Abstract
Beetles (Coleoptera) comprise about one quarter of all described animal species. One of the main contributors to their evolutionary success is the elytra, or hardened forewings, which have protective functions while maintaining their ability to fly. Unlike other beetles, some ship-timber beetles (Lymexylidae) have extremely small elytra and largely exposed functional hindwings. There is little fossil evidence illuminating the evolutionary history of short elytra in lymexylids. Here, I report five well-preserved lymexylid fossils in mid-Cretaceous and Cenozoic ambers from Myanmar (ca. 99 million years ago [Mya]), Russia (ca. 44 Mya), and the Dominican Republic (ca. 16 Mya). Three Cretaceous fossils have strongly reduced, shortened elytra, with unexpected variation in elytral size and shape, whereas very small, modified elytra are found only in much younger Dominican amber. These morphologically diverse extinct lymexylids shed new light on the early origin and evolutionary history of elytra reduction and its diverse variation in the ship-timber beetles. Based on the striking morphological similarities with extant lymexylids, these extinct taxa might have had the same, or similar, ecological, behavioural, and flight modes as the extant ship-timber beetles.
Similar content being viewed by others
Introduction
With nearly 400,000 named living species, beetles (Coleoptera) comprise the largest monophyletic order in the Tree of Life, showing astonishing morphological, ecological, and behavioural diversity in nearly all terrestrial and freshwater ecosystems1,2,3. Elytra, which are heavily sclerotised forewings, characterise beetles and cover the dorsal surface of the abdomen. These shield-like structures have various innovative functions in beetles. They protect the hindwings and dorsal abdomen from predators or harsh environmental conditions. Elytra also support desiccation tolerance, minimise the effect of rapid temperature shifts, and even play roles in mimicry or camouflage4,5,6,7. The strong sclerotisation enabled early beetles to penetrate and adapt to life under bark, like the adults of extant archostematan beetles (Cupedidae and Ommatidae)8. Therefore, the presence of elytra makes a major contribution to adaptations to a variety of habitats or micro-environments. Despite these advantages, some beetles possess shortened or reduced elytra. The partial reduction of the elytra, i.e. brachelytry, is widely scattered across Coleoptera. For example, the rove beetle family Staphylinidae alone contains over 64,000 species and most are brachelytrous. However, the hindwings of rove beetles are hidden under shortened elytra. Although there are some exceptions (see Discussion), this condition of elytra, i.e. brachelytry with hidden hindwings, are almost universal for the entire Coleoptera. Female trilobite beetles (Lycidae, Platerodrilus spp.) are exceptional because they lack trace of elytra9. Excluding Platerodrilus, the degree of elytral reduction ranges from moderate to almost completely reduced, as seen in the ship-timber beetle subfamily Atractocerinae (Lymexylidae)7; the latter have exposed their abdomens and functional hindwings (Fig. 1i,j and Supplementary Fig. 1b).
Diverse Lymexylidae beetles, dorsal views. (a–c) Atractocerinae from mid-Cretaceous Burmese amber, d Atractocerinae from mid-Eocene Baltic amber, e Atractocerinae from early Middle Miocene Dominican amber, (f–k) extant representatives of Lymexylidae, taken from each subfamily. (a) Vetatractocerus burmiticus gen. et sp. nov., holotype, FMNHINS-3965988. (b) Raractocetus extinctus sp. nov., holotype, FMNHINS-3965989. (c) R. fossilis sp. nov., holotype, FMNHINS-3965990. (d) R. balticus sp. nov., holotype, FMNHINS-3965991. (e) Atractocerus sp., FMNHINS-3965992. (f) Melittomma sericeum (Harris), Melittommatinae, from Indiana, U.S.A., showing fully developed, complete elytra. (g) Hylecoetus dermestoides (L.), Hylecoetinae, from Derbyshire, U.K., showing well developed, but slightly reduced elytra. (h) Lymexylon navale (L.), Lymexylinae, from Suffolk, U.K., showing well developed, but moderately reduced elytra. (i–k) A. brasiliensis Lepeletier & Audinet-Serville, Atractocerinae, from Bluefields, Nicaragua, showing distinctly reduced, minute elytra; (i) dorsal habitus, (j) enlargement of elytron from (i), (k) elytron. Photos credits: (g,h) by Udo Schmidt, licensed under the Creative Commons Attribution-Share Alike 2.0 Generic license (CC BY-SA 2.0, https://creativecommons.org/licenses/by-sa/2.0/), derived from Wikimedia Commons, (h) modified. Scale bars: 3 mm (a,c,j), 5 mm (b,d,e–h), 1 cm (i), 1.5 mm (k).
Lymexylidae is a small, monophyletic beetle family, with only 72 species in 12 genera distributed globally (excluding fossils)10,11. Based on several distinct morphological characters (e.g. highly modified maxillary palpus), lymexylids constitute the sole member of the superfamily Lymexyloidea. This group is so specialised that even a different insect order, Strepsiptera (Stylopidae), was once placed in this superfamily1, although this was based on an invalid argument. In some recent studies, Lymexylidae was fully nested within Tenebrionoidea12,13,14 or formed a clade with Tenebrionoidea and Cleroidea15. However, the latest, most comprehensive genomic study still recovered it as a distinct superfamily16. Lymexylids currently comprise four subfamilies: Atractocerinae (seven genera, one extinct), Hylecoetinae (one genus), Lymexylinae (one genus), and Melittommatinae (five genera, one extinct). Atractocerinae is characterised by exceedingly reduced elytra and is sister to Lymexylinae10,11. Direct fossil evidence of the reduced, short elytra in lymexylids has never been provided.
