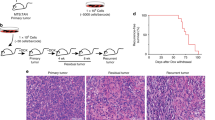
Abstract
Chemotherapy can alter the makeup of a tumor cell population by exerting selection pressure. We examined the change in Shannon index, a mathematical diversity measure used in ecology, for c-MYC copy number variation (CNV) after neoadjuvant chemotherapy and evaluated its clinical significance in breast cancer. Associations between Shannon indices for c-MYC CNV in pre- and post-neoadjuvant chemotherapy breast cancer samples and clinicopathologic features of tumors as well as patient survival were analyzed in 144 patients. A change in c-MYC amplification and copy number gain status was found in 14.3% and 33.6% with most cases showing positive to negative conversion. The chemo-sensitive group showed a significant decrease in Shannon index after neoadjuvant chemotherapy. However, there was no difference in diversity indices between pre- and post-neoadjuvant chemotherapy specimens in the chemo-resistant group. In survival analyses, high Shannon indices for c-MYC CNV in post-neoadjuvant chemotherapy samples as well as those in pre-neoadjuvant chemotherapy samples were revealed as independent prognostic factors for poor disease-free survival not only in the whole group but also in the chemo-resistant subgroup. These findings suggest that a change in Shannon index for c-MYC CNV after neoadjuvant chemotherapy reflects chemo-responsiveness and that Shannon indices after neoadjuvant chemotherapy have a prognostic value in breast cancer patients who receive neoadjuvant chemotherapy.
Similar content being viewed by others
Introduction
Intratumoral heterogeneity refers to the presence of phenotypically and/or genetically distinct tumor cell populations within a tumor that may result in tumor progression1,2 and therapeutic resistance3. With recent technical advances including next generation sequencing, large amounts of data on intratumoral heterogeneity have accumulated, and our understanding of intratumoral heterogeneity and tumor evolution has increased. Most studies thus far have confirmed that intratumoral heterogeneity exists in many tumors, revealing driver genetic events and evolutionary mechanisms4,5,6,7,8. However, only a few have investigated the impact of intratumoral heterogeneity on treatment response or clinical outcome9,10, partly due to the difficulties with quantifying intratumoral heterogeneity via bioinformatics approach. Besides a whole genomic approach, a diversity index calculation-based approach using copy numbers of specific genetic loci is also promising in measuring intratumoral heterogeneity and correlating it with tumor progression11,12,13. Recently, we examined c-MYC copy number variation (CNV) in two cohorts of invasive breast cancer patients using an ecological diversity index, the Shannon index, and we found that a high Shannon index for c-MYC was a significant poor prognostic factor, which suggests that a diversity index of even a single gene can be a measure of intratumoral heterogeneity and can be used as a prognostic indicator14.
Intratumoral clonal heterogeneity can be altered when tumor cells undergo selection pressure driven by chemotherapy, the immune system, hypoxia, nutrient deprivation, or geographic isolation; the most potent selection pressure may be attributed to chemotherapy15. It has been suggested that chemotherapy imposes a bottleneck effect in the process of tumor progression16, which in turn, leads to a different composition of tumor cell populations in residual tumors after chemotherapy compared to those prior to treatment. However, comparative analyses of intratumoral heterogeneity in pre- and post-chemotherapy samples remain scarce in breast cancers. Previously, Almendro et al. analyzed cellular heterogeneity with genetic and phenotypic features as well as the spatial distribution of tumor cells in pre- and post-neoadjuvant chemotherapy breast cancer17. They reported that there was no change in genetic diversity after chemotherapy, contradicting the general concept that chemo-sensitive subclones regress and resistant ones persist after chemotherapy, thereby leading to decreased genetic diversity after treatment. Although it was a comprehensive study incorporating genetic and phenotypic diversity in breast cancer pre- and post-neoadjuvant chemotherapy, patient survival in relation to cellular diversity after chemotherapy was not evaluated.
Here, we focused on the relationship between chemo-responsiveness and the changes in diversity index during chemotherapy using c-MYC which is located at one of the most unstable chromosomal regions (8q24) and frequently harbors copy number gain or amplification in breast cancer regardless of subtype18,19,20, in an attempt to find the clinicopathologic significance of post-treatment genetic diversity.
Results
Clinicopathologic characteristics
Of the 144 patients, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were positive in 107 (74.3%), 90 (62.5%), and 31 (21.5%) patients, respectively. Luminal B subtype was the largest subtype comprising 44.4% of the cases. As for the chemotherapeutic regimen, 42 (29.2%) patients received the ‘AC’ regimen consisting of doxorubicin and cyclophophamide for 4 to 6 cycles, 70 (48.6%) patients received the ‘ACT’ regimen of 4 cycles of AC followed by 4 cycles of docetaxel, and lastly, the remaining 32 (22.2%) patients were treated with the ‘AD’ regimen of doxorubicin and docetaxel 3 to 6 cycles. Table 1 lists the baseline characteristics.
c-MYC CNV before and after neoadjuvant chemotherapy
In pre-neoadjuvant chemotherapy biopsy specimens, evaluation of c-MYC CNV was possible in 119 cases. c-MYC amplification was present in 18 (15.1%) cases while c-MYC copy number gain was present in 64 (53.8%) cases. Of the 144 post-neoadjuvant chemotherapy samples, c-MYC amplification and copy number gain were detected in 11 (7.6%) and 58 (40.3%) cases, showing decreased frequencies in post-neoadjuvant chemotherapy samples compared to pre-neoadjuvant chemotherapy samples (P = 0.054, P = 0.029, respectively). None of the cases showed c-MYC copy number loss in pre- and post-neoadjuvant chemotherapy samples.
