individuals present a decrease of combined activities of Complex I + III (CI + III) and II + III (CII + III)17 (two CoQ10 – dependent reactions).
Patients with primary CoQ10 deficiency respond well to ubiquinone oral administration18,19,20, but this approach is not completely effective, in part also because of the poor bioavailability of CoQ10 related to its extreme hydrophobicity21,22. Consequently, CoQ10 supplementation requires high doses (up to 30–50 mg/kg/day)14 and in the last years several studies have investigated the efficacy of water-soluble formulations and of different ubiquinone analogs23,24,25,26,27. Ubiquinol-10, the reduced form of CoQ10, was found to have the same efficiency at a lower dosage than its oxidized form in a patient with CoQ10 deficiency, due to its better bioavailability28. It was also shown to be more effective in ameliorating the phenotype of a CoQ-deficient mouse model with mitochondrial encephalopathy29. However, experience in patients with primary forms is currently limited. The CoQ10 precursor 4-hydroxybenzoic acid (4-HB) and its analogs 2,4-dihydroxybenzoic acid (2,4-diHB), 3,4-diHB and vanillic acid exhibit beneficial effects on CoQ10 deficient cells in vitro, and 2,4-diHB rescued the mutant phenotypes of two different mice models of ubiquinone deficiency15,30,31,32.
Vitamin K2 (menaquinone-4, MK-4) is an isoprenoid quinone like CoQ10, containing a naphtoquinone ring and shorter isoprenoid side chain. Thus, vitamin K2 is less lipophilic than CoQ10 and it acts as electron carrier in some bacterial species33. A previous study suggested that vitamin K2, similarly to CoQ10, contributes to electron transport in the MRC of D. melanogaster34 and hence it could represent a promising drug to treat mitochondrial diseases, and specifically defects in ubiquinone biosynthesis.
In this work we tested whether vitamin K2 and Coenzyme Q4 (CoQ4, an analog of CoQ10 with a shorter isoprenoid side chain) could substitute CoQ function in human and yeast cells with CoQ deficiency.
Results
Vitamin K2 is incorporated into human mitochondria
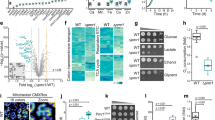
In a first set of experiments, we checked whether vitamin K2 enters the cells and if it reaches mitochondria when it is added to the culture media as an aqueous solution. Cells were supplemented with vitamin K2 (5 μM) for 1 week and, after lipid extraction, vitamin K2 levels were determined by reverse phase HPLC with electrochemical detection (ECD). Untreated cells had no detectable vitamin K2, while in vitamin K2-treated cells the chromatogram displayed a clear peak at 4.7 min identical to the standard (Fig. 1a).
Vitamin K2 incorporates into human cells. (a) Chromatograms of a standard solution of vitamin K2 and its uptake in HeLa cells treated or not with MK-4 (5 μM) for 7 days. Cellular fractions of vitamin K2 were analyzed by reverse phase HPLC with ECD. The arrow indicates the peak corresponding to vitamin K2. (b) Proteins (50 μg) from subcellular fractions of HeLa treated with MK-4 (5 μM) for 7 days were separated by SDS–PAGE and immunoblotted with the indicated antibodies, specific for each fraction. F1 is constituted by nuclei and unbroken cells, F2 is an enriched mitochondrial fraction, and F3 includes cytosol, endoplasmic reticulum (ER), the other organelles and light membranes. (c) Mean ± s.e.m. (n = 3) of vitamin K2 content in cell lipid extracts prepared from each subcellular fraction was determined by HPLC with ECD.
We then analyzed the subcellular distribution of vitamin K2. A cellular fractionation of MK-4-treated cells was carried out by differential centrifugation, resulting in three different fractions: F1 constituted by nuclei and unbroken cells, an enriched-mitochondrial fraction F2, and F3 that included cytosol, endoplasmic reticulum, Golgi apparatus, peroxisomes, lysosomes and the other light membranes. These fractions were analyzed by western blot using antibodies against Complex IV subunit 4 (COX4) as mitochondrial marker, lamin A/C as nuclear marker, and APAF1 as cytosolic marker (Fig. 1b). Lipid extracts were prepared from each fraction, and the incorporation of vitamin K2 was determined by HPLC. As shown in Fig. 1c, vitamin K2 accumulates in F1 and in mitochondria, confirming previous data35.