Here, I report five atractocerine beetles in mid-Cretaceous Burmese, mid-Eocene Baltic, and Middle Miocene Dominican ambers. All of the specimens reported here have extremely reduced elytra. The three Burmese amber atractocerines clearly show the reduced elytra, but they also demonstrate some variation in the shape and size of the elytra, ranging from much longer elytra than in the extant taxa to very similar ones. Shorter elytra are also seen in the Baltic amber fossil. Exceedingly minute, shrunken, deformed elytra are represented by only a single occurrence in the much younger Dominican amber. This series of new lymexylid fossils indicates the antiquity of the elytra reduction in the ship-timber beetles and demonstrates various patterns of elytra during the Cretaceous. The extremely reduced lymexylid elytra as in the extant Atractocerus are thought to have a post-Cretaceous origin. Although elytra reduction exposing the hindwings and abdomen is seen only in a tiny fraction of the mega-diverse beetles, my discovery broadens our knowledge of the evolution of elytra in Coleoptera.
Results
Systematic Palaeontology
Order Coleoptera Linnaeus, 1758
Superfamily Lymexyloidea Fleming, 1821
Family Lymexylidae Fleming, 1821
Subfamily Atractocerinae Laporte, 1840
Vetatractocerus gen. nov.
LSID (Life Science Identifier): urn:lsid:zoobank.org:act:EF9F7C08-DA9E-4D63-81D8-D674B74E18EC.
Type species
Vetatractocerus burmiticus sp. nov.
Etymology
The generic name is a combination of the Latin adjective vetus (meaning, ancient) and the genus Atractocerus.
Diagnosis
Vetatractocerus is distinguished from all other atractocerines by the following combination of characters: body small (ca. 8.5 mm) and narrow, uniformly pale yellowish brown; head large, rather vertical, slightly wider than pronotum; eyes large, occupying almost the entire frons, eyes nearly contiguous anteriorly; antenna filiform, as long as the head and pronotum combined, each antennomere distinctly elongate; pronotum subparallel sided, moderately produced anteriorly; mesoscutellum small and narrow, occupying about half the width of the elytral base; each elytron reduced, but relatively long and slender, approximately 8.7 times longer than wide, exposing large parts of the abdomen, dorsum lacking marking; and metacoxae remarkably modified, strongly projecting posteriorly.
Remarks
Among all extant atractocerines, Vetatractocerus gen. nov. is morphologically similar to the extant genus Urtea Paulus from Greece in having distinctly modified metacoxae, large, contiguous eyes, and anteriorly produced pronotum, but it is easily separated from Urtea as follows11: body much smaller (16 mm in Urtea); eyes smaller, occupying less of the posterior frons; and antennae slenderer and longer. In addition, Vetatractocerus gen. nov. can be distinguished from all other Atractocerinae genera as follows17: from Arractocetus, Fusicornis, and Hymaloxylon in having large, contiguous eyes; from Atractocerus and Raractocetus in having slenderer antennae and markedly modified metacoxae; from Cratoatractocerus in the absence of M + Cu folk of hindwing, but alternatively having much smaller body (28 mm in Cratoatractocerus) and elongate pronotum. In addition, Vetatractocerus gen. nov. differs from all extant genera in the presence of longer, slenderer elytra.
Vetatractocerus burmiticus sp. nov.
(Figures 1a and 2a,f and Supplementary Figs 2a,3)
Diversity of reduced elytra in extinct Atractocerinae beetles, dorsal views. (a–c) Atractocerinae from mid-Cretaceous Burmese amber, showing short to markedly short elytra, (d) Atractocerinae from mid-Eocene Baltic amber, showing very short elytra, e Atractocerinae from early Middle Miocene Dominican amber, showing distinctly short and minute elytra. (a,f) Vetatractocerus burmiticus gen. et sp. nov., holotype, FMNHINS-3965988; (f) enlargement of elytron. (b,g) Raractocetus extinctus sp. nov., holotype, FMNHINS-3965989; (g) enlargement of elytron. (c,h) R. fossilis sp. nov., holotype, FMNHINS-3965990; (h) enlargement of elytron. (d,i) R. balticus sp. nov., holotype, FMNHINS-3965991; (i) enlargement of elytron. (e,j) Atractocerus sp., FMNHINS-3965992; (j) enlargement of elytron. Scale bars: 3 mm (a,c), 5 mm (b,d,e), 1 mm (f,g,i), 0.5 mm (h,j).
LSID (Life Science Identifier): urn:lsid:zoobank.org:act:BC26AB2F-E7B3-4A19-B033-5E0574602A17.
Etymology
The specific epithet is derived from the occurrence of the fossil in Burmese amber known as ‘burmite’.
Material
Holotype: FMNHINS-3965988 (Supplementary Fig. 2a), a completely preserved female adult. Mid-Cretaceous (earliest Cenomanian) amber [ca. 99 million years ago (Mya)18], from the Hukawng Valley, Kachin State, northern Myanmar.
Diagnosis
As for the genus (see above), with the following minor additions: pronotal disc with blackish and longitudinal complete lines along midline and lateral margins; mesoscutellum with complete, thick, longitudinal blackish line along midline.
Description
Refer to online Supplemental Note for a complete description.
