Abstract
Isolated spherical carbonate concretions are frequently observed in finer grained marine sediments of widely varying geological age. Recent studies on various kinds of spherical carbonate (CaCO3) concretions revealed that they formed very rapidly under tightly constrained conditions. However, the formation ages of the isolated spherical carbonate concretions have never been determined. Here we use 87Sr/86Sr ratios to determine the ages of these spherical concretions. The studied concretions formed in the Yatsuo Group of Miocene age in central Japan. Some formed post-mortem around tusk-shells (Fissidentalium spp.), while other concretions have no shell fossils inside. The deformation of sedimentary layers around the concretions, combined with geochemical analyses, reveal that Sr was incorporated into the CaCO3 concretions during their rapid formation. Strontium isotopic stratigraphy using 87Sr/86Sr ratios of all concretions indicates an age of 17.02 ± 0.27 Ma, with higher accuracy than the ages estimated using micro-fossils from the Yatsuo Group. The results imply that the 87Sr/86Sr ratio of isolated spherical carbonate concretions can be applied generally to determine the numerical ages of marine sediments, when concretions formed soon after sedimentation. The 87Sr/86Sr age determinations have high accuracy, even in cases without any fossils evidence.
Similar content being viewed by others
Introduction
Spherical carbonate (CaCO3) concretions are observed widely throughout the world in marine sediments of varying geological ages. The concretions are typically enriched in Sr compared to the surrounding sedimentary rock matrices. The Ca and Sr in the concretions originate in marine water. Typically the concretions have sharp boundaries with the surrounding sedimentary rock matrices1,2,3,4. Over several decades there have been many mineralogical and geochemical studies of the concretion formation process during sediment burial and diagenesis5,6,7,8,9,10,11. However, the 87Sr/86Sr isotopic ratios have so far not been used to determine the ages of concretions.
Yoshida et al.4 determined that generally, isolated spherical carbonate concretions are formed around decaying organic matter, very rapidly, within several years, under highly constrained conditions, even when no skeletal forms occur inside. Recently, Yoshida et al.3 also found that spherical carbonate (calcite) concretions in the Miocene-age Yatsuo Group, in southern Toyama Pref., central Japan (Fig. 1), formed around the mouths of tusk-shells very rapidly over a period of weeks to months post-mortem. Owing to the very rapid formation of the concretions in the Yatsuo Group we were able to determine their ages using strontium (Sr) isotopic stratigraphy12,13. We propose that the method can be used generally to determine the ages of isolated carbonate concretions in marine sediments even when there are no index fossils.
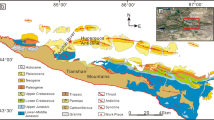
Ca and Sr distribution in and around the tusk-shell concretion. (a) Occurrence of a tusk-shell concretion formed around the mouth of a tusk-shell (Fissidentalium spp.) and, (b) a cross section through the tusk-shell showing the internal texture. (c,d) SXAM Ca and Sr X-ray intensity in and around the cut surface of a tusk-shell concretion. A sharp boundary between the concretion and matrix is also defined by the both elemental distribution. Index map is based on the data of Geospatial Information Authority of Japan website (http://www.gsi.go.jp/ENGLISH/index.html). Figures a–c are referred from Yoshida et al.3. All photographs (a,b) shown here are taken by H. Yoshida.
The studied tusk-shell concretions occur in the upper part of the Kurosedani Formation of the Yatsuo Group and range in size from 1.5 to 3.7 cm (Fig. 1a,b)3,4. The upper part of the Kurosedani Formation consists of clayey sedimentary rocks that have been determined to be of lower to middle Miocene age (17 to 15 Ma) using diatom biostratigraphy and magnetostratigraphy14,15,16,17. Pyroclastic rocks in the Iwaine Formation, which is below the Kurosedani Formation, have a K-Ar age of 16.4 ± 0.9 Ma14,18.
