Abstract
Argon exerts neuroprotection. Thus, it might improve patients’ neurological outcome after cerebral disorders or cardiopulmonary resuscitation. However, limited data are available concerning its effect on pulmonary vessel and airways. We used rat isolated perfused lungs (IPL) and precision-cut lung slices (PCLS) of rats and humans to assess this topic. IPL: Airway and perfusion parameters, oedema formation and the pulmonary capillary pressure (Pcap) were measured and the precapillary and postcapillary resistance (Rpost) was calculated. In IPLs and PCLS, the pulmonary vessel tone was enhanced with ET-1 or remained unchanged. IPLs were ventilated and PCLS were gassed with argon-mixture or room-air. IPL: Argon reduced the ET-1-induced increase of Pcap, Rpost and oedema formation (p < 0.05). PCLS (rat): Argon relaxed naïve pulmonary arteries (PAs) (p < 0.05). PCLS (rat/human): Argon attenuated the ET-1-induced contraction in PAs (p < 0.05). Inhibition of GABAB-receptors abolished argon-induced relaxation (p < 0.05) in naïve or ET-1-pre-contracted PAs; whereas inhibition of GABAA-receptors only affected ET-1-pre-contracted PAs (p < 0.01). GABAA/B-receptor agonists attenuated ET-1-induced contraction in PAs and baclofen (GABAB-agonist) even in pulmonary veins (p < 0.001). PLCS (rat): Argon did not affect the airways. Finally, argon decreases the pulmonary vessel tone by activation of GABA-receptors. Hence, argon might be applicable in patients with pulmonary hypertension and right ventricular failure.
Similar content being viewed by others
Introduction
Noble gases were considered to be inert due to their filled outer electron shell: Meanwhile it is recognised that they exert physiological effects by van der Waals forces1,2. The protective effects of argon and xenon on cellular integrity have been shown for numerous conditions being at high risk for organ dysfunction or poor cerebral outcome, e.g. cardiac surgery3,4,5,6, cardiac resuscitation7,8,9,10, transplantation11 or neurological disorders12,13,14,15,16,17,18. The mechanisms beyond xenon-induced neuroprotection comprise NMDA-antagonism and activation of two-pore potassium channels (TREK-1) or KATP-channels1. Referring the neuroprotective effects of argon, several mechanisms are discussed; e.g. activation of ERK1/2 and PI3K-AKT3,19,20, stimulation of TLR2/417,21,22 and up-regulation of the anti-apoptotic gene Bcl-218. Recently, a common mechanism of argon and xenon has been identified. Both noble gases desensitise acid-sensing ion channels which was shown to be neuroprotective in mouse models of ischaemic stroke23. Regarding the anaesthetic effect of argon under hyperbaric conditions, GABAA-receptors appear to be involved24.
The use of xenon is limited due to its rarity of 0.09 ppm in the atmosphere; in contrast argon is abundant at 0.93%25. The clinical application of argon appears to be more conceivable. The fact that it is non-anaesthetic at normobaric pressure might be even advantageous, as patients requiring neuroprotection are rather harmed from additional sedation. Concerning the neuroprotective effects of argon, only experimental data are available thus far. Yet, one study in humans confirmed that short-term exposure to argon does not affect cerebral circulation or metabolism26.
Usually, neuroprotection is warranted in patients suffering primary neurological disorder (traumatic brain injury, cerebral ischaemia and bleeding) or secondary cerebral ischaemia due to cardiac arrest and cardiac surgery. These patients are often affected by cardiovascular and pulmonary disorders; e.g. left heart disease (LHD), right ventricular (RV) failure, pulmonary hypertension (PH), chronical asthma or chronic obstructive lung disease. Hence, the effects of inhaled argon on airway or pulmonary haemodynamic parameters should be considered. Currently, clinical studies addressing this topic are lacking and experimental trials are rare27,28. One trial in newborn pigs assessed the systemic vascular effects of argon and showed that ventilation with argon (80%) does not affect heart rate or mean arterial blood pressure12,29. Further, Martens et al.27,28 showed that ventilation with argon (70% or 79%) does not affect total pulmonary vascular resistance (PVR). However segmental PVR, expressed as precapillary (Rpre) and postcapillary resistance (Rpost) gives much more evidence about the pulmonary arterial and venous bed. This topic is even more relevant, as pulmonary arteries (PAs) and veins (PVs) react quite differently30,31,32 and PH due to LHD primarily affects PVs33,34.
We studied the effects of argon on Rpre and Rpost in isolated perfused lungs (IPL)35 which allows for the measurement of the capillary pressure (Pcap) and the calculation of Rpre and Rpost. The direct effects of argon and the role of GABA were studied in precision-cut lung slices (PCLS) from rats or humans31,36. PCLS are viable for 72 hours and enable the real time evaluation of the tone of PAs, PVs and airways31,36,37.
Results
We evaluated the effects of argon on pulmonary haemodynamic and airway parameters using rat isolated perfused lungs (IPL) and precision-cut lung slices (PCLS) of rats and humans. In both models, healthy lungs with or without ET-1 pre-treatment were studied. In addition, we addressed the role of GABA-receptor activation within argon-induced relaxation in naïve or ET-1 pre-contracted rat PCLS.
The effects of argon on pulmonary haemodynamics and airway parameters in the IPL
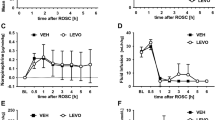
In IPLs, ventilation with argon was started, if baseline parameters were stable for 20 minutes. Control lungs were forward ventilated with room-air. Ventilation with argon did not alter pulmonary haemodynamics (Fig. 1A–E), e.g. pulmonary arterial pressure (PPA), PVR, Pcap, Rpre, Rpost or oedema formation, indicated by the wet-to-dry ratio (W/D-ratio; Fig. 1F). However, ventilation with argon reduced the tidal volume (TV) (Fig. 2A; p < 0.001) and the lung compliance (C) (Fig. 2B; p < 0.01), but had no effect on the lung resistance (R) (Fig. 2C).
Influence of argon on pulmonary haemodynamics in the IPL of the rat. (A) Pulmonary arterial pressure (PPA); (B) PVR; (C) Pulmonary capillary pressure (Pcap); (D) Precapillary resistance (Rpre); (E) Postcapillary resistance (Rpost) and (F) Wet-to-dry ratio (W/D). (A–E) (○) control (n = 12), ( ) argon (n = 13) (■) ET-1 20 nM (n = 10), (
) argon (n = 13) (■) ET-1 20 nM (n = 10), ( ) ET-1 20 nM/argon (n = 8). Statistics were performed by a LMM (A–E) or by the Mann-Whitney U test (F). *p < 0.05, **p < 0.001 and ***p < 0.001.
) ET-1 20 nM/argon (n = 8). Statistics were performed by a LMM (A–E) or by the Mann-Whitney U test (F). *p < 0.05, **p < 0.001 and ***p < 0.001.
Influence of argon on airway parameters in the IPL of the rat. (A) Tidal volume (TV); (B) Lung compliance (C) and (C) Airway resistance (R). (A–C) (○) control (n = 12), ( ) argon (n = 13), (■) ET-1 20 nM (n = 10), (
) argon (n = 13), (■) ET-1 20 nM (n = 10), ( ) ET-1 20 nM/argon (n = 8). (A–C) Statistics were performed by a LMM. **p < 0.01 and ***p < 0.001.
) ET-1 20 nM/argon (n = 8). (A–C) Statistics were performed by a LMM. **p < 0.01 and ***p < 0.001.