Genus Raractocetus Kurosawa, 1985
Remarks
This genus was once synonymised10, but it was later resurrected11. With only two extant species, Raractocetus is a small genus in Lymexylidae. The distribution is restricted mainly to the Oriental region, but it is also found in southernmost Australia11. The Cretaceous fossils described below are biogeographically consistent with the modern species. By contrast, the occurrence of a Raractocetus fossil from Baltic amber is intriguing. The current distribution of Atractocerinae is relatively cosmopolitan, and only a single species of the subfamily is found in Europe, namely Urtea gracea Paulus from Greece11. Therefore, the discovery of Raractocetus in northern Europe is surprising and noteworthy, although this distribution pattern has been known for several beetle groups19,20,21.
Raractocetus extinctus sp. nov.
(Figures 1b and 2b,g and Supplementary Figs 2b,4,5).
LSID (Life Science Identifier): urn:lsid:zoobankorg:act: urn:lsid:zoobank.org:act:94130B93-6FFE-4C43-89E6-8B0987C7FEAD.
Etymology
The specific epithet refers to the Latin adjective extinct, highlighting it as a Cretaceous fossil.
Material
Holotype, FMNHINS-3965989 (Supplementary Fig. 2b), a completely preserved female adult. Mid-Cretaceous (earliest Cenomanian) amber (ca. 99 Mya18), from the Hukawng Valley, Kachin State, northern Myanmar.
Diagnosis
This new species of Atractocerinae can be assigned to the extant genus Raractocetus based on the following combination of characters17: head rather vertical, moderately wider than pronotum; eyes large, contiguous, occupying almost the entire frons; antenna slender, somewhat fusiform; mesoscutellum relatively narrow, occupying about two thirds the width of the elytral base. Raractocetus extinctus sp. nov. can be distinguished from other Raractocetus species by the following combination of characters: antennae rather fusiform, thicker; head wide, moderately wider than pronotum; pronotum weakly elongate, anterior margin weakly rounded, pronotal disc with thick deep longitudinal blackish line/groove along the midline; mesoscutellum slightly wider than long, occupying about two thirds the width of the elytral base, dorsum with conspicuous blackish marking along midline; each elytron short, narrowly elongate, approximately 3.2 times longer than wide, gradually narrowing posteriorly, without marking; mesoventrite carinate along midline; and metacoxae rather strongly modified, relatively strongly projecting posteriorly.
Description
Refer to online Supplemental Note for a complete description.
Raractocetus fossilis sp. nov.
(Figures 1c and 2c,h and Supplementary Figs 2c,6,7)
LSID (Life Science Identifier): urn:lsid:zoobank.org:act:ACB32243-DDD8-461B-9D31-FE006252913E.
Etymology
The specific epithet is derived from the fact that it is a fossil species.
Material
FMNHINS-3965990 (Supplementary Fig. 2c), a nearly complete adult, but partially damaged, sex undetermined. Mid-Cretaceous (earliest Cenomanian) amber (ca. 99 Mya18), from the Hukawng Valley, Kachin State, northern Myanmar.
Diagnosis
This new species of Atractocerinae can be assigned to the extant genus Raractocetus based on the following combination of characters17: head rather vertical, slightly wider than pronotum; eyes large, contiguous, occupying almost the entire frons; mesoscutellum relatively small and narrow, occupying about two thirds the width of the elytral base. Raractocetus fossilis sp. nov. can be distinguished from other Raractocetus species by the following combination of characters: antennae relatively strongly fusiform, slender; head wide, dark brown, slightly wider than pronotum; pronotum subquadrate, very weakly elongate, gradually widened apically, widest and nearly truncate at anterior margin, pronotal disc with thin complete darker line/groove along midline; mesoscutellum as long as wide, occupying about two thirds the width of the elytral base, dorsum with blackish conspicuous marking on base; each elytron slender, approximately 3.9 times longer than wide, narrowest around middle, anterior half with elongate-oval, whitish marking, forming relatively clear pattern; and metacoxae moderately modified, relatively strongly projecting posteriorly.
Description
Refer to online Supplemental Note for a complete description.
Raractocetus balticus sp. nov.
(Figures 1d and 2d,i and Supplementary Figs 2d,8,9).
LSID (Life Science Identifier): urn:lsid:zoobank.org:act:F77CB344-EF73-4EC0-8E6B-AEFDD3AB2D5C.
Etymology
The specific epithet is derived from the English adjective baltic in reference to Baltic amber.
Material
FMNHINS-3965991 (Supplementary Fig. 2d), a completely preserved female adult. Mid-Eocene Baltic amber (ca. 44 Mya22), from Yantarny, Kaliningrad Oblast, Russia.
Diagnosis
This new species of Atractocerinae can be assigned to the extant genus Raractocetus based on the following combination of characters17: head rather vertical, slightly wider than pronotum; eyes large, contiguous, occupying almost the entire frons; antenna slender, relatively strongly fusiform; mesoscutellum relatively wide, occupying little more than two thirds the width of the elytral base. Raractocetus balticus sp. nov. can be distinguished from other congeners by the following combination of characters: antennae rather strongly fusiform, but slender; head wide, slightly wider than pronotum; pronotum subquadrate, nearly as long as wide, anterior margin weakly rounded, lateral margins gently arcuate, pronotal disc with deep, complete, longitudinal groove along midline (while lacking blackish line); mesoscutellum transverse, occupying little more than two thirds the width of the elytral base, dorsum without markings or maculation; elytron slender, approximately 3.5 times longer than wide, nearly subparallel sided, lacking pattern; and metacoxae moderately modified, relatively strongly projecting posteriorly.