The total thickness of the Kurosedani Formation is ca. 500 m and tusk-shell concretions are observed only in a clayey layer (about 20 metres thick) in the middle to lower-upper level of the formation. In outcrops the tusk-shells (Fissidentalium spp.)15 are seen to lie almost horizontally in the compacted clayey matrix, nearly parallel to the sedimentary layers. However, the fine layers around the concretions are bent due to compaction after concretion formation, suggesting the early formation of concretions, before consolidation of the sediment3,4. Isolated carbonate concretions without shell fossils inside are also found commonly in the Kurosedani Formation, with sizes varying from 2 to 7 cm.
The Yatsuo tusk-shell concretions were investigated by the following methods: rock thin-section examination by optical microscopy to determine the overall structures of the concretions and surrounding matrices; subsequently, Scanning Electron Microscope (SEM) determination of detailed matrix textures and pore geometries relevant to mass transport; followed by chemical analysis using X-ray fluorescence analyzers SXAM and XRF; and finally Sr isotopic measurements for age determination. Detailed analysis methods are given in the METHOD SUMMARY.
All tusk-shell concretions have sharp boundaries that clearly divide them from the surrounding matrices (Fig. 1), allowing their spherical shapes to be seen readily at outcrop. Optical microscopic observations of thin-section show that the tusk-shells in the concretions are well preserved as aragonite, without alteration. The micro-pores of the concretions’ matrices are tightly filled by micritic calcite. In contrast, almost no calcite fillings are observed in the surrounding host rock matrices3.
Major and trace element concentrations in the spherical concretions and surrounding matrices are shown in Table 1. The concretions and matrices have clearly different concentrations of Si, Al, Fe, Mn, Ca, Na, K and P, among major elements, and Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr and Pb, among trace elements. Major elements except for Ca and P are mostly located in detrital grains in the concretions and matrices. All concretions have high concentrations of CaO, up to ca. 50 wt%, which are as much as 20 times higher than the concentrations in the surrounding rock matrices. Si and Al, which originate in detrital grains such as quartz and feldspar that are incorporated within the concretion, are lower than those of the surrounding matrices, reflecting the relatively high abundances of CaCO3 in the concretion. Among the trace elements, Sr and Y are remarkably concentrated in the concretions. In particular, Sr has been accumulated from the marine water and attains concentrations up to 950 ppm in the concretions, two to three times higher than in the surrounding rock (Table 1). It is considered that Sr and Y were accumulated from marine porewater during the concretion formation, although the Y concentration process is still unclear. Other trace elements have lower concentrations compared to the surrounding matrices.
A Yatsuo tusk-shell concretion, as distinguished geochemically by high concentrations of Ca and Sr analysed by SXAM, is shown in Fig. 1c,d. The Ca and Sr concentration profiles across all concretions were found to vary little, except in the central part of the concretion which presumably coincides with the region once occupied by soft tissues3,4. The profiles of Ca and Sr across the concretions’ rims also both decrease rapidly towards the surrounding sedimentary matrices (Fig. 1c,d). Such Sr accumulation is well known, particularly in carbonate-rich marine sediments19. Many carbonate-rich spherical concretions observed in marine sediments commonly accumulate Sr up to several hundreds of ppm20. If the Sr was co-precipitated during concretion formation, the ages determined from Sr isotopic ratios can be generally used to indicate the sedimentation ages as well as the ages of the concretions.