Vitamin K2 does not restore electron flow in the respiratory chain of cells with CoQ10 deficiency
We evaluated the effects of vitamin K2 supplementation on CII + III combined activities in CoQ10 deficient fibroblasts with mutations in COQ2 (P1)36. P1 fibroblasts were incubated with 5 μM CoQ10 or vitamin K2 for one week. CII + III activity was determined spectrophotometrically by following the reduction of 50 μM cyt c at 550 nm. As shown in Fig. 2a, untreated fibroblasts had 20% residual CII + III activity relative to controls (these cells harbor a hypomorphic allele that allows a residual production of CoQ1037). Only CoQ10-treated cells showed a normalization of mitochondrial CII + III activity, whereas vitamin K2 had no effect. As a further control we employed the more soluble decyl-ubiquinone (DUB) that could largely rescue the CII + III enzymatic defect when added to the cuvette.
Vitamin K2 is not able to correct the defective MRC activities in human cells. (a) Complex II + III activity was determined spectrophotometrically in lysates obtained from CoQ10 deficient patient fibroblasts (P1) treated for 7 days with or without CoQ10 or MK-4 (5 μM), or with DUB (150 μM) added to the cuvette. Data are represented as mean ± s.e.m. (n = 3). (b) CoQ10 deficient patient fibroblasts (P2) treated for 7 days with or without CoQ4 or MK-4 (5 μM). Data are represented as mean ± s.e.m. (n = 3). (c) HEK293 cells treated with 4-NB (4 mM) with or without CoQ10 or MK-4 (5 μM) for 7 days (E). Data are represented as mean ± s.e.m. (n = 4). (d) HEK293 COQ4−/− cells treated for 7 days with or without CoQ10, CoQ4 or MK-4 (5 μM). Data are represented as mean ± s.e.m. (n = 3). (e) ATP content was measured in HEK293 cells (CTRL) and in COQ4−/− cells treated with 5 μM CoQ10, MK-4 or CoQ4 for 7 days. Data are represented as mean ± s.e.m. (n = 3). (f) Complex II activity was determined spectrophotometrically in lysates obtained from mitochondrial-enriched fractions of HEK293 cells by using DUB, CoQ10 or vitamin K2 as electron acceptors. Data are represented as mean ± s.e.m. (n = 3).
To exclude a mutation-specific effect, we repeated the experiment using fibroblasts (P2) with a different genetic defect in COQ238, and similar results were obtained. In this case we also tested the effect of CoQ4, a short chain quinone that is less lipophilic than CoQ10 (the isoprene chain is similar to that of vitamin K2). Supplementation with 5 μM CoQ4 for 1 week was able to restore normal CII + III activity (Fig. 2b).
We then employed a different system to induce CoQ10 deficiency. HEK293 cells were treated with 4 mM 4-nitrobenzoate (4-NB), a selective inhibitor of CoQ10 biosynthesis39, for 7 days. This treatment decreased CII + III activity by ~50%, and the addition of CoQ10 could rescue MRC functionality, whereas we did not observe any effect after vitamin K2 supplementation (Fig. 2c).
In the meantime, we were able to obtain a human cell line harboring a genetic ablation of COQ4 (COQ4−/−)40. We therefore tested the effect of vitamin K2 also in this model (which is more stable than primary fibroblasts or 4-NB-treated cells). Cells were treated with 5 μM CoQ10, CoQ4 or vitamin K2 for 1 week and CII + III activity was determined as above. COQ4−/− cells displayed a strong decrease of CII + III activity compared to controls (Fig. 2d). Only cells supplemented with CoQ10 or CoQ4 displayed a rescue of the biochemical defect.
Finally, we also analyzed the ATP content in COQ4−/− cells treated with 5 μM CoQ10, vitamin K2 or CoQ4 for one week. (Fig. 2e). While CoQ10 and CoQ4 were largely able to rescue the bioenergetic defect, increasing ATP to levels comparable to control cells, vitamin K2 was not effective. To further support these results, we repeated the same experiment using patient’s fibroblasts with CoQ10 deficiency (P1) and obtained similar results (see Supplementary Fig. S1).
To test whether treatment with CoQ4 might affect CoQ10 content, we also measured CoQ10 levels in HEK293 cells treated for 7 days with 5 μM CoQ10, vitamin K2 or CoQ4. As expected, CoQ10 content was increased in cells treated with CoQ10, while it was essentially unchanged in cells treated with either vitamin K2 or CoQ4, suggesting that the two compounds are not affecting CoQ10 biosynthesis (see Supplementary Fig. S2).
