Abstract
Biological invasions are a major threat to global biodiversity. Ligustrum lucidum, native to temperate Asia, is one of the most invasive plant species in the world. Climate is an important ecological factor influencing species distribution. Therefore, we investigated the climatic niche of L. lucidum in various regions of the world to determine whether it uses different climatic conditions in its invasive ranges than in its native range. The geographical coordinates of its occurrence were extracted from the Global Biodiversity Information Facility and Southern African Plant Invaders Atlas databases. Climatic variables and altitude data were obtained from WorldClim. We evaluated niche overlap and performed niche similarity tests, and estimated niche shift parameters. L. lucidum occurs mostly in warm temperate climates. Niche overlap between native and invaded areas was low. Niche similarity tests indicated that the species could expand its occurrence into regions with climates similar to and different from that of its native range. We concluded that L. lucidum uses different realized climatic niches in its invasive ranges than in its native range. Warmer and wetter climatic conditions may not necessarily constrain this species from establishing populations outside of its native range.
Similar content being viewed by others
Introduction
At present, biological invasion is considered a major ecological threat because of its substantial impact on ecosystem function and biodiversity1. For this reason, it is useful to elucidate the capacity of a species to shift its climatic niche during invasion. This information may help to predict the ability of an invasive species to expand beyond its geographic range and its responses under different scenarios of global climatic change2.
In general, each species has specific environmental requirements to ensure its long-term viability. These conditions are the result of evolutionary history and reflect the fundamental niche in which the species thrives3. When many species coexist, the occurrence of each species depends on its realized niche, which is the portion of the fundamental niche in which the species can succeed when biological interactions arise4. Climatic factors, such as water availability and temperature, have great impacts on species physiology and survival5. They influence the ability of a species to expand to novel areas. Therefore, climatic conditions are considered highly significant ecological determinants of species distribution6,7.
During invasion, the shift in realized climatic niche can be evaluated by determining the similarities between the climate of the invaded area and that of the native one7. A species may either expand its presence into climatically novel areas or occupy areas that climatically resemble that of its native range7. However, the climate type within the native range of the invasive species may be absent or it could remain unoccupied in the invaded area. Some investigators8,9,10,11 have suggested that exotic species are most likely to invade areas with environmental conditions similar to those found in their native area (i.e., climatic niche stability). Nevertheless, biotic interactions (the release from negative biotic interactions present in the native range and/or positive feedback in the invaded area) and increases in resource availability are important factors favoring biological invasion12,13,14,15. In fact, investigators have demonstrated that climatic niche shift may also occur16,17,18,19. These contrasting results reflect the complexity of this research subject and indicate a relative lack of standardized methodologies for data analysis and interpretation7,20,21.
At present, Ligustrum lucidum W.T. Aiton (broad-leaf privet; Oleaceae) is considered one of the most invasive tree species in the world. It originated in temperate Asia, specifically China22, and was introduced into many parts of the world as an urban ornamental23,24,25,26,27,28,29,30,31. Its leaves and fruit may be toxic to humans, and it produces allergenic pollen32,33. It is widely distributed globally and has invaded various environments, including degraded sites, conserved areas, wetlands, drier areas, open areas (e.g., fields and plains), and forest understories24,34.
Considering the global relevance of L. lucidum as an invasive species, we aimed to evaluate the climatic niche within its native and invaded ranges. To this end, we determined on a global scale whether L. lucidum is found in climatic conditions that differ between its invasive ranges and the region of its origin in South Asia. This would demonstrate that its native range only represents a fraction of the elusive fundamental niche of this species.
Results
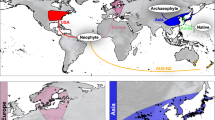
L. lucidum is present on all continents except Antarctica, occurring mostly in warm temperate climates (Köppen-Geiger climate type C) (Fig. 1). Even within the same macroclimate, there was low climatic overlap between the invaded regions of the plant and its native range. The D values varied from 0.00 (Southeastern South America) to 0.12 (Oceania) (Table 1). Except for South Africa and the North American West Coast, the low D values reflected high proportions of expansion (E > 70%) and unfilled (U > 90%) climatic conditions. In South Africa and the West Coast of North America, the expansion values (E %) were relatively low (<35%). Therefore, the climates of these regions were highly similar to those of the native area.