The 87Sr/86Sr ratios of calcite in the tusk-shell concretions in the Kurosedani Formation vary little and lie within 0.70865~0.70867, as shown in Table 2. The values are similar to those of tusk-shell aragonite, which are in the range 0.70865~0.70868. In addition, the isolated carbonate concretions without shell fossils have 87Sr/86Sr ratios of 0.70865~0.70868, which are also well consistent with the tusk-shell aragonite values. The calcite in the matrices has a wider 87Sr/86Sr range (0.70863~0.70912) than those in the tusk-shell concretions and tusk-shells. On the other hand, 87Sr/86Sr values of detrital grains of silicate minerals from the concretion and surrounding matrices lie in the range 0.70741~0.70768 (Table 3), which is clearly different from the range of values given by carbonate in the concretions and tusk-shells. We consider that the calcites in the concretions are unlikely to contain significant Sr of terrigenous (detrital) origin. This conclusion follows from the fact that if calcite in the concretions contains some Sr originating in terrigenous material, with a lower 87Sr/86Sr value of around 0.7075, the Sr isotopic ratios of the calcite will be lower than the seawater values at the formation time of the concretions and will indicate an older age as described later. However, all of the Sr isotope ages are consistent with the depositional age of the sedimentary layer as estimated from palaeontological information.
Yoshida et al.3,4 revealed that tusk-shell concretions were formed very rapidly, post-mortem, around the mouths of tusk-shells. The concretions formed under highly constrained conditions by reactions between the decay products of organic matter and Ca2+ from the marine water. The similar Sr isotopic ratios obtained in the present study from the concretions (0.70865~0.70868) and the tusk-shells themselves (0.70865~0.70868) are consistent with this model. These similar ratios indicate that the tusk-shells and the surrounding concretions both accumulated Sr from the same marine water. If the concretions had formed much later, after sedimentation, the Sr would have accumulated from water with a different Sr isotopic ratio to that of the marine water in which the tusk-shells grew, in which case the Sr isotopic ratios of the concretions and tusk-shells would have been different.
The 87Sr/86Sr variations of marine water from the Cambrian to the present, based on Sr isotopic stratigraphy, have been well established using Sr isotopic data and the fossil record from marine sediments worldwide (Fig. 2)12,21. An accurate Sr isotopic timescale has been determined by correlating Sr isotopic variations with stratigraphic ages based on other evidence12. The Sr isotopic curve is widely accepted for determining the ages of carbonate rich fossils and calcareous marine sediments12,21. In particular, the Sr stratigraphy from the Paleogene to the present has been well analysed and ages are accurate to within ±0.1~0.2 Ma12,21 (Fig. 2). Based on this Sr isotope stratigraphy and the measured 87Sr/86Sr values, the age of the tusk-shell concretions lies within a narrow range of 17.08 (+0.27, −0.28) Ma (Fig. 2 and Table 2). The age determined from the 87Sr/86Sr of the tusk-shells themselves (16.86 ± 0.34 Ma) is almost the same (Table 2). Such a high coincidence of the numerical ages between the concretions and the tusk-shells implies that all the concretions formed sufficiently rapidly to accumulate Sr from the marine water present at the time the tusk-shell lived. Both the Sr isotopic ratios of concretions and the tusk-shells consistently indicate that the concretions were formed within a very short time after the decomposition of the life forms. All evidence suggests that Sr isotopes were rapidly fixed during concretion formation and that the isotopic ratios have not changed thereafter by further calcite precipitation within the spherical concretions.
Sr isotopic stratigraphy and numerical age determination by concretions. (a) Numerical age determined by Sr stratigraphy based on the well-known 87Sr/86Sr variations of seawater during the Paleogene to the present12,13 with the accuracy within ±0.1~0.2 Ma. (b) The age of the tusk-shell concretions and concretions without fossils from Yatsuo Group lie within a narrow range of 17.02 ± 0.27 Ma based on the Sr isotopic stratigraphy. The centre, lower and upper lines (black, blue and red) show a best-fit line, lower-age and upper-age limits on the Sr-isotope curve13, respectively.
The estimated sedimentation age of the Yatsuo Group based on microfossil biostratigraphy and magnetostratigraphy14,15,16,17 has a relatively wide range of 17 to 15 Ma, because of the limited accuracy of fossil time ranges and magnetostratigraphical intervals15. On the other hand, Sr isotopic data shown in this study have a much higher resolution than the fossil age estimations. Our study also indicates that isolated carbonate concretions without index fossils inside, in marine sediments, can be used directly to determine Sr isotopic numerical ages with high accuracy. The age determined from the 87Sr/86Sr of concretions without fossils, 16.95 (+0.36, −0.37) Ma, is almost the same as that of the tusk-shell concretions (Table 2). Strontium isotopic stratigraphy using 87Sr/86Sr ratios of all concretions with tusk-shell and without fossils indicates an age of 17.02 ± 0.27 Ma.