Vitamin K2 does not restore respiratory growth of yeast COQ mutants
In a final set of experiments, we employed the S. cerevisiae ΔCOQ6 strain as additional model to test the effect of exogenous vitamin K2 on lower eukaryotes. This strain is unable to grow on non-fermentable carbon sources (ethanol and glycerol) if not supplemented with coenzyme Q6 (CoQ6)41. The ΔCOQ6 strain was transformed with the WT yCOQ6 as control or its mutant versions: the catalytically inactive but structurally stable F455X allele42 and the G386A_N388D double mutant43. Yeast growth was analyzed on a medium containing glycerol as a non-fermentable carbon source (YPG), with or without 50 μM vitamin K2. Vitamin K2 was not able to rescue the defective growth in the ΔCOQ6 strain (see Supplementary Fig. S3).
To exclude that the lack of rescue by exogenous vitamin K2 could be due to the inability of the compound to reach yeast MIM, we treated wild-type yeast with 50 μM vitamin K2 for 2 days and quantified vitamin K2 content by HPLC separation and ECD, after mitochondria purification and lipid extraction. As in human cells, we found that yeast mitochondria also uptake vitamin K2 significantly (see Supplementary Fig. S3).
Overall, these findings indicate that vitamin K2 is not active as electron carrier in the MRC of S. cerevisiae, or in that of human cells.
Vitamin K2 is not an efficient substrate for complex II in human cells in vitro
Finally, we repeated the experiment performed by Vos and coworkers to test the electron acceptor capability of vitamin K2 compared to CoQ10. However, we also included as further controls DUB, a good electron acceptor for Complex II (CII) in vitro, and CoQ4. We measured CII activity by following the reduction of the 2,6-dichlorophenolindophenol (DCPIP) at 600 nm in mitochondrial-enriched fractions from HEK293 cells. As seen in Fig. 2f, neither CoQ10 nor vitamin K2 are good substrates for this reaction, since the activity measured with these compounds is almost two orders of magnitude inferior to that measured with DUB, at comparable concentrations.
Discussion
Primary CoQ10 deficiency is one of the few treatable mitochondrial disorders, since oral supplementation with CoQ10 improves the symptoms of patients with different COQ genes defects44. Moreover, CoQ10 has been widely used for the treatment of patients with mitochondrial disorders because they frequently display secondary deficiency45. However, CoQ10 bioavailability and mitochondrial targeting are low due to its high hydrophobicity. Therefore, in the last years many efforts have been made to identify more water-soluble analogs of CoQ10. The use of certain oxidized or reduced CoQ10 dosages and formulations is able to increase ubiquinone levels in all human tissues after oral administration46. Also the use of 4-HB analogs is a promising strategy to bypass defective steps in the CoQ10 biosynthetic pathway47.
Vos and coauthors have proposed that vitamin K2 can function as an electron carrier in the MRC of a multicellular eukaryote, D. melanogaster34. The structure of vitamin K2 is similar to CoQ10, but it has a shorter hydrophobic carbon chain tail with four prenyl units that confer higher hydrophilicity. Because of the better bioavailability of vitamin K2, these findings opened the possibility that it could substitute CoQ10 in deficient patients.
To test this hypothesis, we have employed cells in which CoQ10 biosynthesis was disrupted or inhibited either by genetic or pharmacological means. Our results do not support the notion that vitamin K2 can act as an electron carrier in eukaryotic cells. In fact, even though it can easily reach mitochondria, vitamin K2 could not restore either electron flow or ATP biosynthesis in CoQ10-deficient cells.
One of the experiments performed by Vos and coworkers to support their claims showed that CoQ10 and vitamin K2 act as electron acceptors from complex II in vitro34. However, we now show that neither compound is a good in vitro substrate for Complex II compared to what is considered a good one, DUB48. In fact, the ability of vitamin K2 to accept electrons is about two orders of magnitude lower than that of DUB, and the situation for CoQ10 is only slightly better. Therefore, it is impossible to draw any conclusions from this experiment.
In agreement with our results, vitamin K2 failed to rescue mouse embryonic fibroblasts (MEFs) that are deficient for MCLK1 (the orthologue of human COQ7), where it could not restore the respiratory defect caused by CoQ10 deficiency24.
One of the limitations of our study is that we could not use D. melanogaster cells to exclude that the different results obtained in that model could be due to a species-specific effect. Nevertheless, our data clearly show that the role of vitamin K2 as an electron carrier (if confirmed) is probably restricted to Drosophila, and is not a general phenomenon in eukaryotic cells.