Description
Refer to online Supplemental Note for a complete description.
Remarks
Raractocetus balticus sp. nov. is the first atractocerine described from Baltic amber.
Genus Atractocerus Palisot de Beauvois, 1802
Atractocerus sp.
(Figures 1e and 2e,j and Supplementary Figs 2e,10)
Material
FMNHINS-3965992 (Supplementary Fig. 2e), a complete, relatively well, preserved female adult. Ventral side is largely not visible. Early Middle Miocene amber (ca. 16 Mya23), from the Dominican Republic, no further details are available.
Diagnosis
The fossil can be placed in the extant genus Atractocerus using the following diagnostic characters17: head clearly narrower than pronotum; large, contiguous eyes; short fusiform antenna; broadened mesoscutellum that occupies more than two thirds the width of the elytral base; and extremely shortened, small elytra. This fossil taxon may be distinguished from other congeners by the following characters: smaller body; strongly fusiform, but distally slender, antennae; minute, modified and elevated elytra; and the structures of the female genitalia. The ventral side is not fully visible. Further comparison with the modern congeners is difficult because of a poor visibility of the ventral side and the ambiguity of the species limit of the extant Atractocerus.
Description
Refer to online Supplemental Note for a complete description.
Remarks
The distribution of Atractocerus in Central and South America (including the Dominican Republic), Africa, Madagascar, India, Sumatra, and northern Australia suggests Gondwanan relictualism11. Therefore, the occurrence of Atractocerus in Dominican amber is congruent with the modern congeners. Grimaldi & Engel2 mentioned a much larger (ca. 29 mm) unnamed Atractocerus in Dominican amber, but the elytra were not illustrated.
Discussion
Three Cretaceous and two Cenozoic beetles unambiguously belong to Lymexylidae, based on their narrowly elongate bodies, cylindrical projecting procoxae, filiform or fusiform, fairly short antennae, highly modified maxillary palp organ, and female genital structures10,24. They can be further assigned to Atractocerinae based on the markedly reduced elytra with the exposed hindwings, large bulging eyes, and distinctly modified maxillary palp organ in the female10. The diverse atractocerines from Burmese, Baltic, and Dominican ambers reported here represent the first described and named amber inclusions in the subfamily.
The oldest members of the ship-timber beetles were found in Lower Cretaceous Lebanese amber (~125 Mya)25. They are not formally described and therefore no detailed morphological information is available, although they have long, nearly complete elytra25. If these fossils are truly lymexylids, this is consistent with the Jurassic-Early Cretaceous origin of the crown group of the family, suggested by previous molecular studies16,26. Currently, the earliest definitive fossil of the family is Cratoatractocerus grimaldii Wolf-Schwenninger described based on a compression fossil from the Lower Cretaceous Crato Formation (~113 Mya27) in Brazil28 (Fig. 3a). This fossil species was assigned to the subfamily Atractocerinae based on several characters, including general body shape, large contiguous eyes, two longitudinal ridges on the mesoscutellum, and heavily sclerotised female genitalia28. This assignment appears correct, although, unfortunately, the elytra of this fossil taxon were originally missing28. Until now, this uncertainty prevented us from elucidating the origin and evolutionary history of elytra reduction in Atractocerinae, since no other fossil lymexylid has been described from the Mesozoic. An undescribed Atractocerinae fossil from Burmese amber has been reported, the authors did not include a figure of the elytra of this fossil2. All other family records are confined to Eocene–Miocene (~16 Mya) compression and amber fossils with either long elytra or without sufficient information about them2,29,30,31,32,33. My study provides the first documented evidence of the elytral reduction in Lymexylidae, which can now be reliably traced back to the mid-Cretaceous (~earliest Cenomanian, ca. 99 Mya18).
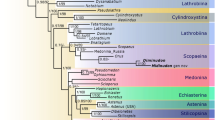
Phylogenetic framework of Lymexylidae and Atractocerinae, based on Wheeler10, Paulus11, and Wolf-Schwenninger28. (a) Time-dated cladogram of Lymexylidae, (b) hypothesised generic cladogram of Atractocerinae. (a) 1: Oldest Atractocerinae from Lower Cretaceous (~113 Mya) Crato Formation, Brazil28; 2: Atractocerinae from mid-Cretaceous (ca. 99 Mya) Burmese amber; 3: Atractocerinae from mid-Eocene (ca. 44 Mya) Baltic amber; 4: Atractocerinae from early Middle Miocene (ca. 16 Mya) Dominican amber; 5: oldest Melittommatinae from mid-Eocene Baltic amber33. Two older records of Hylecoetinae29 and Melittommatinae31,32 were omitted due to uncertainty in their systematic positions. Red lines show lineages supported by fossil records. Square, compression fossil. Circles, amber inclusions. (b) The elytra of five fossil species reported here are mapped onto the cladogram. Vetatractocerus gen. nov. is tentatively placed as a sister taxon to Urtea based on the distinctly modified metacoxae, large, contiguous eyes, and anteriorly produced pronotum. Cratoatractocerus was considered the most basal member of Atractocerinae based on its hindwing venation, although the general characters resemble those of Raractocetus28. Since there is significant lack of morphological information on this fossil genus, I tentatively followed the view of Wolf-Schwenninger28.