In summary, Sr isotopic data from all concretions and the tusk-shells show that the concretions were formed sufficiently rapidly to preserve the isotopic ratio of marine water Sr at the time of sedimentation. These results are consistent with our recent examination of several kinds of concretions from marine sediments of different ages. This earlier study revealed that spherical carbonate concretions without fossils inside were formed rapidly after sedimentation by reactions involving the decay products of organic matter once present inside them4. In marine clayey sediments, isolated spherical carbonate concretions are commonly observed but do not always contain index fossils that can be used to estimate sedimentation ages. Hence, concretions without fossils inside have never previously been used for age determination. However numerical ages derived from the 87Sr/86Sr isotopic ratios of the concretions examined here indicate that the methodology can be applied to estimate the ages of all kinds of spherical concretions in marine sediments.
Method Summary
Thin-sections were prepared from Epoxy resin-impregnated rock samples by cutting the samples across the centres of tusk-shell concretions. The sections were orientated to characterize the textures preserved inside the concretions and tusk-shells. The rock thin-sections were also used to determine the major and Sr distributions in and around the concretions by SXAM (XGT-2000V Horiba Japan) at the Department of Education, Gifu University, Gifu, Japan. SXAM intensity maps of concretions were reduced to one-dimensional element profiles in a direction perpendicular to the concentric ring patterns identified in elemental maps of the concretions, using the lamination trace technique22. Each measurement was made with a high-intensity continuous X-ray beam (Rh anode 50 kV 1 mA) with 100 μm in diameter. The beam was focused with a guide tube perpendicular to the surface of a sample, which was located on a PC-controllable X-Y stage. X-ray fluorescence from the sample surface was analyzed with the hp-Si detector of an energy-dispersion spectrometer. The results show semi-quantitatively the two-dimensional distribution of all elements across the whole surface of each sample.
Samples were carefully collected and prepared from each concretion and the surrounding matrix. XRF analyses were undertaken to measure major and minor element contents quantitatively using a Rigaku ZSX Primus II WD-XRF spectrometer equipped with a Rh X-ray tube at the Graduate School of Environmental Studies, Nagoya University. Glass beads were prepared by mixing a portion of each sample, which was ignited at 950 °C to decompose carbonates, with anhydrous lithium tetraborate flux and then fusing. Measurements were calibrated with mixed samples of rock reference sample JLs-1 with sedimentary and igneous ones issued by the Geological Survey of Japan (GSJ: Geochemical Reference Sample Data Base, https://gbank.gsj.jp/geostandards/welcome.html). Analytical uncertainties were estimated to be 1 to 2% for SiO2 and Al2O3, 5% for the rest of the major elements, and 10% for trace elements.
Samples of tusk-shell, surrounding concretion and rock matrix from outside the concretion, were used for Sr isotope analysis. Each sample, weighing 10–50 mg, was dissolved in 2 ml of 10% acetic acid at room temperature for 3 h. Then the sample was centrifuged for 15 min at 3500 rpm, and the residue was removed. The dissolved fractions from the tusk-shells, concretions and rock matrices are mostly calcite or aragonite. The strontium in the dissolved fraction was purified using cation-exchange chromatography (BioRad AG50W- X8, 200–400 mesh) with a 2.4 M HCl eluent. The residue was dried at 85 °C for 12 h and weighed, and the residue weight percentage was determined. The residual fractions from the concretion and surrounding rock matrices are mostly detrital silicate. Dissolution of the tusk-shell leaves hardly any residue. The residual silicate fraction was decomposed by HF + HClO4, and then strontium was separated from the silicate fraction by cation-exchange chromatography.