Köppen-Geiger climate classification (1976–2000)35 and Ligustrum lucidum geographic distribution focusing on the area of its native occurrence in China and other invaded areas for which the climate niche dynamics were evaluated. This figure was generated using R 3.5.1, with code adapted from http://www.rforscience.com/portfolio/koppen-geiger/ 52.
According to the niche similarity test, however, only the North American West Coast has climatic conditions similar to those in the native range in China (Sim.test: p < 0.05). Therefore, the expanded climatic conditions (E %) in this region correspond to the same conditions observed in the native range. However, the climatic conditions of the other invaded areas were dissimilar to those observed in the native range (Sim.test: p > 0.05). The climates of the expansion areas (E %) differed significantly from those of the native range. In these cases, L. lucidum shifted its climatic niche during the invasion process.
For regions in which L. lucidum shifted its climatic niche (Fig. 2a–g), the first two axes of the multivariate ordinations explained a large proportion of the total inertia (>60%). In general, L. lucidum expanded its occurrence to warmer and wetter areas (Oceania, Europe, and Southeastern South America) and areas with comparatively lower seasonality of precipitation (North American East Coast) and temperature (Central America and Mexico) than that of the native area.
Principal component analysis ordination indicating the climatic variables most relevant to the Ligustrum lucidum niche shift during invasion (green = unfilled climatic condition in the invaded area; red = climatic expansion in the invaded area; blue = climatic stability) into different regions (a - Oceania; b - Europe; c - Southeastern South America; d - Central America and Mexico; e - North American West Coast; f - North American East Coast; and g - South Africa). The first two axes of each PCA represent the environmental space. The continuous lines represent the available climatic space. The dashed lines represent the species occurrence density. The arrows indicate the change in the centroid from the native range to the invaded area. (bio 1 = Annual Mean Temperature; bio 3: Isothermality; bio 4: Temperature Seasonality (standard deviation ×100); bio 5: Maximum Temperature of the Warmest Month; bio 6: Minimum Temperature of the Coldest Month; bio 7: Annual Temperature Range; bio 8: Mean Temperature of the Wettest Quarter; bio 9: Mean Temperature of the Driest Quarter; bio 10: Mean Temperature of the Warmest Quarter; bio 11: Mean Temperature of the Coldest Quarter; bio 12: Annual Precipitation; bio 14: Precipitation of the Driest Month; bio 15: Precipitation Seasonality; bio 17: Precipitation of Driest Quarter; bio 19: Precipitation of the Coldest Quarter).
On the North American West Coast, L. lucidum occupied the same climatic niche as its native range (Fig. 2e). In this area, expansion occurred under similar climatic conditions. The first two axes of the multivariate ordinations explained a large proportion of the total inertia (66.6%; Axis 1 = 45.2%, and Axis 2 = 21.4%). Figure 2e shows that the climatic conditions of L. lucidum on the North American West Coast were mainly analogous (blue color) to those in the native distribution area.
Discussion
L. lucidum tends to be found within the same type of macroclimate, namely warm temperate35. Nevertheless, we showed that it can invade regions with climatic conditions both similar to and different from those found within its native range. Considering that our analysis was conducted using field data that included biotic interactions, this fact suggests that the native range of L. lucidum only represents a portion of the fundamental niche of the species. In this sense, our study supports the findings of other investigators who demonstrated the potential of species to use different realized climatic niches during invasion16,36,37.
A complex interaction among ecological-evolutionary forces such as biological associations, propagule pressure, and adaptive evolution processes15,38,39,40,41 can explain why an invasive species may not retain the realized niche from its native range. When introduced into a new community, an invasive species may be favored by the lack of negative biological interactions present in its native range and by the potential presence of new mutualists12,13,14,15. In addition, the repeated introduction of an invasive species may increase propagule pressure39 and foster evolutionary processes that lead to invaders with more adaptive potential40. Moreover, an invasive species may be preadapted to conditions that are present in the invaded range but are no longer readily available in the native one4.