Finally, we found that CoQ4 supplementation rescues both CII + III activity and ATP content in CoQ10 deficient cells. It was previously shown that CoQ2 cannot mimic CoQ10 function in the MRC of human fibroblasts with ubiquinone deficiency49. Our data highlight that the effectiveness of electron transport in the MRC critically depends on the length of the isoprenoid chain, suggesting that two additional isoprenoid groups are sufficient to ensure the interaction with respiratory complexes binding sites. However, the mechanisms underlying these differences remain to be elucidated.
Although short chain quinones are toxic, this is particularly true for compounds with 0–3 isoprene units, whereas CoQ4 displays only minimal toxicity, evident only at concentrations of around 200 μM50, 40 times higher than those employed in this study.
Our findings indicate that CoQ4 could provide an interesting alternative to CoQ10 for the treatment of CoQ10 deficiency in humans. Further studies on animal models are warranted to unravel this issue.
Materials and Methods
Cell culture and chemicals
HEK293, HeLa cells and human fibroblasts were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 2 mM L-glutamine, 75 U/mL penicillin, 50 µg/mL streptomycin (Invitrogen) at 37 °C in a 5% CO2 incubator. Fibroblasts of patients with CoQ10 deficiency were previously described38,51. They had been obtained with the informed consent of the parents of the patients. The study was reviewed by the local ethical committee (Comitato Etico per la Sperimentazione Clinica della Provincia di Padova) – protocol AOPD2019 #0020044). All experiments were performed in accordance with the relevant guidelines and regulations of the University of Padova.
HEK293 COQ4−/− were generated in our laboratory as described40 and were cultured in the presence of pyruvate (1 mM) and uridine (50 μg/mL) with 4% FBS, to minimize CoQ10 content in the medium.
For MRC activity and ATP measurement, cells were cultured for 2 days in DMEM medium containing low glucose (2 mM), to force mitochondrial respiration.
CoQ10, MK-4, CoQ4 and DUB were purchased from Sigma (Milan, Italy). They were dissolved in ethanol and added to the cells as less than 1% of total cell culture volume.
Cell lysates, isolation of mitochondria, western blot, biochemical assays and ATP measurements
Whole-cell lysates were prepared in CHAPS buffer (1% CHAPS, 100 mM KCl, 50 mM HEPES pH 7.5, 1 mM EGTA) supplemented with the complete protease-inhibitor mixture (Sigma). Isolation of mitochondria was performed as detailed elsewhere52. Proteins were quantified using the Bradford method (Biorad) and fifty micrograms of proteins were separated by SDS-PAGE. Gels were probed with the following antibodies: COX4 (Thermo Fisher), Lamin A/C (Santa Cruz, USA), Apaf1 (Vinci-Biochem) and secondary HRP-conjugated anti-mouse, anti-rabbit or anti-goat antibodies (Santa Cruz, USA). Visualization of antibody protein complexes was achieved by enhanced chemiluminescence (LiteAblot Turbo, Euroclone) and the ChemiDocTM XRSþSystem (Bio-Rad). The enzymatic activity of RC complexes and citrate synthase (CS) was measured spectrophotometrically as previously described53, using a Cary UV 100 spectrophotometer (Varian). Where indicated, RC activities were normalized to CS. Cellular ATP was measured using the ATPlite kit (PerkinElmer), following the manufacturer’s instructions. ATP levels were normalized by total protein concentration.
Lipid extraction and measurement of vitamin K2 by HPLC
A mixture of ethanol:hexane (2:5) was added to samples treated with MK-4 and after vortexing for 1 min, they were centrifuged at 2000 g for 5 min at 4 °C. The upper phase was recovered and dried. Lipid extracts were suspended in 150 μl of methanol prior to HPLC injection. Lipid components of the extracts were separated with a Waters Alliance e2695 HPLC system equipped with a vacuum degasser and an electrochemical detector (Antec Decade II). Chromatographic separations were conducted on a C18 4.6 × 150 mm analytical column, 3.5 µm particle size (Varian, Palo Alto, California) maintained at 30 °C. The pump flow rate was 1 mL/min and injection volume was comprised between 2 µL (human cells) and 10 µL (yeast cells). The separation was performed by isocratic elution with a mobile phase constituted by 96% methanol + 4% ethanol and 0.1 M LiClO4. The standard solution with vitamin K2 was injected to generate a standard curve that was used to quantify vitamin K2.