In comparison with the mega-diversity of beetles, only a small minority of beetles have reduced elytra with exposed hindwings. Nonetheless, this phenomenon is found repeatedly across the order, indicating multiple independent origins. Because the condition and degree of development of the elytra varies greatly among beetles, a previous study classified the reduced elytra into three morpho-types7: 1) elytra reduced along with a reduction in the hindwings, e.g. Lycidae34 and Lampyridae35; 2) elytra truncated but completely covering the folded functional hindwings, e.g. the hyper-diverse Staphylinidae36; and 3) reduced elytra exposing (or partially exposing) functional hindwings, e.g. some Cerambycidae (i.e. Necydalinae37), Ripiphoridae38, or Lymexylidae10. The third form is the rarest and most interesting elytral type because it apparently does not have a protective function and other merits. Despite these disadvantages, this elytral form is found in unrelated beetle lineages7. The origin and role of this type of brachelytry have still not been adequately explored. However, it was suggested that elytra reduction affects the evolution of beetle hindwings by optimising the aerodynamic efficiency or changes in flight mechanics induced by elytra loss7. In atractocerine species, extreme cases of reduced elytra are known. Indeed, the elytra are so minute that most of the hindwings and abdomen are exposed dorsally. A remarkable dipteran haltere-like role of minute elytra was revealed by surgically removing the elytra of Atractocerus brevicornis (L.)39,40. These shortened elytra vibrate during flight and may perform a sensory function essential for stabilising flight, producing the unique flight mode of atractocerines39,40. In fact, A. brevicornis was incapable of steady flight after removing the elytra39,40. Based on the minute elytra, my discovery of two new species of Raractocetus from Burmese amber suggests that they had a flight mode similar to that of A. brevicornis during the mid-Cretaceous, as this seems to be a common flight mechanism for the subfamily as a whole.
The fossils described here show a remarkable variation in shape and length of elytra. For example, Vetatractocerus gen. nov. differs greatly from the remaining extant and extinct Atractocerinae. With a very slender, unusually long (8.7 times longer than wide) elytron, this new genus is distinct among the atractocerines. More significantly, the length of the elytra and their coverage on the abdomen seems to be intermediate between the subfamily Lymexylinae (Fig. 1h), which is a sister taxon to Atractocerinae, and the other members of Atractocerinae. Nonetheless, the elytra of Vetatractocerus gen. nov. are still much shorter than in Lymexylinae, with more than four abdominal tergites exposed dorsally. The presence of a highly modified maxillary palp organ in the female prevents the systematic placement of Vetatractocerus gen. nov. within Lymexylinae, but it is definitely placed in Atractocerinae. This new genus might form a sister group with Urtea based on the distinctly modified metacoxae, large, contiguous eyes, and anteriorly produced pronotum. These two genera may be nested together at the basal position of Atractocerinae (Fig. 3b), although future studies need to assess the detailed systematic position of Vetatractocerus gen. nov. The two Cretaceous Raractocetus species have a pair of short, but weakly posteriorly narrowing elytron (Fig. 2b,c,g,h), while one Eocene Raractocetus species has a nearly parallel-sided elytron (Fig. 2d,i). Compared with these taxa, one Miocene Atractocerus has a shorter (2.1 times longer than wide), distinctly pointed, modified elytron, with an irregularly dorsally raised surface (Fig. 2e,j). Consequently, the modified elytron as seen in extant taxa is considered a derived condition that may have originated by the Middle Miocene, based on my fossil in Dominican amber (ca. 16 Mya).
Of note, there are already some Cretaceous examples of brachelytrous beetles with exposed hindwings. The most notable cases are several wedge-shaped beetles (Ripiphoridae) from mid-Cretaceous French and Burmese ambers38,41,42. They clearly exhibit the typical form of reduced elytra with exposed hindwings, although they are much less reduced than the examples presented here. Another example is found in soldier beetles (Cantharidae) from Burmese amber; for example, the genera Ornatomalthinus43 and Sanaungulus44 have shortened elytra exposing the hindwings dorsally. These discoveries indicate that reduction in elytral length with exposed hindwings, occurred before the mid-Cretaceous independently in distantly related beetle groups.
Elytra reduction may affect the evolution of hindwing structures7. A characteristic feature of recent Atractocerinae is the extremely reduced hindwing venation with a complete lack of transverse folds28,45,46. Hindwing shape differs markedly between related brachelytrous and macroelytrous beetles, with the exception of Atractocerus, having a wider hindwing than in macroelytrous congeners and having an elongated anal field28. Although I could not adequately observe the hindwing shape in the fossils described here, the wing venation patterns were simple and the wings folded lengthwise, as in all extant atractocerine genera; they lack a M + Cu fork basad of the level of the r-m crossvein, clearly differentiated from the Lower Cretaceous Cratoatractocerus, and also lack a radial cell along the costal margin of the C + Sc + R vein. The general length of the hindwings is the same as in the extant atractocerines, with at least two free abdominal tergites uncovered by the hindwings. This demonstrates the mid-Cretaceous origin of the modern atractocerine hindwings. Because these atractocerines are unlike examples of beetle taxa inhabiting mesic micro-environments47,48,49,50, the close morphological similarities with the extant taxa are interesting.