Strontium isotopic ratios, 87Sr/86Sr, were measured by a VG Sector 54-30 thermal-ionization mass spectrometer and normalized to a 86Sr/88Sr value of 0.1194. The mean value of the NIST-SRM 987 standard run with the samples was 87Sr/86Sr = 0.710261 ± 0.000005 (2 SE, n = 14). The 87Sr/86Sr values of the samples have been corrected for inter-laboratory bias by adjusting the mean value of the NIST-SRM987 standard run with the samples to the value of 0.710248 stated by McArthur et al.13.
Ages are determined by the method of McArthur et al.13. The age error for each calcite/aragonite sample is based on 2 SE for the repeated analysis of multiple samples and that of NIST-SRM987. For example, in the case of calcite fractions for tusk-shell concretions with a value of 87Sr/86Sr = 0.708657 ± 0.000009, the averaged value of 0.708657 gives a mean age of 17.08 Ma, the value of 0.708666 (average + 2 SE) gives a lower age limit of 16.80 (=17.08 − 0.28) Ma, and the value of 0.708648 (average − 2 SE) gives an upper age limit of 17.35 (=17.08 + 0.27) Ma. Averaged ages for calcite fractions of the tusk-shell concretions, concretions without fossils, and for aragonite fractions of tusk-shells, are simple mean values with 2 SE.
Change history
07 May 2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Raiswell, R. The microbiological formation of carbonate concretions in the Upper Lias of NE England. Chemical Geology 18, 227–244 (1976).
Dix, G. R. & Mullins, H. T. Shallow, subsurface growth and burial alteration of Middle Devonian calcite concretions. Jour. Sedimentary Petrology 57, 140–152 (1987).
Yoshida, H. et al. Early post-mortem formation of carbonate concretions around tusk-shells over week-month timescales. Scientific Reports 5, 14123, https://doi.org/10.1038/srep14123 (2015).
Yoshida, H. et al. Generalized conditions of spherical carbonate concretions formation around decaying organic matter in early diagenesis, Scientific Reports, 8, 6308, https://doi.org/10.1038/s41598-018-24205-5 (2018).
Raiswell, R. The growth of Cambrian and Liassic concretions. Sedimentology 17, 147–171 (1971).
Irwin, H., Curtis, C. & Coleman, M. Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments. Nature 269, 209–213 (1977).
Coleman, M. L. Microbial processes: Controls on the shape and composition of carbonate concretions. Marine Geology 113, 127–140 (1993).
Curtis, D. C., Petrowski, C. & Oertel, G. Stable carbon isotope ratios within carbonate concretions: a clue to place and time of formation. Nature 235, 98–100 (1972).
Curtis, C. D., Coleman, M. L. & Love, L. G. Pore water evolution during sediment burial from isotopic and mineral chemistry of calcite, dolomite and siderite concretions. Geochim. Cosmochim. Acta 50, 2321–2334 (1986).
Mozley, P. & Burns, S. J. Oxygen and carbon isotopic composition of marine carbonate concretions: An overview. J. Sedim. Petrol. 63, 73–83 (1993).
Raiswell, R. & Fisher, Q. J. Mudrock-hosted carbonate concretions: a review of growth mechanisms and their influence on chemical and isotopic composition. Jour. Geological Society, London 157, 239–251 (2000).
McArthur, J. M., Howarth, R. I. & Shields, G. A. Strontium isotope stratigraphy. Geologic Time Scale 2012 Chapter 7, 127–144, https://doi.org/10.1016/B978-0-444-59425-9.00007-X (2012).
McArthur, J. M., Howarth, R. I. & Bailey, T. R. Strontium isotope stratigraphy: LOWESS version 3: Best fit to the marine Sr-isotope curve for 0-509 Ma and accompanying look-up table for deriving numerical age. Journal of Geology 109, 155–170 (2001).