Based on our results and those previously reported, we inferred that the realized niche shifts and worldwide distribution of L. lucidum are the result of a biological invasion “perfect storm” created by intentional human distribution, positive biotic interactions, and strong abiotic plasticity. This species has been widely planted as an ornamental tree by humans23,24,25,26,28,29,30,31. In certain municipalities of southern Brazil, L. lucidum is the most abundantly planted urban tree42. Moreover, the fruit of L. lucidum may be avidly consumed by local birds43,44, which disperse tree propagules in natural areas where they grow rapidly under various ecological conditions24,34,45. It is, therefore, evident that L. lucidum is potentially a serious invasive species.
Overall, our results showed low climatic niche overlap (D) and expansion into areas possessing different climatic conditions (E %). L. lucidum expanded its presence into more humid and warmer areas with less temperature and precipitation seasonality. Therefore, the species may be tending towards the invasion of wet tropical areas. Tropical ecosystems constitute a vital part of global biodiversity46 and the invasion of plant species such as L. lucidum could place even more pressure on these ecosystems47. Examples of these ecosystems can be found in regions in the Brazilian Atlantic Forests, Mesoamerican Forests, East Australian Forests, Madagascar, and the islands of the Indian Ocean. These observations are alarming when one considers biological invasion together with ongoing climate change. An invasive species may be able to thrive under many new environmental conditions in the future48.
In conclusion, on a global scale, L. lucidum has invaded and occupied regions with climates similar to and distinct from that of its native habitat. In Oceania, Europe, Southeastern South America, Central America and Mexico, the North American East Coast, and South Africa, L. lucidum thrives under various climatic conditions. In these areas, invasion occurred primarily along temperature and precipitation gradients, towards wetter and warmer areas with lower seasonality in temperature and rainfall. This indicates that the native range of L. lucidum only represents a portion of its fundamental niche. Considering the invasive potential of this species, policies are required to limit or restrict the use, sale, and transport of L. lucidum outside its native range. In this way, its establishment can be prevented in areas where it has not yet been introduced.
Methods
Species geographic occurrence and climatic data
The global occurrence data for L. lucidum were obtained from georeferenced points in the Global Biodiversity Information Facility49 and Southern African Plant Invaders Atlas50 databases. Climatic data (1970–2000) were obtained from the WorldClim 2.0 database51 at a resolution of 10 min (~20 km). A Köppen-Geiger climate classification map (1976–2000)35 was plotted with an R code52. Nineteen climatic variables referring to minimum, average, and maximum temperatures and precipitation (bio 1: Annual Mean Temperature; bio 2: Mean Diurnal Range (mean of monthly (max temp − min temp); bio 3: Isothermality (bio 2/bio 7) (×100); bio 4: Temperature Seasonality (standard deviation ×100); bio 5: Maximum Temperature of the Warmest Month; bio 6: Minimum Temperature of the Coldest Month; bio 7: Annual Temperature Range (bio 5–bio 6); bio 8: Mean Temperature of the Wettest Quarter; bio 9: Mean Temperature of the Driest Quarter; bio 10: Mean Temperature of the Warmest Quarter; bio 11: Mean Temperature of the Coldest Quarter; bio 12: Annual Precipitation; bio 13: Precipitation of the Wettest Month; bio 14: Precipitation of the Driest Month; bio 15: Precipitation Seasonality (Coefficient of Variation); bio 16: Precipitation of the Wettest Quarter; bio 17: Precipitation of the Driest Quarter; bio 18: Precipitation of the Warmest Quarter; bio 19: Precipitation of the Coldest Quarter; and alt: Altitude). These variables were used in the data analysis because they are ecologically relevant to species distribution.
Data analysis
A single occurrence point was filtered for each 10 km × 10 km grid to reduce geographic sampling bias (collections near research centers, universities, herbaria, and others). Polygonal borders were established for regions with significant L. lucidum occurrence. Eight areas were considered: (1) native tree range in southern China, (2) Oceania, (3) Europe, (4) Southeastern South America, (5) Central America and Mexico, (6) North American West Coast, (7) North American East Coast, and (8) South Africa. Areas without extensive occurrence of this species were excluded to avoid analytical bias.