To study the effects of argon under conditions of increased PVR and to mimic a feature of PH38,39,40, ET-1 was added to the perfusion buffer (final concentration: 20 nM) as soon as baseline values were stable for 10 minutes. Again 10 minutes later, argon-ventilation was started. Aside from the contractile effects of ET-1 on the pulmonary vascular bed, ET-1 provokes bronchoconstriction38.
ET-1 significantly increased PPA, PVR, Pcap, Rpre and Rpost (Fig. 1A–E; all: p < 0.001), as well as the W/D-ratio (Fig. 1F; p < 0.05). Beyond that, ET-1 decreased TV and C (Fig. 2A/B; both: p < 0.01) and increased R (Fig. 2C; p < 0.001). Ventilation with argon did not alter the increasing effects of ET-1 on PPA, PVR and Rpre (Fig. 1A/B and D), but it significantly attenuated the raising effects of ET-1 on Pcap, Rpost and W/D-ratio (Fig. 1C/E/F; all: p < 0.05). In addition, ventilation with argon did not alter the broncho-pulmonary effects of ET-1 on TV, C or R (Fig. 2A–C).
The effects of argon on ET-1-induced lung oedema and vascular permeability
Argon affected the formation of ET-1-induced lung oedema (Fig. 1F). To distinguish if this effect derives from reduced Pcap (Fig. 1C) or from reduced vascular permeability, we perfused some lungs in addition and determined the filtration coefficient (Kfc). Perfusion with ET-1 increased Kfc (p < 0.01; Fig. 3) and this effect was highly attenuated, if lungs were ventilated with an argon-mixture (p < 0.01, Fig. 3).
The effects of argon in PCLS: Role of GABA-receptor inhibition
PCLS were gassed with argon in an incubation chamber; subsequently the effects of argon on the tone of airways (AWs), PVs and PAs were analysed (Fig. 4A–C). Argon did not alter the tone of AWs (Fig. 4A) or PVs (Fig. 4B). However, argon relaxed PAs, indicated by an increase of the initial vessel area (IVA) between the time points 2 h and 6 h (Fig. 4C, p < 0.05).
Effects of argon in PCLS of rats: Role of GABA-receptor inhibition. (A) Naïve AW: (◊) control (n = 15), ( ) argon (n = 13). (B) Naïve PV: (□) control (n = 12), (
) argon (n = 13). (B) Naïve PV: (□) control (n = 12), ( ) argon (n = 13). (C) Naïve PA: (○) control (n = 13), (
) argon (n = 13). (C) Naïve PA: (○) control (n = 13), ( ) argon (n = 14). (D) Influence of GABAA-receptor inhibition on the relaxant effect of argon in PAs: (
) argon (n = 14). (D) Influence of GABAA-receptor inhibition on the relaxant effect of argon in PAs: ( ) argon (n = 14), (▼) argon/gabazine (n = 5). (E) Influence of GABAB-receptor inhibition on the relaxant effect of argon in PAs: (
) argon (n = 14), (▼) argon/gabazine (n = 5). (E) Influence of GABAB-receptor inhibition on the relaxant effect of argon in PAs: ( ) argon (n = 14), (▲) argon/saclofen (n = 6). (A–E) Statistics were performed by a LMM. *p < 0.05 and **p < 0.001.
) argon (n = 14), (▲) argon/saclofen (n = 6). (A–E) Statistics were performed by a LMM. *p < 0.05 and **p < 0.001.
Next, we studied in PAs, if activation of GABA-receptors plays a role within argon-induced relaxation. Thus, PCLS were treated with the GABAA-receptor inhibitor gabazine or the GABAB-receptor inhibitor saclofen prior to exposure to argon. Control PCLS only underwent argon-gassing. Regarding IVA, the gabazine/argon group did not differ from the argon group (Fig. 4D), although a trend towards gabazine-induced inhibition of argon-induced relaxation was observable. In contrast, GABAB-inhibition interacted with the relaxant effect of argon (Fig. 4E; p < 0.01) and PAs even contracted slightly.
Interaction of argon and ET-1 in PCLS: Role of GABA-receptor inhibition
ET-1 contracted AWs to 13% of IAA (Fig. 5A) and PVs or PAs to 61% or 63% of IVA, respectively (Fig. 5B/C). Simultaneous gassing with argon reduced the contractile effect of ET-1 in PAs at time points 1 h, 2 h and 6 h (Fig. 5C; p < 0.05), but it did not alter the effect of ET-1 in PVs (Fig. 5B) or AWs (Fig. 5A). We wanted to highlight if activation of GABA-receptors contributes to the effect of argon within ET-1-induced contraction of PAs, thus, we pre-treated PAs with gabazine and ET-1 (Fig. 5D) or with saclofen and ET-1 (Fig. 5E) prior to their exposure to argon. Inhibition of GABAA-receptors (gabazine) prevented the effect of argon on ET-1-induced contraction of PAs (Fig. 5D; p < 0.05) and even increased it (Fig. 5D; p < 0.01). Inhibition of GABAB-receptors (saclofen) also prevented the effect of argon on ET-1-induced contraction (Fig. 5E; p < 0.05), but did not increase it (Fig. 5E; p > 0.05). In contrast, if PCLS were not exposed to argon, inhibition of GABAA/B-receptors did not affect the tone of naïve PAs (data not shown) and did not alter the contractile effect of ET-1 in PAs (Fig. 5F).
Interaction of argon and ET-1 in PCLS of rats: Role of GABA-receptor inhibition. (A) AW: (◆) ET-1 200 nM (n = 13), ( ) ET-1 200 nM/argon (n = 12); (B) PV: (■) ET-1 200 nM (n = 14), (
) ET-1 200 nM/argon (n = 12); (B) PV: (■) ET-1 200 nM (n = 14), ( ) ET-1 200 nM/argon (n = 13); (C) PA: (●) ET-1 200 nM (n = 15), (
) ET-1 200 nM/argon (n = 13); (C) PA: (●) ET-1 200 nM (n = 15), ( ) ET-1 200 nM/argon (n = 13). (D) PA: (●) ET-1 200 nM (n = 15), (
) ET-1 200 nM/argon (n = 13). (D) PA: (●) ET-1 200 nM (n = 15), ( ) ET-1 200 nM/argon (n = 13), (▼) ET-1 200 nM/argon/gabazine (n = 4). (E) PA: (●) ET-1 200 nM (n = 15), (
) ET-1 200 nM/argon (n = 13), (▼) ET-1 200 nM/argon/gabazine (n = 4). (E) PA: (●) ET-1 200 nM (n = 15), ( ) ET-1 200 nM/argon (n = 13), (▲) ET-1 200 nM/argon/saclofen (n = 5). (F) PA: (●) ET-1 200 nM (n = 15), (▼) ET-1 200 nM/gabazine (n = 5), (▲) ET-1 200 nM/saclofen (n = 4). (A–F) Statistics were performed by a LMM. *p < 0.05, **p < 0.001 and ***p < 0.001.
) ET-1 200 nM/argon (n = 13), (▲) ET-1 200 nM/argon/saclofen (n = 5). (F) PA: (●) ET-1 200 nM (n = 15), (▼) ET-1 200 nM/gabazine (n = 5), (▲) ET-1 200 nM/saclofen (n = 4). (A–F) Statistics were performed by a LMM. *p < 0.05, **p < 0.001 and ***p < 0.001.