Yanagisawa, Y. & Akiba, F. Refined Neogene diatom biostratigraphy for the northwest Pacific Japan, with an introduction of code numbers for selected diatom biohorizons. Jour. Geol., Soc., Japan 104, 395–414 (1998).
Itoh, Y., Yanagisawa, Y. & Watanabe, M. Magnetostratigraphy and diatom biostratigraphy of Neogene rocks distributed in the Yatsuo area, central Japan. Bull. Geol. Surv. Japan 50, 215–223 (1999).
Watanabe, M. & Yanagizawa, Y. Refined early to middle Miocene diatom biochronology for the middle-to high-latitude North Pacific. The Island Arc 14, 91–101 (2005).
Tamaki, M., Itoh, Y. & Watanabe, M. Paleomagnetism of the Lower to Middle Miocene Series in the Yatsuo area, eastern part of southwest Japan: clockwise rotation and marine transgression during a short period. Bull. Geol. Surv. 57, 73–88 (2006).
Shibata, K. K-Ar ages of volcanic rocks from the Hokuriku Group. The Memoirs of the Geological Society of Japan 8, 143–149 (1973).
Turekian, K. K. & Kulp, J. L. The geochemistry of strontium. Geochim. Cosmochim. Acta 10, 245–296 (1956).
Holland, H. D., Holland, H. J. & Munoz, J. L. The coprecipitation of cations with CaCO3 – II. The coprecipitation of Sr with calcite between 90 and 100 °C. Geochim. Cosmochim. Acta 28, 1287–1301 (1964).
Howarth, R. J. & McArthur, J. M. Statistic for strontium isotope stratigraphy: A robust LOWESS fit to the marine strontium isotope curve for the period 0 to 206 Ma, with look-up table for the derivation of numerical age. Journal of Geology 105, 441–456 (1997).
Katsuta, N., Takano, M., Okaniwa, T. & Kumazawa, M. Image processing to extract sequential profiles with high spatial resolution from the 2D map of deformed laminated patterns. Computers & Geosciences 29, 725–740 (2003).
Acknowledgements
We are very grateful for helpful discussions with Profs. I. Maruyama, S. Sirono, Y. Yamaguchi of Nagoya University and H. Hasegawa of Kochi University and Dr. S. Nishimoto of Nagoya City Science Centre for XRD measurement. In particular, Prof. A. Ujihara of Nagoya University is thanked for using palaeontological data to determine the geological age of the Yatsuo Group. S. Yogo and M. Nozaki prepared samples for optical examination in thin-sections and SXAM mapping. The research was supported by JSPS KAKENHI grant 266-30464.
Author information
Authors and Affiliations
Contributions
H. Yoshida designed the research and experiments, and wrote the manuscript with Y. Asahara, K. Yamamoto and R. Metcalfe. N. Katsuta contributed the analysis of SXAM geochemical maps, Y. Asahara and M. Minami carried out strontium isotope analysis, and K. Yamamoto and R. Metcalfe carried out all other geochemical analysis and interpretation. All authors contributed and discussed the results and provided inputs on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshida, H., Asahara, Y., Yamamoto, K. et al. 87Sr/86Sr age determination by rapidly formed spherical carbonate concretions. Sci Rep 9, 1003 (2019). https://doi.org/10.1038/s41598-019-38593-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-38593-9
This article is cited by
-
Visualization and identification of components in a gigantic spherical dolomite concretion by Raman imaging in combination with MCR or CLS methods
Scientific Reports (2024)
-
Strontium isotope compositions of Late Permian evaporites from the northernmost Thuringian Basin (Germany) indicate continental influence on the marine Zechstein Sea
International Journal of Earth Sciences (2024)
-
Biogenic apatite in carbonate concretions with and without fossils investigated in situ by micro-Raman spectroscopy
Scientific Reports (2023)
-
A part per trillion isotope ratio analysis of 90Sr/88Sr using energy-filtered thermal ionization mass spectrometry
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.