Statistical analysis
Results are expressed as the mean ± SEM values of the indicated number (n) of independent experiments. Statistical significance was determined by unpaired Student’s t test between the indicated samples and differences were considered statistically significant for p < 0.05.
Data Availability
All data analyzed during this study are included in this published article and supplemental materials.
References
Ernster, L. & Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta 1271, 195–204 (1995).
Quinzii, C. M. & Hirano, M. Coenzyme Q and mitochondrial disease. Dev Disabil Res Rev 16, 183–8 (2010).
Bentinger, M., Tekle, M. & Dallner, G. Coenzyme Q–biosynthesis and functions. Biochem Biophys Res Commun 396, 74–9 (2010).
Luna-Sanchez, M. et al. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol Med 9, 78–95 (2017).
Kleiner, G. et al. CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway. Biochim Biophys Acta Mol Basis Dis 1864, 3708–3722.
Bentinger, M., Brismar, K. & Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 7(Suppl), S41–50 (2007).
Payet, L. A. et al. Mechanistic Details of Early Steps in Coenzyme Q Biosynthesis Pathway in Yeast. Cell Chem Biol 23, 1241–1250 (2016).
Doimo, M. et al. Genetics of coenzyme q10 deficiency. Mol Syndromol 5, 156–62 (2014).
Stefely, J. A. & Pagliarini, D. J. Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem Sci 42, 824–843 (2017).
Kawamukai, M. Biosynthesis of coenzyme Q in eukaryotes. Biosci Biotechnol Biochem 80, 23–33 (2015).
Acosta, M. J. et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta 1857, 1079–85 (2016).
DiMauro, S., Quinzii, C. M. & Hirano, M. Mutations in coenzyme Q10 biosynthetic genes. J Clin Invest 117, 587–9 (2007).
Quinzii, C. M., DiMauro, S. & Hirano, M. Human coenzyme Q10 deficiency. Neurochem Res 32, 723–7 (2007).
Desbats, M. A., Lunardi, G., Doimo, M., Trevisson, E. & Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J Inherit Metab Dis 38, 145–56 (2015).
Freyer, C. et al. Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J Med Genet 52, 779–83 (2015).
Emmanuele, V. et al. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol 69, 978–83 (2012).
Montero, R. et al. Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes. Clin Biochem 41, 697–700 (2008).
Pineda, M. et al. Coenzyme Q(10)-responsive ataxia: 2-year-treatment follow-up. Mov Disord 25, 1262–8 (2010).
Montini, G., Malaventura, C. & Salviati, L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med 358, 2849–50 (2008).
Rotig, A. et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 356, 391–5 (2000).
Bentinger, M., Dallner, G., Chojnacki, T. & Swiezewska, E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med 34, 563–75 (2003).
Santos-Ocana, C., Do, T. Q., Padilla, S., Navas, P. & Clarke, C. F. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem 277, 10973–81 (2002).
Geromel, V. et al. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab 77, 21–30 (2002).
Wang, Y. & Hekimi, S. Mitochondrial respiration without ubiquinone biosynthesis. Hum Mol Genet 22, 4768–83 (2013).
Villalba, J. M., Parrado, C., Santos-Gonzalez, M. & Alcain, F. J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig Drugs 19, 535–54 (2010).
Beg, S., Javed, S. & Kohli, K. Bioavailability enhancement of coenzyme Q10: an extensive review of patents. Recent Pat Drug Deliv Formul 4, 245–55 (2010).
Bhagavan, H. N., Chopra, R. K., Craft, N. E., Chitchumroonchokchai, C. & Failla, M. L. Assessment of coenzyme Q10 absorption using an in vitro digestion-Caco-2 cell model. Int J Pharm 333, 112–7 (2007).
Salviati, L. et al. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J Med Genet 49, 187–91 (2012).
Garcia-Corzo, L. et al. Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency. Biochim Biophys Acta 1842, 893–901.
Wang, Y., Oxer, D. & Hekimi, S. Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat Commun 6, 6393 (2015).
Herebian, D. et al. 4-Hydroxybenzoic acid restores CoQ10 biosynthesis in human COQ2 deficiency. Ann Clin Transl Neurol 4, 902–908 (2017).
Widmeier, E. et al. Treatment with 2,4-Dihydroxybenzoic Acid Prevents FSGS Progression and Renal Fibrosis in Podocyte-Specific Coq6 Knockout Mice. J Am Soc Nephrol 30, 393–405 (2019).
Kurosu, M. & Begari, E. Vitamin K2 in electron transport system: are enzymes involved in vitamin K2 biosynthesis promising drug targets? Molecules 15, 1531–53 (2010).