Modulation of ET-1-induced contraction in PCLS: Role of GABA-receptor activation
Inhibition of GABAA/B-receptors did not modulate the contractile effect of ET-1 in PAs (Fig. 5F). Next, we studied, if activation of GABAA/B-receptors alters ET-1-induced contraction in PAs, PVs or airways. Thus, PCLS were treated with ET-1 alone, ET-1/gabazine or ET-1/saclofen prior to the treatment with the GABAA-receptor agonist muscimol or the GABAB-receptor agonist baclofen (R/S baclofen). In PAs, muscimol decreased the contractile effect of ET-1 compared to their exposure only to ET-1 (Fig. 6A; p < 0.001). The effect of muscimol was prevented, if GABAA-receptors were blocked by gabazine (Fig. 6A; p < 0.001). Accordingly, exposure to the GABAB-receptor agonist baclofen reduced the contractile effect of ET-1 in PAs (Fig. 6B; p < 0.0001). This effect was prevented, if GABAB-receptors were blocked by saclofen (Fig. 6B; p < 0.001). In PVs, muscimol did not attenuate ET-1-induced contraction (Fig. 6C; p > 0.05), but baclofen reduced it (Fig. 6D; p < 0.001) which was also prevented by saclofen (Fig. 6D). In addition, muscimol or baclofen did not alter ET-1-induced bronchoconstriction (Fig. 6E/F).
Modulation of ET-1-induced contraction in PCLS of rats: Role of GABA-receptor activation. (A) PA: (●) ET-1 200 nM (n = 15), (∇) ET-1 200 nM/muscimol 5 nM (n = 8), (▼) ET-1 200 nM/muscimol 5 nM/gabazine 5 µM (n = 5). (B) PA: (●) ET-1 200 nM (n = 15), (Δ) ET-1 200 nM/baclofen 5 nM (n = 7), (▲) ET-1 200 nM/baclofen 5 µM/saclofen 5 µM (n = 5). (C) PV: (■) ET-1 200 nM (n = 11), (∇) ET-1 200 nM/muscimol 5 nM (n = 7). (D) PV: (■) ET-1 200 nM (n = 11), (Δ) ET-1 200 nM/baclofen 5 nM (n = 8), (▲) ET-1 200 nM/baclofen 5 µM/saclofen 5 µM (n = 3). (E) AW: (♦) ET-1 200 nM (n = 15), (∇) ET-1 200 nM/muscimol 5 nM (n = 8). F) AW: (♦) ET-1 200 nM (n = 15), (Δ) ET-1 200 nM/baclofen 5 nM (n = 7). (A–F) Statistics were performed by a LMM. ***p < 0.001.
Effect of argon in untreated and ET-1 pre-treated human pulmonary arteries (PCLS)
Human PAs were gassed with argon and analysed subsequently. Argon did not change the tone of naïve human PAs (Fig. 7A). ET-1 (100 nM) contracted PAs to 23% of IVA (Fig. 7B; p < 0.001) and simultaneous exposure to argon did not alter the initial contractile effect of ET-1 (Fig. 6B). However, if PAs were exposed for 24 h to argon, the contractile effect of ET-1 was attenuated (Fig. 7B; p < 0.05).
Discussion
In this study, argon relaxed the pulmonary circulation. Gassing with an argon-mixture (argon 74%, CO2 5%, O2 21%) reduced the tone of rat PAs and lowered ET-1-induced contraction in PAs from rats or humans. In IPLs, ventilation with an argon-mixture reduced the ET-1-induced increase of Pcap, Rpost and the W/D-ratio. Regarding argon-induced relaxation, GABA-receptors appear to be involved, as 1) inhibition of GABAB-receptors prevented the relaxant effect of argon in naïve PAs and 2) inhibition of GABAA/B-receptors blocked the attenuating effect of argon on ET-1-induced contraction. Beyond that, GABAA/B seems to interact with ET-1, as stimulation of GABAA/B- or GABAB-receptors reduced ET-1-induced contraction in rat PAs or PVs, respectively. In the IPL, argon exerted some effects on the airway tone which were not confirmed in PCLS and discussed later.
In rat PAs, argon exerted relaxation and reduced the contractile effect of ET-1. Both effects were evident, if argon-gassing was performed for 2 hours, whereas argon did not alter the tone of PVs, emphasizing the different response of PAs or PVs to various stimuli30,32,41. In line with our results from rats, argon attenuated the contractile effect of ET-1 in human PAs, although a longer duration of argon-gassing was necessary, but it did not relax naïve human PAs. The differential behaviour of PAs from both species might be due to several reasons. (1) ET-1-induced contraction differed among PAs of rats or humans and was about 63% or 25% of IVA, respectively. This fact could explain the delayed effect of argon on ET-1-induced contraction in human PAs. (2) Rat PAs relaxed without pre-contraction suggesting a certain resting tone, as it was shown for PVs from guinea pigs30,31. (3) Human PAs only relaxed due to argon, if they were pre-contracted, as it was shown for milrinone31 and other relaxant stimuli (our own unpublished data). Most likely, they do not dispose of a resting tone, 4) PAs from both species belong to different parts of the pulmonary arterial bed. PAs from rats derive from a central part of the lung, whereas human PAs derive from a more peripheral part of the lung. Regarding the diverging responses to argon, we can expect species-dependent differences31,36, but also that various pulmonary vascular segments react differently to similar stimuli42.
Despite the intriguing effects of argon on the pulmonary arterial tone of rats or humans, argon did not influence PPA or Rpre, regardless if PVR was increased by ET-1 or not. In the IPL, PPA represents the central part of the pulmonary arterial bed which corresponds to rat PAs studied in PCLS, whereas Rpre rather displays peripheral PAs. So, it was somewhat unexpected that ventilation with argon did not affect PPA in untreated lungs or did not lower the effect of ET-1 on PPA. Regarding these discrepancies, the following ideas should be considered. When PCLS are exposed to argon the argon-mixture reaches the PAs, PVs or AWs directly, thus allowing the study of argon’s effects with confidence. Unlike PCLS, inhaled argon initially must pass the pulmonary vasculature at the capillary and postcapillary level where, argon effectively relaxed the tissue targets. However, it is uncertain, if argon acts in other parts of the pulmonary circulation which are more distant to the alveolo-capillary membrane to the same extent, or whether higher concentrations are required, e.g. realised by an extended application period. Potentially, the pulmonary arterial relaxant effects of argon shown in PCLS are less relevant for clinical practice. Irrespective of these considerations, argon did not deteriorate pulmonary haemodynamics, as it was shown for xenon43,44. It rather exerted beneficial effects on Pcap, Rpost and oedema formation which are not debatable and of interest in patients with LHD often suffering from postcapillary PH and/or lung oedema45.
In the IPL, argon reduced the ET-1-induced increase of Pcap and prevented the effects of ET-1 on the W/D-ratio and on Kfc. Apart from that, argon reduced the ET-1-induced increase of Rpost resembling the postcapillary vascular bed, and thus the smallest PVs. Our data from PCLS (rats) do not reflect these results, as argon did not relax PVs and did not attenuate the contractile effect of ET-1 in PVs. Nonetheless, they become more distinct, if some other factors are considered. (1) Rpost is determined by the tone of the smallest PVs which are not reflected in PCLS, as PCLS from rats allow the study of central, large PVs, whereas human PCLS enable the study of more peripheral, but not the smallest PVs. (2) PLA reflects the central pulmonary venous system. However, if constant flow is applied during negative pressure ventilation, it is essential to establish a pressure balancing chamber in the perfusion outflow to prevent negative pressure lung oedema. This pressure balancing chamber is connected by tubes to the artificial thorax chamber. Hence, PLA conforms to the pressure in the thorax chamber. Finally, we studied in PCLS central or medium-sized PVs, whereas in the IPL, the smallest parts of the pulmonary venous bed were addressed.