Considering the native and invaded range climates, principal component analyses (PCAs) were performed pairwise for each study region to investigate variations in realized climatic niche53,54. In this way, a two-dimensional climatic species distribution space was created. Ordinations were determined using kernel density estimation for species occurrence21. Niche overlap was determined according to Schoener’s D index, which ranges from 0 (no overlap) to 1 (complete overlap).
Niche similarity tests were performed54 (α = 0.05) by comparing observed and randomly simulated (n = 100) D values. For this test, an observed D value greater than the randomly simulated D values was considered a null hypothesis, meaning that the invaded niche resembled the native niche; therefore, there was no climate niche shift. We also determined expansion into climatic conditions that are distinct from those in the native range (E) and the unfilled climatic conditions in the native area of the species that are absent in the invaded area (U).
All analyses were carried out using the R statistical programming language v 3.5.155 and the following packages: SDMtools56, raster57, maptools58, ecospat59, rgbif60, spThin61, and splancs62.
Data Availability
The data used in this research are available upon request by contacting the corresponding author.
References
Simberloff, D. et al. Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28, 58–66 (2013).
Bradley, B. A., Blumenthal, D. M., Wilcove, D. S. & Ziska, L. H. Predicting plant invasions in an era of global change. Trends Ecol Evol 25, 310–318 (2010).
Hutchinson, G. E. Concluding remarks. Cold Spring Harbor symposium. Quant Biol 22, 425–427 (1957).
Pearman, P. B., Guisan, A., Broennimann, O. & Randin, C. F. Niche dynamics in space and time. Trends Ecol Evol 23, 149–158 (2008).
Guisan, A. & Zimmermann, N. E. Predictive habitat distribution models in ecology. Ecol Model 135, 147–186 (2000).
Prentice, K. C. Bioclimatic distribution of vegetation for general circulation models. J Geophys Res 95, 811–830 (1990).
Guisan, A., Petitpierre, B. & Broennimann, O. Unifying niche shift studies: insights from biological invasions. Trends Ecol Evol 29, 260–269 (2014).
Peterson, A. T., Soberon, J. & Sanchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 (1999).
Wiens, J. J. & Graham, C. H. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36, 519–539 (2005).
Petitpierre, B., Kueffer, C. & Broennimann, O. Climatic niche shifts are rare among terrestrial plant invaders. Science 335, 1344–1348 (2012).
Faleiro, F. V., Silva, D. P., Carvalho, R. A., Särkinen, T. & Marco, P. Jr. Ring out the bells, we are being invaded! Niche conservatism in exotic populations of the Yellow Bells, Tecoma stans (Bignoniaceae). Nat Conservacao 13, 24–29 (2015).
Mitchell, C. E. et al. Biotic interactions and plant invasions. Ecol Lett 9, 726–740 (2006).
Theoharides, K. A. & Dukes, J. S. Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol 176, 256–273 (2007).
Blumenthal, D., Mitchell, C. E., Pyšek, P. & Jarošík, V. Synergy between pathogen release and resource availability in plant invasion. Proc Natl Acad Sci USA 106, 7899–7904 (2009).
Prior, K. M., Powell, T. H., Joseph, A. L. & Hellmann, J. J. Insights from community ecology into the role of enemy release in causing invasion success: the importance of native enemy effects. Biol Invasions 17, 1283–1297 (2015).
Broennimann, O. et al. Evidence of climatic niche shift during biological invasion. Ecol Lett 10, 701–709 (2007).
Guisan, A. et al. Response to comment on “Climatic niche shifts are rare among terrestrial plant invaders”. Science 338, 193 (2012).
Dellinger, A. S. et al. Niche dynamics of alien species do not differ among sexual and apomictic flowering plants. New Phytol 209, 1313–1323, https://doi.org/10.1111/nph.13694 (2016).