The fossil evidence reported here sheds light on the macroevolution of ship-timber beetles, especially the subfamily Atractocerinae. These morphologically diverse extinct lymexylids highlight the early diversification of elytra reduction in this subfamily. The diverse series of variation in elytral forms reported here also suggest much greater morphological diversity of the elytral shape in the past than seen at present. Interestingly, although only suggestive, the very minute, modified elytra seen in extant Atractocerus were found only from younger Dominican amber. The reported fossils here also clearly show several synapomorphic characters of lymexylids, including the modified maxillary palp organ functions as chemoreceptors51,52, which represent an autapomorphy of the family (Supplementary Fig. 1c,d)10. Another insight is the possible bright colouration of Vetatractocerus burmiticus gen. et sp. nov., Raractocetus extinctus sp. nov., and R. fossilis sp. nov. when they were alive, based on their maculation and patterns of the head, pronotum, mesoscutellum, and elytra. It has been hypothesised that the pale species of Atractocerinae, e.g. Atractocerus and Raractocetus, with large eyes appear to mimic certain nocturnal wasps (Vespidae and Ichneumonidae)17,52. Hence, they may even be considered potential mimics, although more evidence is needed to support this hypothesis. All known lymexylid larvae are wood borers and are believed to form symbiotic associations with ambrosia fungi, Ascoidea spp. (Ascomycetes: Saccharomycetales) that grow on the walls of their tunnels24,52,53. Considering this, it is possible that these fossil taxa had the same, or similar, ecological lifestyles as the modern ship-timber beetles. Indeed, the Raractocetus fossils show striking general morphological similarities to the recent congeners, suggesting they had the same ecological, behavioural, and even flight modes in the mid-Cretaceous.
Methods
Materials
The three Cretaceous fossils described herein were obtained from amber from the Hukawng Valley, Kachin State, northern Myanmar (26°21′33.41″N, 96°43′11.88″E). Burmese amber, or burmite, dates to the mid-Cretaceous, currently recognised as the earliest Cenomanian (98.79 ± 0.62 Mya) age based on U–Pb dating of zircon crystals from the volcaniclastic matrix of the amber outcrops18. The Baltic amber specimen described here was from Yantarny, Kaliningrad Oblast, westernmost Russia. Although the age of Baltic amber has been the subject of much debate, I follow the mid-Eocene (44.1 ± 1.1 Mya) age from the most recent estimates for the amber-bearing ‘Blue Earth’ formation based on the absolute dating analyses22. The Dominican amber fossil comes from the Dominican Republic and further details are unknown. The age of Dominican amber is also somewhat controversial, but I follow the early Middle Miocene (ca. 16 Mya) age23. All of the fossil specimens used in this paper are deposited in the insect collection of the Gantz Family Collections Center, Field Museum of Natural History (FMNH), Chicago, IL, USA, with consecutive numbers from FMNHINS-3965988 to FMNHINS-3965992.
Imaging
Specimens were photographed using a Canon 80D digital camera with a Canon MP-E 65 mm macro lens (F2.8, 1–5 × ) and a Canon MT-24EX macro twin lite as the light source (Figs 1a–e, 2a–e,g–j and Supplementary Figs 3,4,5a–e,g,6a,b,d,7h,8,9). Alternatively, a Dun Ink BK PLUS Lab System mounted on a Canon 6D digital camera, attached with either CF4 or 5 × lens, was used (Fig. 2f and Supplementary Figs 5f, 6c,e,f,7a–g,10). All images were later stacked using the auto-montage software Combine ZM or Helicon Focus 5.3. Figures were edited and assembled with Adobe Photoshop® Elements 15. Specimens were studied under a Leica MZ16 stereomicroscope.
Classification
I follow the classifications of Kurosawa17 and Paulus11.
Morphological terminology
The morphological terminology generally follows Kurosawa17 and Wheeler10 for general body parts, and Wolf-Schwenninger28 for wing venation. Abbreviations of the hindwing venation are as follows: C, costa; Cu, cubitus; M, median vein; R, radius; r-m, a cross vein that connects M and Cu; Sc, subcosta.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the International Code of Zoological Nomenclature (ICZN). The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix ‘http://zoobank.org/’. The LSIDs for this publication are: urn:lsid:zoobank.org:pub:256304D4-479E-44B5-A14D-893D9E898479; urn:lsid:zoobank.org:act:EF9F7C08-DA9E-4D63-81D8-D674B74E18EC; urn:lsid:zoobank.org:act:BC26AB2F-E7B3-4A19-B033-5E0574602A17; urn:lsid:zoobank.org:act:94130B93-6FFE-4C43-89E6-8B0987C7FEAD; urn:lsid:zoobank.org:act:ACB32243-DDD8-461B-9D31-FE006252913E;
urn:lsid:zoobank.org:act:F77CB344-EF73-4EC0-8E6B-AEFDD3AB2D5C.
Data Availability
All fossil materials are deposited in the FMNH. The data supporting the study findings are provided in both the paper and Supplementary Information. Higher-resolution figures have been deposited in the figshare database (https://doi.org/10.6084/m9.figshare.7800830).
References
Crowson, R. A. The Biology of the Coleoptera. IX + 802 (Academic Press, 1981).
Grimaldi, D. & Engel, M. S. Evolution of the Insects. XV + 755 (Cambridge University Press, 2005).
Ślipiński, S. A., Leschen, R. A. B. & Lawrence, J. F. In Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (ed. Zhang, Z.-Q.), 203–208 (Zootaxa 3148, 2011).
Bezzerides, A. L., McGraw, K. J., Parker, R. S. & Husseini, J. Elytra color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 61, 1401–1408 (2007).
Wilts, B. D., Michielsen, K., Kuipers, J., De Raedt, H. & Stavenga, D. G. Brilliant camouflage: photonic crystals in the diamond weevil, Entimus imperialis. Proc. R. Soc. B. 279, 2524–2530 (2012).