Here, argon lowered the ET-induced increase of Rpost, but did not influence PVR. At first look this appears to be a discrepancy, but it is explainable. PVR is determined as followed: PVR = (PPA − PLA) × 80/flow. Due to the facts that (1) argon had no effect on PPA, (2) PLA was fixed due to the pressure balancing chamber and (3) the flow was constant, PVR could not change. In contrast, Rpost is calculated as followed: Rpost = (Pcap − PLA)/flow. Based on the facts that argon lowered the ET-1-induced increase of Pcap, whereas PLA and the flow remained constant, Rpost unavoidably decreased. We hypothesise that argon would have decreased the effect of ET-1 on PVR, if we had applied positive pressure ventilation.
Other studies addressing the pulmonary vascular effects of argon are scarce. Martens et al.27 studied the organoprotective effects of argon in a porcine model of ex vivo lung perfusion. PVR was increased by a warm ischaemic period of 2 hours. Afterwards, argon ventilation was started, but without effects on PVR. In a similar work, Martens et al.28 studied the organoprotective effects of argon within the context of pre-conditioning. Again, argon did not alter PVR. The studies from Martens et al.27,28 and our study show diverging results and are difficult to compare. (1) Martens et al. increased PVR by a warm ischaemic period, whereas we used ET-1. (2) They used a model without fixed PLA, but in our set-up PLA was fixed. (3) They did not determine segmental PVR (Rpre, Rpost) and (4) both studies were done in different species.
In this study, PVR was increased by ET-1 to mimic a characteristic of PH. Notably, increased ET-1-levels play a role in sepsis, sepsis-related organ dysfunction46,47,48 and within the pathogenesis of acute lung injury46,49. This issue is emphasised by the fact that ET-1-antagonists attenuate the occurrence of acute lung injury50,51,52. Here, argon ventilation completely prevented the effect of ET-1 on the W/D-ratio and on Kfc. Thus, argon prevented the formation of lung oedema by a decrease of Pcap which is the main pressure driving fluid from the pulmonary capillaries to the interstitium53 and by decreased vascular permeability. This topic is clinical relevant, as patients suffering to neurological illness often develop neurogenic lung oedema being crucial for their prognosis54. Our results are in conflict to those of Martens et al.27,28 who did not observe argon-related effects on the W/D-ratio. Possibly, the diverging results rely on the various modes of induction of lung oedema. These conflicting results suggest that argon should be further explored within acute lung injury or lung oedema.
Argon-induced downstream signalling is unexplored. In view of neuroprotection, a role of ERK1/2, PI3K-AKT3,19,20, TLR2/417,21,22 or Bcl-218 is discussed, though all these targets are fairly unspecific. The anaesthetic effect of argon is referred to the activation of GABAA-receptors24. GABA (γ-aminobutyric acid) is the main inhibitory neurotransmitter in the mammalian brain55. Beyond that, GABA-receptors are found in the lungs56,57. There, activation of GABAA-receptors leads to airway smooth muscle relaxation58,59,60,61,62 and plays a role in the fetal development of the lung63,64.
Here, inhibition of GABAA/B-receptors did not affect the tone of naïve PAs and did not alter the contractile effect of ET-1 in PAs. Thus, the basal activation of GABAA/B-receptors appears to be not relevant. However, inhibition of GABAB-receptors (saclofen) reduced the relaxant effect of argon in rat PAs indicating a certain role of GABAB-receptors within argon-induced relaxation. The relevance of GABA for the regulation of the pulmonary vascular tone is supported by Starke et al.65 who proved in PAs from rabbits that their contractile force is reduced, if PAs were treated with GABA and further, that GABAA-inhibition by bicuculline or picrotoxin did not prevent this effect. Hence, a dominant role of GABAB appears to be possible. Here, argon lowered the contractile effect of ET-1 in rat PAs and this effect was prevented, if GABAA/B-receptors were blocked by gabazine or saclofen. These data allow us to conclude that argon relaxes rat PAs via activation of GABAA- and GABAB-receptors. They are in line with data from Kaye et al.66, who found in the feline pulmonary vascular bed that both the GABAA-agonist muscimol and the GABAB-agonist SKF-97541 relaxed the pulmonary vascular bed, if it was pre-contracted with the thromboxane analogue U46619. Regarding the pulmonary vasorelaxant effects of GABA, further studies are lacking. Though, Suzuki et al.67 showed that monocrotaline-induced pulmonary vascular remodelling was attenuated, if rats were pre-treated with GABA leading to decreased levels of norepinephrine. To the best of our knowledge, the presence of GABA-receptors has been proven in the lung56,57, but not specifically in the pulmonary circulation, even if the activity of GABA-transaminase was verified in PAs or PVs of guinea pigs, with dominance for PAs suggesting the presence of GABA-receptors68. In addition, there is evidence that stimulation of GABA-receptors alters the tone of systemic vessels69,70.
Beyond the pulmonary vasorelaxant effects of argon, argon reduced the effects of ET-1 on the formation of lung oedema and on Kfc. Within this context, the role of GABA is supported by several studies indicating its protective effect on the development of lung oedema, e.g. Chintagari et al.71 reported that intratracheal instillation of GABA attenuated the effect of high-tidal volume ventilation on the formation of lung oedema by an increased alveolar fluid clearance. Conversely, this effect was prevented if GABA was instilled together with the GABAA-antagonist bicuculline71. However, there is also evidence that activation of GABAA-receptors aggravate lung oedema64. These contrasting results might be explained by a switch of the Cl− conductance pattern of GABAA-receptors according to the intracellular Cl− concentration63. In addition, Zhang and colleagues72 showed that propofol also acting on GABAA-receptors73 reduces the occurrence of neurogenic pulmonary oedema.
In the IPL, we found a significant reduction in TV and C with the administration of the argon-mixture. These changes should not be related to the different viscosity of both gases, as (1) we performed a viscosity based correction of our data (explained in the method section) and (2), if we did not correct the data, TV and C would have been rather increased due to the higher viscosity of the argon-mixture. Our results from PCLS do not show any changes of the airway tone due to the exposure to the argon-mixture. They are further in line with others12,27,28,29 who did not find altered lung mechanics or blood gas analyses due to inhalation of argon. Hence, it must be questioned which phenomena might account for the noticed reduction of TV and C?
The ventilation of the IPL is initiated by a negative pressure in the lung chamber. This amounts to a pressure controlled ventilation mode, such that the TV normally should be the same (based on the lung compliance) independent of the viscosity of the argon-mixture in spite of the change in airway resistance, though the time to filling could increase. However, due to the physiologically realistic breathing frequency of 70 per minute, the increased time to fill might have caused the small difference in TV that was observed.
Though, regarding the role of GABAA/B-receptors within the pulmonary vasorelaxant effect of argon, it is somewhat unexpected that argon did not exert bronchorelaxation, as activation of GABAA-receptors was shown to relax airway smooth muscle in several studies58,59,60,61,62. In view of the pulmonary circulation, it seems that argon-mediated activation of GABAB-receptors is more dominant than stimulation of GABAA-receptors. Anyhow, if argon stimulates GABAA-receptors in PAs, we assume that this should be also the case in AWs. Possibly, the lack of bronchorelaxant effects of argon relies on the intensity of ET-1-induced bronchoconstriction. Obviously, ET-1 contracted PAs and PVs to 60–65% of IVA, whereas the AWs contracted to 10–15% of IAA. Most probably, bronchoconstriction was too strong for a later relaxation.