Vos, M. et al. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 336, 1306–10 (2012).
Suhara, Y., Murakami, A., Nakagawa, K., Mizuguchi, Y. & Okano, T. Comparative uptake, metabolism, and utilization of menaquinone-4 and phylloquinone in human cultured cell lines. Bioorg Med Chem 14, 6601–7 (2006).
Quinzii, C. et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q(10) deficiency. American Journal of Human Genetics 78, 345–349 (2006).
Desbats, M. A. et al. The COQ2 genotype predicts the severity of coenzyme Q10 deficiency. Hum Mol Genet 25, 4256–4265 (2016).
Desbats, M. A. et al. Primary coenzyme Q10 deficiency presenting as fatal neonatal multiorgan failure. Eur J Hum Genet 23, 1254–8 (2015).
Forsman, U., Sjoberg, M., Turunen, M. & Sindelar, P. J. 4-Nitrobenzoate inhibits coenzyme Q biosynthesis in mammalian cell cultures. Nat Chem Biol 6, 515–7 (2010).
Giorgio, V. et al. The idebenone metabolite QS10 restores electron transfer in complex I and coenzyme Q defects. Biochim Biophys Acta 1859, 901–908 (2018).
Doimo, M. et al. Effect of vanillic acid on COQ6 mutants identified in patients with coenzyme Qw deficiency. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1842, 1–6 (2014).
Heeringa, S. F. et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest 121, 2013–24 (2011).
Ozeir, M. et al. Coenzyme Q biosynthesis: Coq6 is required for the C5-hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem Biol 18, 1134–42 (2011).
Emma, F., Montini, G., Parikh, S. M. & Salviati, L. Mitochondrial dysfunction in inherited renal disease and acute kidney injury. Nat Rev Nephrol 12, 267–80 (2016).
Yubero, D. et al. Secondary coenzyme Q10 deficiencies in oxidative phosphorylation (OXPHOS) and non-OXPHOS disorders. Mitochondrion 30, 51–8 (2016).
Zaki, N. M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv 23, 1868–81 (2014).
Pierrel, F. Impact of Chemical Analogs of 4-Hydroxybenzoic Acid on Coenzyme Q Biosynthesis: From Inhibition to Bypass of Coenzyme Q Deficiency. Front Physiol 8, 436 (2017).
Spinazzi, M. et al. Optimization of respiratory chain enzymatic assays in muscle for the diagnosis of mitochondrial disorders. Mitochondrion 11, 893–904 (2011).
Lopez, L. C. et al. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLoS One 5, e11897 (2010).
Takahashi, T., Mine, Y. & Okamoto, T. Intracellular reduction of coenzyme Q homologues with a short isoprenoid side chain induces apoptosis of HeLa cells. J Biochem 163, 329–339 (2018).
Quinzii, C. et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet 78, 345–9 (2006).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2, 287–95 (2007).
Spinazzi, M., Casarin, A., Pertegato, V., Salviati, L. & Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7, 1235–46 (2012).
Acknowledgements
This work was supported by grants from the Italian Ministry of Health [grant number GR-2009-1578914] and the University of Padova [grant number CPDA140508/14] to E.T and by grants from Telethon Italy [GGP14187C] and from Fondazione IRP Città della Speranza to L.S.
Author information
Authors and Affiliations
Contributions
C.C. Data curation; Formal analysis, writing. She performed the bulk of the biochemical experiments. L.V.F., A.C. and G.V. methodology. A.C., F.P. and G.V. acquired and analyzed the HPLC data, whereas L.V.F. performed the genome editing of human cells. L.S. funding acquisition, review and editing. E.T. Conceptualization, Funding acquisition, supervision of the experiments, manuscript writing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cerqua, C., Casarin, A., Pierrel, F. et al. Vitamin K2 cannot substitute Coenzyme Q10 as electron carrier in the mitochondrial respiratory chain of mammalian cells. Sci Rep 9, 6553 (2019). https://doi.org/10.1038/s41598-019-43014-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43014-y
This article is cited by
-
Diverse biological functions of vitamin K: from coagulation to ferroptosis
Nature Metabolism (2023)
-
Delivery of the reduced form of vitamin K2(20) to NIH/3T3 cells partially protects against rotenone induced cell death
Scientific Reports (2022)
-
New pathogenic variants in COQ4 cause ataxia and neurodevelopmental disorder without detectable CoQ10 deficiency in muscle or skin fibroblasts
Journal of Neurology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.