Wan, J., Wang, C., Tan, J. & Yu, F. Climatic niche divergence and habitat suitability of eight alien invasive weeds in China under climate change. Ecol Evol 7, 1541–1552 (2017).
Peterson, A. T. Ecological niche conservatism: a time-structured review of evidence. J Biogeogr 38, 817–827 (2011).
Guisan, A., Thuiller, W. & Zimmermann, N. E. Habitat suitability and distribution models. With applications in R. Cambridge, Cambridge University Press (2017).
GRIN. Germplasm Resources Information Network. United States Department of Agriculture, Agricultural Research Service, National Germplasm Resources Laboratory, Beltsville, MD, http://www.ars-grin.gov Accessed 17 July (2017).
Panetta, F. D. Fates of fruits and seeds of Ligustrum lucidum W.T.Ait. and L. sinense Lour. maintained under natural rainfall or irrigation. Aust J Bot 48, 701–706 (2000).
Aragón, R. & Groom, M. Invasion by Ligustrum lucidum (Oleaceae) in NW Argentina: early stage characteristics in different habitat types. Rev Biol Trop 51, 59–70 (2003).
Nel, J. L. et al. A proposed classification of invasive alien plant species in South Africa: towards prioritizing species and areas for management action. S Afr J Sci 100, 53–64 (2004).
Andreu, J. & Vilà, M. Análisis de la gestión de las plantas exóticas en los espacios naturales españoles. Ecosistemas 16, 109–124 (2007).
Henderson, L. Invasive, naturalized and casual alien plants in southern Africa: a summary based on the Southern African Plant Invaders Atlas (SAPIA). Bothalia 37, 215–248 (2007).
Sullivan, J. J., Meurk, C., Whaley, K. J. & Simcock, R. Restoring native ecosystems in urban Auckland: urban soils, isolation, and weeds as impediments to forest establishment. N Z J Ecol 33, 60–71 (2009).
Maddox, V., Byrd, J. Jr. & Serviss, B. Identification and control of invasive privets (Ligustrum spp.) in the middle southern United States. Invas Plant Sci Mana 3, 482–488 (2010).
Sung, C. Y., Li, M. H., Rogers, G. O., Volder, A. & Wang, Z. Investigating alien plant invasion in urban riparian forests in a hot and semi-arid region. Landsc Urban Plan 100, 278–286 (2011).
Ayup, M. M., Montti, L., Aragón, R. & Grau, H. R. Invasion of Ligustrum lucidum (Oleaceae) in the southern Yungas: changes in habitat properties and decline in bird diversity. Acta Oecol 54, 72–81 (2014).
Cariñanos, P., Alcázar, P., Galán, C. & Domínguez, E. Privet pollen (Ligustrum sp.) as potential cause of pollinosis in the city of Cordoba, southwest Spain. Allergy 57, 92–97 (2002).
Mani, B. M. et al. Identification of Ligustrum lucidum pollen allergens using a proteomics approach. Biochem Biophys Res Comm 468, 788–792 (2015).
Guidini, A. L. et al. Invasão por espécies arbóreas exóticas em remanescentes florestais no Planalto Sul Catarinense. Rev Árvore 38, 469–478 (2014).
Rubel, F. & Kottek, M. Observed and projected climate shifts 1901-2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol Z 19, 135–141, https://doi.org/10.1127/0941-2948/2010/0430 (2010).
Beaumont, L. J. et al. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15, 409–420 (2009).
Gallagher, R. V., Beaumont, L. J., Hughes, L. & Leishman, M. R. Evidence for climatic niche and biome shifts between native and novel ranges in plant species introduced to Australia. J Ecol 98, 790–799 (2010).
Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10, 1115–1123 (2007).
Lockwood, J. L., Cassey, P. & Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20, 223–228 (2005).
Lavergne, S. & Molofsky, J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA 104, 3883–3888 (2007).
Alexander, J. M. Evolution under changing climates: climatic niche stasis despite rapid evolution in a non-native plant. Proc Biol Sci 280, 20131446 (2013).
Santos, E. M. et al. Quali-quantitative analysis of urban trees in Lages, SC. Revista de Ciências Agroveterinárias 12, 59–67 (2013).