In rat PAs, activation of GABAA/B-receptors (muscimol/baclofen) reduced the contractile effect of ET-1. In contrast, in PVs this was only the case, if GABAB-receptors were stimulated. Conversely, muscimol and baclofen did not alter ET-1-induced contraction, if GABA-receptors were blocked with gabazine or saclofen, emphasising the specific activation of GABAA/B. In this view, it must be questioned if ET-1 acts at all on GABAA/B-receptors, or rather if activation of GABAA/B-receptors alters ET-1-induced contraction. From the literature there is some evidence that GABA interacts anyhow with ET-1, e.g. it was reported74 that the application of the GABAA-antagonist bicuculline led to the generation of lung oedema. In that study, ET-1 levels were increased in the bronchoalveolar lavage of bicuculline-treated rats74 and conversely, the occurrence of lung oedema was attenuated by phosphoramidon or by the ETA-antagonist BQ-12374. In addition, GABA reduces the release of norepinephrine65,67 which highly contributes to the formation of neurogenic lung oedema75,76.
In conclusion, argon decreased the pulmonary vascular tone of the rat, if PVR was enhanced, but it did not affect the airway tone. In view of the pulmonary vasorelaxant potential of argon, activation of GABAA/B-receptors plays a pivotal role. Finally, our results support the application of argon for neuroprotection in patients with critical pulmonary haemodynamics based on PH, RV failure or LHD. The relevance of our findings is strengthened by the fact that argon also relaxed human PAs.
Methods
Animals and human lung tissue
Female Wistar rats (250 ± 50 g) were purchased from Charles River (Sulzfeld, Germany) and used as lung donors. Rat lungs were randomly assigned to one of the groups.
Human PCLS were prepared from patients undergoing lobectomy due to cancer. After pathological inspection, cancer free tissue from a peripheral pulmonary part was used. None of the patients showed any signs of PH (echocardiographic or histological evaluation). The study was approved by the local ethics committee (EK 61/09) of the Medical Faculty Aachen, Rhenish-Westphalian Technical University Aachen. All patients gave written informed consent.
All animal studies and experimental procedures were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz North Rhine-Westphalia (ID: 84-02.04.2013.A146; ID: 8.87-51.05.20.10.245; ID: 50086A4) and performed due to the Directive 2010/63/EU of the European Parliament.
IPL: Preparation
IPLs were prepared as described before31,35,77. Briefly, female rats were anaesthetised with 95 mgkg−1 pentobarbital (Narcoren; Garbsen, Germany) and bled, if reflex checks were unresponsive. The trachea was cannulated and the lungs were ventilated with positive pressure (70 breath/min). In addition, a PEEP of 3 cmH2O and an I:E of 1:1 were applied. As soon, as cannulas were inserted into the pulmonary artery (inflow) and the left atrium (outflow), the lungs were perfused at constant flow (20 ml/min) with 200 ml Krebs-Henseleit buffer, containing 2% bovine serum albumin, 0.1% glucose, 0.3% HEPES and 50 nM salbutamol to prevent bronchoconstriction78. The buffers’ temperature was kept at 37 °C by a water bath and the pH was maintained between 7.35 and 7.45 by gassing with CO2. After pulmonary ventilation and perfusion was established, heart and lungs were removed and set into a negative-pressure chamber which was adjusted for Pmin = −7 cmH2O and Pmax = −2 cmH2O. To prevent lung oedema during constant flow perfusion and negative pressure ventilation, a pressure balancing chamber was set in the perfusion outflow and connected by tubes to the artificial thorax chamber. To prevent atelectasis, every 5 minutes a deep breath was applied. Airway (TV, C or R) and pulmonary haemodynamic parameters (PPA, PLA and flow) were recorded with the Pulmodyn Software 2.0 (Hugo-Sachs Elektronik Harvard Apparatus, Germany). If all parameters were stable, the PVR was enhanced with ET-1 (final buffer concentration: 20 nM)79.
IPL: Calculation of airway parameters
The pressure inside the thorax chamber was measured by a pressure transducer (Hugo-Sachs Elektronik Harvard Apparatus, Germany). The inhalation flow (Q) was measured by a pneumatograph (Hugo-Sachs Elektronik Harvard Apparatus, Germany). Finally, TV was calculated from the integration of Q. The compliance (C) of the lung expresses its elasticity. C is defined by the TV and the change (Δ) of the transpulmonary pressure (Ptp). In the IPL, ΔPtp reflects the difference of the maximal and minimal pressure (P) in the thorax chamber. So, C was calculated: C = TV/(Pmax − Pmin) and R (resistance) was calculated by the inhalation flow (Q) in relation to Pmax and Pmin: R = (Pmax − Pmin)/Q35.
IPL: Ventilation with argon and correction of airway parameters
To ventilate the lungs with argon, a pressure regulator and a flow meter were used. Argon (flow 0.3 L/min) was applied via a tube which was connected to the pneumatograph. Inhalation flow was measured using a pneumatograph consisting of a single tube with small enough diameter to maintain laminar flow such that the pressure drop across the tube is linearly proportional for the flow rate. The complication in this application occurs because the proportionality is dependent on the gas viscosity (µ); i.e., if the gas viscosity is increased the pressure drop needed to drive the flow would be increased. The pneumatograph was originally calibrated for air; thus for the argon mixture that has a greater viscosity (2.227 kg/(s-m)10−5 for argon at 32 °C versus 1.84 kg/(s-m)10−5 for air at 32 °C) the measured pressure drop will be greater than that for air at the same flow rate. Therefore, a recalibration of the pneumatograph for the argon-mixture would be necessary; however this is not applicable without interrupting the experiment. Another possibility is to correct the pressure drop data by multiplying by the viscosity ratio of air over argon to obtain the flow rate. Alternatively, TV, R and C can be corrected. We corrected TV, R and C as followed: TVcorr = TVmeas × (µair/µargon-mix); Rcorr = Rmeas × ( µair/µargon-mix) and Ccorr = TVcorr/(Pmax − Pmin)80.
IPL: Calculation of PVR, precapillary and postcapillary resistance
PVR was calculated as followed: \({\rm{PVR}}=({{\rm{P}}}_{{\rm{PA}}}-{{\rm{P}}}_{{\rm{LA}}})\ast 80/{\rm{flow}}\). To determine Rpre and Rpost, Pcap was recorded every 10 minutes by the double occlusion method. Rpre and Rpost were calculated according to the following equation: \({{\rm{R}}}_{{\rm{pre}}}=({{\rm{P}}}_{{\rm{PA}}}-{{\rm{P}}}_{{\rm{cap}}})/{\rm{flow}}\) and \({{\rm{R}}}_{{\rm{post}}}=({{\rm{P}}}_{{\rm{cap}}}-{{\rm{P}}}_{{\rm{LA}}})/{\rm{flow}}\)27,81.
IPL: Wet-to-dry ratio (W/D-ratio)
After IPLs were perfused for 2 h, the wet weight of the right superior lobe was recorded and subjected to drying at 60 °C for 72 h. The dry weights were monitored and the W/D-ratio was calculated.
IPL: Assessment of the vascular permeability by determination of the filtration coefficient
To distinguish, if lung oedema derives from increased Pcap or increased vascular permeability, the capillary filtration coefficient (Kfc) was determined as described in reference35. Measurements were performed at 0 and 120 minutes of the perfusion using the following equation: Kfc = (dweight/dtime)/dPcap. Due to the fact, that weight gain measurements do not allow the simultaneous application of the double occlusion method, Pcap was calculated according to the Gaar equation82: Pcap = PLA + 0.44 (PPA − PLA).
PCLS of rats and humans: Preparation
Rats received intraperitoneal anaesthesia with pentobarbital, which was verified by missing reflexes. Thereafter, they were prepared as described before31,36. Rat lungs were filled via the trachea and human lungs were filled via a main or lobar bronchus, respectively, with 1.5% low-melting agarose. Afterwards, they were cooled on ice. Tissue cores (diameter 11 mm) were prepared and cut into about 250 µm thick slices with a Krumdieck tissue slicer (Alabama Research & Development, Munford, USA). PCLS were incubated over night at 37 °C and repeated medium changes were performed to wash out the agarose.