Linz, D. M., Hu, A. W., Sitvarin, M. I. & Tomoyasu, Y. Functional value of elytra under various stresses in the red flour beetle, Tribolium castaneum. Sci. Rep. 6, 34813, https://doi.org/10.1038/srep34813 (2016).
Goczał, J., Rossa, R. & Tofilski, A. Elytra reduction may affect the evolution of beetle hind wings. Zoomorphology 137, 131–138 (2018).
Yan, E. V., Lawrence, J. F., Beattie, R. & Beutel, R. G. At the dawn of the great rise: †Ponomarenkia belmonthensis (Insecta: Coleoptera), a remarkable new Late Permian beetle from the Southern Hemisphere. J. Syst. Palaeontol. 16, 611–619 (2018).
Masek, M. & Bocak, L. The taxonomy and diversity of Platerodrilus (Coleoptera, Lycidae) inferred from molecular data and morphology of adults and larvae. ZooKeys 426, 29–63 (2014).
Wheeler, Q. D. Revision of the genera of Lymexylidae (Coleoptera: Cucujiformia). Bull. Am. Mus. Nat. Hist. 183, 113–210 (1986).
Paulus, H. F. Urtea graeca nov. gen. et nov. spec., der erste Vertreter der tropischen Atractocerinae in Europa sowie eine Beschreibung von Hymaloxylon aspoecki nov. spec. aus Yunnan (China) (Coleoptera, Cucujiformia, Lymexylidae, Atractocerinae nov. status). Denisia 13, 277–290 (2004).
Crampton-Platt, A. et al. Soup to tree: the phylogeny of beetles inferred by mitochondrial metagenomics of a Bornean rainforest sample. Mol. Biol. Evol. 32, 2302–2316 (2015).
McKenna, D. D. et al. The beetle tree of life reveals that Coleoptera survived end‐Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 40, 835–880 (2015).
Robertson, J. A. et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 40, 745–778 (2015).
Hunt, T. et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318, 1913–1916 (2007).
Zhang, S.-Q. et al. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 9, 205, https://doi.org/10.1038/s41467-017-02644-4 (2018).
Kurosawa, Y. Revisional notes on the family Lymexylonidae (Coleoptera) in eastern and southeastern Asia. Bull. Natn. Sci. Mus., Tokyo, Ser. A 11, 109–119 (1985).
Shi, G. et al. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretaceous Res. 37, 155–163 (2012).
Alekseev, V. I. Coleoptera from the middle-upper Eocene European ambers: generic composition, zoogeography and climatic implications. Zootaxa 4290, 401–443 (2017).
Brunke, A. J., Chatzimanolis, S., Metscher, B. D., Wolf-Schwenninger, K. & Solodovnikov, A. Dispersal of thermophilic beetles across the intercontinental Arctic forest belt during the early Eocene. Sci. Rep. 7, 12972, https://doi.org/10.1038/s41598-017-13207-4 (2017).
Bogri, A., Solodovnikov, A. & Żyła, D. Baltic amber impact on historical biogeography and palaeoclimate research: oriental rove beetle Dysanabatium found in the Eocene of Europe (Coleoptera, Staphylinidae, Paederinae). Pap. Palaeontol. 4, 433–452 (2018).
Wappler, T. In Fossils X3, 3rd International Congress of Palaeoentomology with 2nd International Meeting on Palaeoarthropodology and 2nd World Congress on Amber and its Inclusions. Programme and Abstracts, 7 th to 11 th Feb 2005, Pretoria South Africa. (eds Brothers, D. & Mostovski, M.), 53 (2005).
Iturralde-Vinent, M. A. Geology of the amber-bearing deposits of the Greater Antilles. Caribbean J. Sci. 37, 141–167 (2001).
Young, D. K. In American Beetles. Volume 2, Polyphaga: Scarabaeoidea through Curculionoidea (eds Arnett, R. H., Frank, J. H., Thomas, M. C. & Skelley, P. E.), 261–262 (CRC Press, 2002).
Kirejtshuk, A. G. & Azar, D. Current knowledge of Coleoptera (Insecta) from the Lower Cretaceous Lebanese amber and taxonomical notes for some Mesozoic groups. Terrestrial Arthropod Rev. 6, 103–134 (2013).
Toussaint, E. F. et al. The peril of dating beetles. Syst. Entomol. 42, 1–10 (2017).
Konikiewicz, M. & Mąkol, J. Insight into fossil fauna of terrestrial Parasitengona mites (Trombidiformes: Prostigmata)–The first representatives of Erythraeina Welbourn, 1991 and Trombidiina Welbourn, 1991 in Burmese amber. Cretaceous Res. 89, 60–74 (2018).
Wolf-Schwenninger, K. The oldest fossil record of Lymexylidae (Insecta: Coleoptera) from the Lower Cretaceous Crato Formation of Brazil. Insect Syst. Evo. 42, 205–212 (2011).
Heer, O. Die Urwelt der Schweiz. XXIX + 622 (F. Schulthess, 1865).
Klebs, R. Über Bernsteineinschlüsse im allgemeinen und die Coleopteren meiner Bersteinsammlung. Schr. Phys.-ökon. Ges. Königsberg 51, 217–242 (1910).
Wickham, H. F. Fossil Coleoptera from Florissant, with descriptions of several new species. Bull. Am. Mus. Nat. Hist. 30, 53–69 (1911).
Wickham, H. F. In Catalogue of the Coleoptera of America North of Mexico (ed. Leng, C. W.) 349–365 (Mount Vernon, 1920).