Ferreras, A. E., Torres, C. & Galetto, L. Fruit removal of an invasive exotic species (Ligustrum lucidum) in a fragmented landscape. J Arid Environ 72, 1573–1580 (2008).
Vogel, H. F., Zawadzki, C. H. & Metri, R. Coexistence between Turdus leucomelas Vieillot, 1818 and Turdus rufiventris Vieillot, 1818 (Aves: Passeriformes) in an urban remnant of Araucária tree Forest, Southern Brazil. Biota Neotrop 11, 35–45 (2011).
Lichstein, J. W., Grau, H. R. & Aragón, R. Recruitment limitation in secondary forests dominated by an exotic tree. J Veg Sci 15, 721–728 (2004).
Gaston, K. J. Global patterns in biodiversity. Nature 405, 220–227 (2000).
Myers, N. et al. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Early, R. & Sax, D. F. Climatic niche shifts between species’ native and naturalized ranges raise concern for ecological forecasts during invasions and climate change. Glob Ecol Biogeogr 23, 1356–1365 (2014).
GBIF Secretariat GBIF Backbone Taxonomy. Checklist dataset, https://doi.org/10.15468/39omei. Accessed 1 July 2018 (2017).
SAPIA. Southern African Plant Invaders Atlas. Sapia database. Accessed 11 April (2018).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int J Climatol 37, 4302–4315 (2017).
Soetaert, K. Climate classification - color key split in two, and countries added, http://www.rforscience.com/portfolio/koppen-geiger/. Accessed 1 July 2018 (2018).
Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883 (2008).
Broennimann, O. et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob Ecol Biogeogr 21, 481–497 (2012).
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL, https://www.R-project.org/ (2018).
Vanderwal, J., Falconi, L., Januchowski, S., Shoo, L. & Storlie, C. SDMTools: tools for processing data associated with species distribution modelling exercises. –R package, ver. 1.1-20. 2014, https://CRAN.R-project.org/package=SDMTools (2014).
Hijmans, R. J. raster: Geographic Data Analysis and Modeling. R package version 2.6-7 2017, https://CRAN.R-project.org/package=raster Accessed 11 July 2017 (2017).
Bivand, R. & Lewin-Koh, R. maptools: Tools for Reading and Handling Spatial Objects. R package version 0.9-2. 2017. Disponível em:, https://CRAN.Rproject.org/package=maptools Accessed July 2017 (2017).
Broennimann, O. et al. ecospat: Spatial Ecology Miscellaneous Methods. R package version 2.2.0, https://cran.r-project.org/web/packages/ecospat/index.html Accessed July 2017 (2014).
Chamberlain, S, Barve, V. & Mcglinn, D. rgbif: Interface to the Global Biodiversity Information Facility API.R package version 0.9.8, https://github.com/ropensci/rgbif Accessed 11 October 2017 (2017).
Aiello‐Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B. & Robert, P. A. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, 541–545 (2015).
Rowlingson, B. & Diggle, P. J. splancs: Spatial and Space-Time Point Pattern. Analysis. R package version 2, 01–16 (2005).
Acknowledgements
The authors thank the Brazilian National Council for Scientific and Technological Development (CNPq) for productivity research grants awarded to PH and ACS, and Programa de Bolsas de Monitoria de Pós-Graduação da Universidade do Estado de Santa Catarina (PROMOP/UDESC), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship awarded to JBBD. We also thank the ARC-Plant Protection Research Institute, Pretoria, South Africa, for access to the Southern African Plant Invaders Atlas (SAPIA), which furnished data on the occurrence of L. lucidum in South Africa.
Author information
Authors and Affiliations
Contributions
P.H., A.C.S. and J.B.B.D. conceived the ideas, designed the methodology, analyzed the data, and contributed to the writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dreyer, J.B.B., Higuchi, P. & Silva, A.C. Ligustrum lucidum W. T. Aiton (broad-leaf privet) demonstrates climatic niche shifts during global-scale invasion. Sci Rep 9, 3813 (2019). https://doi.org/10.1038/s41598-019-40531-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40531-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.