PCLS: Treatment and videomicroscopy
To study the role of GABA within argon-induced relaxation, PCLS were treated with the GABAA-receptor antagonist gabazine (5 µM)83 or with the GABAB-receptor antagonist saclofen (5 µM)84 (Fig. 4D/E). To study the relaxant effect of argon in pre-contracted PAs, PVs and AWs, rat PCLS were pre-contracted with 200 nM ET-1 (Fig. 5A–C) and human PCLS were pre-contracted with 100 nM ET-1 (Fig. 7B). To study the role of GABA-receptors within argon-induced relaxation in ET-1 pre-contracted PAs, PCLS were simultaneously pre-treated with 200 nM ET-1 and 5 µM gabazine (Fig. 5D) or with 200 nM ET-1 and 5 µM saclofen (Fig. 5E). To study the effect of GABA-receptor inhibition or activation within ET-1 induced vasoconstriction and bronchoconstriction, PCLS were pre-treated with ET-1 and gabazine (Fig. 6A), ET-1 and saclofen (Fig. 6B/D) or ET-1 alone (Fig. 6C/E/F) prior to the treatment with the GABAA-receptor agonist muscimol (5 nM)85,86 or the GABAB-receptor agonist baclofen (5 µM)87. In PCLS, all changes of the IVA and IAA were quantified in % and indicated as “Change of IVA [%]” or “Change of IAA [%]”. Thus, an IVA < 100% indicates contraction and an IVA > 100% indicates relaxation. In order to compare the effect of argon in pre-treated vessels, the intraluminal area was defined after pre-treatment again as 100%. In the graphs, all pre-treatments were indicated. The intraluminal area of PAs, PVs and airways was monitored with a digital video camera (Leica Viscam 1280, Leica DFC 280). The images were analysed with Optimas 6.5 (Media Cybernetics, Bothell, WA).
PCLS from rats and humans: Argon gassing
PCLS were transferred into 24 well plates (1 ml medium per well). In order to treat PCLS with argon (Argon 74%, CO2 5%, O2 21%) or air mix (N2 74%, CO2 5%, O2 21%), we used the modular incubator chamber (Billups-Rothenberg, USA) with a filling volume of 6 liters. To avoid evaporation, we added 20 ml of purified water in a petri dish inside the chamber. Next, the incubator chamber with the PCLS inside was purged with the appropriate gas mixture and a gas flow rate of 20 L/min for 5 minutes; the outlet and inlet ports where closed with plastic clamps. Alkalisation of the incubation medium was prevented, as all gas mixtures were enriched with 5% CO2. Afterwards, the whole chamber was transferred in a heat CO2 incubator. In order to investigate the effects of argon on pre-constricted airways or vessels, we pre-treated PCLS with ET-1 and made images at 1 h, 2 h, 3 h, 6 h and even 24 h, in case of human PCLS. To study the role of GABA within the relaxant effect of argon, we pretreated PCLS with the GABA inhibitors gabazine or saclofen and made images according to the pre-treatment with ET-1. Images were recorded by videomicroscopy and the IVA/IAA was calculated with Optimas 6.5.
Reagents
ET-1 was purchased from Biotrends (Wangen, Switzerland). The potency of ET-1 differs strongly with age and lot numbers. GABA receptor agonists/antagonists and standard laboratory chemicals were from Sigma-Aldrich (Steinheim, Germany). Gas mixtures were delivered from Air Liquide GmbH (Simmerath, Germany) or Linde Gas AG (Pullach, Germany).
Statistical analysis
Statistics was conducted using SAS 9.2 (SAS Institute, Cary, North Carolina, USA) and GraphPad Prism 5.01 (GraphPad, La Jolla, USA). All data were analysed by a linear mixed model analysis, except Figs 1F and 3 which were analysed by the Mann Whitney U Test. P-values were adjusted for multiple comparisons (false discovery rate) and presented as mean ± SEM. N indicates the number of animals or lung lobes. P < 0.05 are considered as significant: *p < 0.05, **p < 0.01, ***for p < 0.001.
Ethical approval and informed consent
Animal studies were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz North Rhine-Westphalia (ID: 84-02.04.2013.A146; ID: 8.87-51.05.20.10.245; ID: 50086A4) and performed due to the Directive 2010/63/EU of the European Parliament.
Human PCLS were prepared from patients undergoing lobectomy due to cancer. All patients gave written informed consent and the local ethics committee (EK 61/09) of the Medical Faculty Aachen, Rhenish-Westphalian Technical University Aachen, approved the study.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Dickinson, R. & Franks, N. P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit Care 14, 229 (2010).
Hollig, A. et al. Argon: systematic review on neuro- and organoprotective properties of an “inert” gas. Int. J. Mol. Sci. 15, 18175–18196 (2014).
Lemoine, S. et al. Argon Exposure Induces Postconditioning in Myocardial Ischemia-Reperfusion. J Cardiovasc. Pharmacol. Ther. 22, 564–573 (2017).
Mayer, B. et al. Argon Induces Protective Effects in Cardiomyocytes during the Second Window of Preconditioning. Int. J Mol Sci 17 (2016).
Pagel, P. S. et al. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 105, 562–569 (2007).
Pagel, P. S. Cardioprotection by noble gases. J. Cardiothorac. Vasc. Anesth. 24, 143–163 (2010).
Brucken, A. et al. Argon reduces neurohistopathological damage and preserves functional recovery after cardiac arrest in rats. Br. J Anaesth. 110(Suppl 1), i106–i112 (2013).
Brucken, A. et al. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by K(ATP)-channel opening. Resuscitation 85, 826–832 (2014).
Brucken, A. et al. Delayed argon administration provides robust protection against cardiac arrest-induced neurological damage. Neurocrit. Care 22, 112–120 (2015).
Ristagno, G. et al. Postresuscitation treatment with argon improves early neurological recovery in a porcine model of cardiac arrest. Shock 41, 72–78 (2014).
Irani, Y. et al. Noble gas (argon and xenon)-saturated cold storage solutions reduce ischemia-reperfusion injury in a rat model of renal transplantation. Nephron Extra. 1, 272–282 (2011).
Alderliesten, T. et al. Neuroprotection by argon ventilation after perinatal asphyxia: a safety study in newborn piglets. PLoS One 9, e113575 (2014).
David, H. N. et al. Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS One 7, e30934 (2012).
Hollig, A. et al. Beneficial Properties of Argon After Experimental Subarachnoid Hemorrhage: Early Treatment Reduces Mortality and Influences Hippocampal Protein Expression. Crit Care Med (2016).
Loetscher, P. D. et al. Argon: neuroprotection in in vitro models of cerebral ischemia and traumatic brain injury. Crit Care 13, R206 (2009).
Ryang, Y. M. et al. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med. 39, 1448–1453 (2011).
Ulbrich, F. et al. Argon Mediates Anti-Apoptotic Signaling and Neuroprotection via Inhibition of Toll-Like Receptor 2 and 4. PLoS One 10, e0143887 (2015).
Zhuang, L. et al. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit Care Med. 40, 1724–1730 (2012).
Fahlenkamp, A. V. et al. The noble gas argon modifies extracellular signal-regulated kinase 1/2 signaling in neurons and glial cells. Eur. J. Pharmacol. 674, 104–111 (2012).
Ulbrich, F. et al. Neuroprotective effects of Argon are mediated via an ERK-1/2 dependent regulation of heme-oxygenase-1 in retinal ganglion cells. J Neurochem. 134, 717–727 (2015).