Kirejtshuk, A. G. A new genus and species of timber beetle (Coleoptera: Lymexylidae) from the Baltic Amber. Paleontological J. 42, 63–65 (2008).
Bocak, L., Grebennikov, V. & Masek, M. A new species of Dexoris (Coleoptera: Lycidae) and parallel evolution of brachyptery in the soft-bodied elateroid beetles. Zootaxa 3721, 495–500 (2013).
Branham, M. In Handbook of zoology. Volume IV: Arthropoda: Insecta, Part 38. Coleoptera, beetles. Volume 2. Morphology and systematics (Polyphaga partim) (eds Beutel, R. G., Leschen, R. A. B. & Lawrence, J. F.), 141–145 (Walter de Gruyter, 2010).
Saito, K., Yamamoto, S., Maruyama, M. & Okabe, Y. Asymmetric hindwing foldings in rove beetles. Proc. Natl. Acad. Sci. USA 111, 16349–16352 (2014).
Svacha, P. & Lawrence, J. F. In Handbook of zoology. Volume 4: Arthropoda: Insecta, Part 40. Coleoptera, beetles. Volume 3. Morphology and systematics (Phytophaga) (eds Leschen, R. A. B. & Beutel, R. G.), 77–177 (Walter de Gruyter, 2014).
Cai, C., Yin, Z. & Huang, D. A new ripiphorid beetle from Upper Cretaceous Burmese amber sheds light on early evolution of the extant subfamily Ripidiinae (Coleoptera: Ripiphoridae). C. R. Palevol. 17, 351–356 (2018).
Miller, P. L. The possible stabilising function of the elytra of Atractocerus brevicornis (L.) (Lymexylidae: Coleoptera) in flight. Entomologist 104, 105–110 (1971).
Taylor, G. K. & Krapp, H. G. Sensory systems and flight stability: what do insects measure and why? Adv. Insect Phys. 34, 231–316 (2007).
Perrichot, V., Nel, A. & Néraudeau, D. Two new wedge-shaped beetles in Albo-Cenomanian ambers of France (Coleoptera: Ripiphoridae: Ripiphorinae). Eur. J. Entomol. 101, 577–581 (2004).
Batelka, J., Engel, M. S. & Prokop, J. A remarkable diversity of parasitoid beetles (Ripiphoridae) in Cretaceous amber, with a summary of the Mesozoic record of Tenebrionoidea. Cretaceous Res. 90, 296–310 (2018).
Poinar, G. & Fanti, F. New fossil soldier beetles (Coleoptera: Cantharidae) in Burmese, Baltic and Dominican Amber. Palaeodiversity 9, 1–7 (2016).
Fanti, F., Damgaard, A. L. & Ellenberger, S. Two new genera of Cantharidae from Burmese amber of the Hukawng Valley (Insecta, Coleoptera). Cretaceous Res. 86, 170–177 (2018).
King, E. W. The phylogenetic position of Atractocerus Pails. Coleopts. Bull. 9, 65–74 (1955).
Selander, R. B. Additional remarks on wing structure in. Atractocerus. Coleopts. Bull. 13, 1–5 (1959).
Clarke, D. J. & Chatzimanolis, S. Antiquity and long-term morphological stasis in a group of rove beetles (Coleoptera: Staphylinidae): Description of the oldest Octavius species from Cretaceous Burmese amber and a review of the “Euaesthetine subgroup” fossil record. Cretaceous Res. 30, 1426–1434 (2009).
Yamamoto, S., Takahashi, Y. & Parker, J. Evolutionary stasis in enigmatic jacobsoniid beetles. Gondwana Res. 45, 275–281 (2017).
Yamamoto, S. & Takahashi, Y. First and oldest Leptochirini rove beetles illuminate diverse cephalic structures in the Cretaceous (Coleoptera: Staphylinidae: Osoriinae). Syst. Entomol. https://doi.org/10.1111/syen.12342 (2019).
Cai, C. et al. Basal polyphagan beetles in mid-Cretaceous amber from Myanmar: biogeographic implications and long-term morphological stasis. Proc. R. Soc. B 286, 20182175 (2019).
Slifer, E. H., Gruenwald, T. F. & Sekhon, S. S. The maxillary palp organs of a wood‐boring beetle, Melittomma sericeum (coleoptera, lymexylonidae). J. Morphol. 147, 123–135 (1975).
Lawrence, J. F. In Handbook of zoology. Volume IV: Arthropoda: Insecta, Part 38. Coleoptera, beetles. Volume 2. Morphology and systematics (Polyphaga partim) (eds Beutel, R. G., Leschen, R. A. B. & Lawrence, J. F.), 229–235 (Walter de Gruyter, 2010).
Batra, L. R. & Francke-Grosmann, H. Contributions to our knowledge of ambrosia fungi. I. Ascoidea hylecoeti sp. nov. (Ascomycetes). Am. J. Bot. 48, 453–456 (1961).
Acknowledgements
Thanks are due to Crystal A. Maier (FMNH) for registering the specimen numbers. I am also grateful to Margaret K. Thayer and Alfred F. Newton (FMNH) for general support at FMNH. This work was supported by a Japan Society for the Promotion of Science (Tokyo, Japan) Overseas Research Fellowship (no. 29–212).
Author information
Authors and Affiliations
Contributions
S.Y. devised the project, performed the research, analysed the data, and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamamoto, S. Fossil evidence of elytra reduction in ship-timber beetles. Sci Rep 9, 4938 (2019). https://doi.org/10.1038/s41598-019-41310-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41310-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.