Ulbrich, F. et al. Argon mediates protection by interleukin-8 suppression via a TLR2/TLR4/STAT3/NF-kappaB pathway in a model of apoptosis in neuroblastoma cells in vitro and following ischemia-reperfusion injury in rat retina in vivo. J Neurochem. 138, 859–873 (2016).
Ulbrich, F. & Goebel, U. The Molecular Pathway of Argon-Mediated Neuroprotection. Int. J Mol Sci 17 (2016).
Lehmke, L. et al. Inhalational anesthetics accelerate desensitization of acid-sensing ion channels. Neuropharmacology 135, 496–505 (2018).
Abraini, J. H., Kriem, B., Balon, N., Rostain, J. C. & Risso, J. J. Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth. Analg. 96, 746–9 (2003).
Jephcoat, A. P. Rare-gas solids in the Earth’s deep interior. Nature 393, 355 (1998).
Grune, F. et al. Argon does not affect cerebral circulation or metabolism in male humans. PLoS One 12, e0171962 (2017).
Martens, A. et al. Argon and xenon ventilation during prolonged ex vivo lung perfusion. J. Surg. Res. 201, 44–52 (2016).
Martens, A. et al. A porcine ex vivo lung perfusion model with maximal argon exposure to attenuate ischemia-reperfusion injury. Med Gas Res. 7, 28–36 (2017).
Alderliesten, T. et al. Correction: Neuroprotection by Argon Ventilation after Perinatal Asphyxia: A Safety Study in Newborn Piglets. PLoS One 10, e0133818 (2015).
Rieg, A. D. et al. Levosimendan relaxes pulmonary arteries and veins in precision-cut lung slices – The role of KATP-channels, cAMP and cGMP. PLoS One 8(6), e66195, https://doi.org/10.1371/journal.pone.0066195 (2013).
Rieg, A. D. et al. Milrinone relaxes pulmonary veins in guinea pigs and humans. PLoS Onee 87685 (2013).
Rieg, A. D., Rossaint, R., Uhlig, S. & Martin, C. Cardiovascular agents affect the tone of pulmonary arteries and veins in precision-cut lung slices. PLoS One 6, e29698 (2011).
Adir, Y. & Offer, A. Pulmonary hypertension associated with left heart disease. Eur Respir Monogr 57, 119–137 (2012).
Breitling, S., Ravindran, K., Goldenberg, N. M. & Kuebler, W. M. The pathophysiology of pulmonary hypertension in left heart disease. Am. J. Physiol Lung Cell Mol. Physiol 309, L924–L941 (2015).
Uhlig, S. & Wollin, L. An improved setup for the isolated perfused rat lung. J. Pharmacol. Toxicol. Methods 31, 85–94 (1994).
Schleputz, M. et al. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS). PLoS One 7, e47344 (2012).
Martin, C., Uhlig, S. & Ullrich, V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J 9, 2479–2487 (1996).
Del, B. P. & Argiolas, L. Cardiopulmonary effects of endothelin-1 in the guinea pig: role of thromboxane A2. J Cardiovasc. Pharmacol. 26(Suppl 3), S120–S122 (1995).
Lutz, J. et al. Endothelin-1- and endothelin-receptors in lung biopsies of patients with pulmonary hypertension due to congenital heart disease. Clin. Chem. Lab Med 37, 423–428 (1999).
Rubens, C. et al. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 120, 1562–1569 (2001).
Back, M. et al. Modulation of vascular tone and reactivity by nitric oxide in porcine pulmonary arteries and veins. Acta Physiol Scand 174, 9–15 (2002).
Bonnet, S. & Archer, S. L. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol. Ther. 115, 56–69 (2007).
Hein, M. et al. Xenon alters right ventricular function. Acta Anaesthesiol. Scand. 52, 1056–1063 (2008).
Hein, M. et al. Xenon and isoflurane improved biventricular function during right ventricular ischemia and reperfusion. Acta Anaesthesiol. Scand. 54, 470–478 (2010).
Rosenkranz, S. et al. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J 37, 942–954 (2016).
Comellas, A. P. et al. Endothelin-1 impairs alveolar epithelial function via endothelial ETB receptor. Am. J Respir. Crit Care Med 179, 113–122 (2009).
Kaffarnik, M. F. et al. Correlation between plasma endothelin-1 levels and severity of septic liver failure quantified by maximal liver function capacity (LiMAx test). A prospective study. PLoS One 12, e0178237 (2017).
Lundberg, O. H. et al. Adrenomedullin and endothelin-1 are associated with myocardial injury and death in septic shock patients. Crit Care 20, 178 (2016).
Matsuishi, Y. et al. Landiolol hydrochloride ameliorates acute lung injury in a rat model of early sepsis through the suppression of elevated levels of pulmonary endothelin-1. Life Sci 166, 27–33 (2016).
Deja, M. et al. Inhalation of the endothelin-A receptor antagonist LU-135252 at various doses in experimental acute lung injury. J Cardiovasc. Pharmacol. 44(Suppl 1), S151–S155 (2004).
Kaisers, U. et al. Inhaled endothelin A antagonist improves arterial oxygenation in experimental acute lung injury. Intensive Care Med 26, 1334–1342 (2000).
Kuklin, V. et al. Tezosentan reduces the microvascular filtration coefficient in isolated lungs from rats subjected to cecum ligation and puncture. Crit Care 9, R677–R686 (2005).
Takala, J. Pulmonary capillary pressure. Intensive Care Med. 29, 890–893 (2003).
Busl, K. M. & Bleck, T. P. Neurogenic Pulmonary Edema. Crit Care Med. 43, 1710–1715 (2015).
Chebib, M. & Johnston, G. A. The ‘ABC’ of GABA receptors: a brief review. Clin. Exp. Pharmacol. Physiol 26, 937–940 (1999).
Castelli, M. P., Ingianni, A., Stefanini, E. & Gessa, G. L. Distribution of GABA(B) receptor mRNAs in the rat brain and peripheral organs. Life Sci 64, 1321–1328 (1999).
Chapman, R. W., Hey, J. A., Rizzo, C. A. & Bolser, D. C. GABAB receptors in the lung. Trends Pharmacol. Sci 14, 26–29 (1993).
Gallos, G. et al. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am. J. Physiol Lung Cell Mol. Physiol 304, L191–L197 (2013).
Gallos, G. et al. Selective targeting of the alpha5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am. J. Physiol Lung Cell Mol. Physiol 308, L931–L942 (2015).
Jahan, R. et al. Optimization of substituted imidazobenzodiazepines as novel asthma treatments. Eur. J Med Chem. 126, 550–560 (2017).
Yocum, G. T. et al. Targeting the GABA Receptor alpha4 Subunit in Airway Smooth Muscle to Alleviate Bronchoconstriction. Am. J. Respir. Cell Mol. Biol (2015).
Yocum, G. T. et al. GABAA receptor alpha4-subunit knockout enhances lung inflammation and airway reactivity in a murine asthma model. Am. J Physiol Lung Cell Mol Physiol 313, L406–L415 (2017).
Chintagari, N. R. et al. Role of GABA receptors in fetal lung development in rats. PLoS One 5, e14171 (2010).
Jin, N. et al. Ionotropic GABA receptor expression in the lung during development. Gene Expr. Patterns. 8, 397–403 (2008).
Starke, K. & Weitzell, R. gamma-Aminobutyric acid and postganglionic sympathetic transmission in the pulmonary artery of the rabbit. J. Auton. Pharmacol. 1, 45–51 (1980).
Kaye, A. D., Hoover, J. M., Baber, S. R., Ibrahim, I. N. & Fields, A. M. Analysis of gamma-aminobutyric acid-mediated responses in the pulmonary vascular bed of the cat. Anesth. Analg. 99, 758–63 (2004).
Suzuki, R. et al. Beneficial effects of gamma-aminobutyric acid on right ventricular pressure and pulmonary vascular remodeling in experimental pulmonary hypertension. Life Sci 91, 693–698 (2012).
Erdo, S. L. & Amenta, F. Specific, vascular localization of GABA-transaminase in the guinea pig lung. Neurosci. Lett. 68, 202–206 (1986).
Bek, T. & Holmgaard, K. GABA-induced relaxation of porcine retinal arterioles in vitro depends on inhibition from the perivascular retina and is mediated by GABAC receptors. Invest Ophthalmol. Vis. Sci 53, 3309–3315 (2012).
Elliott, K. A. & Hobbiger, F. gamma Aminobutyric acid; circulatory and respiratory effects in different species; re-investigation of the anti-strychnine action in mice. J Physiol 146, 70–84 (1959).
Chintagari, N. R. & Liu, L. GABA receptor ameliorates ventilator-induced lung injury in rats by improving alveolar fluid clearance. Crit Care 16, R55 (2012).
Zhang, L. et al. Effects of Propofol on Excitatory and Inhibitory Amino Acid Neurotransmitter Balance in Rats with Neurogenic Pulmonary Edema Induced by Subarachnoid Hemorrhage. Neurocrit. Care (2015).
Franks, N. P. Structural comparisons of ligand-gated ion channels in open, closed, and desensitized states identify a novel propofol-binding site on mammalian gamma-aminobutyric acid type A receptors. Anesthesiology 122, 787–794 (2015).
Herbst, C., Tippler, B., Shams, H. & Simmet, T. A role for endothelin in bicuculline-induced neurogenic pulmonary oedema in rats. Br. J Pharmacol. 115, 753–760 (1995).
Inamasu, J. et al. The role of catecholamines in the pathogenesis of neurogenic pulmonary edema associated with subarachnoid hemorrhage. Acta Neurochir. (Wien.) 154, 2179–2184 (2012).
Rassler, B. Contribution of alpha - and beta -Adrenergic Mechanisms to the Development of Pulmonary Edema. Scientifica. (Cairo.) 2012, 829504 (2012).
Maihofer, N. A. et al. Imatinib relaxes the pulmonary venous bed of guinea pigs. Respir. Res. 18, 32 (2017).
Atzori, L. et al. Sulfur dioxide-induced bronchoconstriction in the isolated perfused and ventilated guinea-pig lung. Respiration 59, 16–21 (1992).
Horgan, M. J., Pinheiro, J. M. & Malik, A. B. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ. Res 69, 157–164 (1991).
Baumert, J. H. et al. Increased airway resistance during xenon anaesthesia in pigs is attributed to physical properties of the gas. Br. J Anaesth. 88, 540–545 (2002).
Hakim, T. S., Michel, R. P. & Chang, H. K. Partitioning of pulmonary vascular resistance in dogs by arterial and venous occlusion. J Appl. Physiol Respir. Environ. Exerc. Physiol 52, 710–715 (1982).
Gaar, K. A. Jr., Taylor, A. E., Owens, L. J. & Guyton, A. C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am. J Physiol 213, 910–914 (1967).
Zhang, K. M. et al. Nicotine enhances GABAergic inhibition of oxytocin mRNA-expressing neuron in the hypothalamic paraventricular nucleus in vitro in rats. Neurosci. Lett. 638, 5–11 (2017).
Kerr, D. I. et al. GABAB receptor antagonism by resolved (R)-saclofen in the guinea-pig ileum. Eur. J Pharmacol. 308, R1–R2 (1996).
Defeudis, F. V. Binsing studies with muscimol; relation ty synaptic gamma-aminobutyrate receptors. Neuroscience 5, 675–688 (1980).
King, T. S. et al. Concentration-dependent effects of muscimol to enhance pulsatile GnRH release from GT1-7 neurons in vitro. Brain Res. 824, 56–62 (1999).
Chapman, R. W. et al. Prejunctional GABA-B inhibition of cholinergic, neurally-mediated airway contractions in guinea-pigs. Pulm. Pharmacol. 4, 218–224 (1991).
Acknowledgements
We gratefully acknowledge Hanna Czajkowska for excellent technical assistance. This work was funded by the START programme (grant 44/12 (691216) of the RWTH-Aachen. The funders had no influence of the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.S. performed the experiments, analysed the data, interpreted the data and wrote the manuscript. S.e.K. analysed the data and interpreted the data. I.K. analysed the data in view of the viscosity and density properties of the used gases and helped with his experience to calculate the airway measurements. G.B. analysed the data, interpreted the data, and helped with the human lung tissue. J.K. analysed the data, interpreted the data and helped with the human lung tissue. S.v.S. analysed the data, interpreted the data, helped with the human lung tissue and critically reviewed the manuscript. Sv.K. analysed the data, interpreted the data and helped with the human lung tissue. T.B. analysed the data, interpreted the data and helped with the human lung tissue. A.B. analysed the data, interpreted the data and critically reviewed the manuscript. J.S. analysed the data, interpreted the data and helped with the human lung tissue. S.K. analysed the data, interpreted the data and helped with the human lung tissue. T.S. analysed the data, interpreted the data and helped with the human lung tissue. M.M. analysed the data, interpreted the data and critically reviewed the manuscript. M.C. analysed the data, interpreted the data and critically reviewed the manuscript. S.U. analysed the data, interpreted the data and critically reviewed the manuscript. C.M. designed the study, analysed the data, interpreted the data and critically reviewed the manuscript. A.D.R. designed the study, analysed the data, interpreted the data and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Said Suleiman has no financial and non-financial interests. Sergej Klassen has no financial and non-financial interests. Ira Katz works for Air Liquide. Even he could have a commercial interest; he did not influence the design or the outcome of the study. Ira Katz was very helpful with his experience in view of physiochemical properties of laboratory gases to explain and interpret some unexpected effects of argon on the airway parameters. Galina Balakirski has no financial and non-financial interests. Julia Krabbe has no financial and non-financial interests. Saskia von Stillfried has no financial and non-financial interests. Svetlana Kintsler has no financial and non-financial interests. Till Braunschweig has no financial and non-financial interests. Aaron Babendreyer has no financial and non-financial interests. Jan Spillner has no financial and non-financial interests. Sebastian Kalverkamp has no financial and non-financial interests. Thomas Schröder has no financial and non-financial interests. Manfred Moeller has no financial and non-financial interests. Mark Coburn has no financial and non-financial interests. Stefan Uhlig has no financial and non-financial interests. Christian Martin has no financial and non-financial interests. Annette D Rieg has no financial and non-financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suleiman, S., Klassen, S., Katz, I. et al. Argon reduces the pulmonary vascular tone in rats and humans by GABA-receptor activation. Sci Rep 9, 1902 (2019). https://doi.org/10.1038/s41598-018-38267-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-38267-y
This article is cited by
-
Platelet-derived growth factor (PDGF)-BB regulates the airway tone via activation of MAP2K, thromboxane, actin polymerisation and Ca2+-sensitisation
Respiratory Research (2022)
-
Update of the organoprotective properties of xenon and argon: from bench to beside
Intensive Care Medicine Experimental (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.









 ) argon (n = 5). B) PA: (
) argon (n = 5). B) PA: ( ) ET-1 100 nM/argon (n = 5). (A,B) Statistics were performed by a LMM. *p < 0.05 and ***p < 0.001.
) ET-1 100 nM/argon (n = 5). (A,B) Statistics were performed by a LMM. *p < 0.05 and ***p < 0.001.