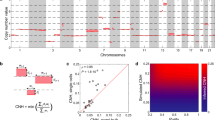
Table 2 shows paired analyses of c-MYC CNV before and after neoadjuvant chemotherapy. Among the 119 patients in whom comparison between pre- and post-neoadjuvant chemotherapy samples was possible, a change in c-MYC amplification and copy number gain status between pre- and post-neoadjuvant chemotherapy specimens was found in 17 (14.3%) and 40 (33.6%) cases, respectively. Of the 17 patients with altered c-MYC amplification status, 13 showed conversion from amplified to non-amplified status, and 4 revealed conversion from non-amplified to amplified status (Fig. 1). All of the four cases showing alteration from a non-amplified to amplified status belonged to the chemo-resistant group (10.5% vs. 0%, chemo-resistant group vs. chemo-sensitive group; P = 0.009). Of the 40 patients with alterations in c-MYC copy number gain status, 30 showed changes from a positive to negative status, and 10 revealed changes from a negative to positive status.
Shannon index for c-MYC CNV before and after neoadjuvant chemotherapy
Shannon index calculated with c-MYC CNV ranged from 0.786 to 2.496 (median 1.340) in pre-neoadjuvant chemotherapy samples, and it ranged from 0.120 to 2.772 (median 1.231) in post-neoadjuvant chemotherapy samples. Shannon indices in pre- and post-ST samples showed a weak positive correlation (r = 0.431, P < 0.001; Supplementary Fig. S1). When we compared the changes in Shannon indices after neoadjuvant chemotherapy, they were decreased in the post-neoadjuvant chemotherapy samples compared to the pre-neoadjuvant chemotherapy samples as a whole (1.426 ± 0.443 vs. 1.321 ± 0.435, pre-neoadjuvant chemotherapy vs. post-neoadjuvant chemotherapy; P = 0.016, paired sample t-test; Fig. 2A). We also evaluated the change in Shannon indices according to chemo-responsiveness. The chemo-sensitive group showed a significant decrease in Shannon index after neoadjuvant chemotherapy (1.451 ± 0.441 vs. 1.266 ± 0.386, pre-neoadjuvant chemotherapy vs. post-neoadjuvant chemotherapy; P < 0.001, paired sample t-test; Fig. 2B) while the chemo-resistant group showed no difference in Shannon indices between pre- and post-neoadjuvant chemotherapy specimens (1.375 ± 0.449 vs. 1.440 ± 0.510; P = 0.385, paired sample t-test; Fig. 2C). However, there was no difference in Shannon indices after neoadjuvant chemotherapy between the chemo-resistant group and chemo-sensitive group (1.394 ± 0.573 vs. 1.287 ± 0.105; P = 0.242, independent sample t-test).
Change in Shannon indices for c-MYC copy number variation (CNV) after neoadjuvant chemotherapy according to chemo-responsiveness. (A) In the whole group, Shannon indices for c-MYC CNV after neoadjuvant chemotherapy (NAC) are significantly decreased compared to those before NAC. (B) In the chemo-sensitive group composed of Miller-Payne regression grade 3 and 4, Shannon indices are significantly decreased in post-NAC samples compared to pre-NAC samples. (C) In the chemo-resistant group including Miller-Payne regression grade 1 and 2, there is no difference between Shannon indices before and after NAC. The box shows the first to third quartiles and the horizontal line inside the box represents the median. The whiskers extend to minimum and maximum values within 1.5 times the interquartile range from the first and third quartiles, and the outliers are represented by small circles.
Clinicopathological characteristics of tumors according to c-MYC CNV
We also evaluated the association between c-MYC CNV and clinicopathological characteristics of tumors before and after neoadjuvant chemotherapy (Supplementary Tables S1–S2). In pre-neoadjuvant chemotherapy status, c-MYC copy number gain was associated with high histologic grade (adj. P = 0.016), high Ki-67 index (adj. P = 0.004), and p53 overexpression (adj. P = 0.016). High Shannon index for c-MYC CNV (above median) in pre-neoadjuvant chemotherapy specimens was associated with high histologic grade (adj. P = 0.008), high Ki-67 index (adj. P = 0.016), and p53 overexpression (adj. P = 0.032). However, c-MYC amplification in pre-neoadjuvant chemotherapy samples was not associated with any of the clinicopathologic characteristics.
In post-neoadjuvant chemotherapy specimens, c-MYC amplification was associated with high yp T stage (adj. P = 0.027). c-MYC copy number gain in post-neoadjuvant chemotherapy specimens was associated with high histologic grade (adj. P < 0.001) and high Ki-67 index (adj. P = 0.009). High Shannon index (above median) in post-neoadjuvant chemotherapy specimens showed a significant association with high histologic grade (adj. P = 0.009) and high Ki-67 index (adj. P = 0.027).
Prognostic significance of c-MYC CNV before and after neoadjuvant chemotherapy
Most of the patients were treated according to standard guidelines and were followed up regularly. One hundred and nineteen (82.6%) patients received radiation therapy, and 105 (72.9%) received endocrine therapy following surgery. The Kaplan-Meier plots for disease-free survival according to c-MYC CNV before and after neoadjuvant chemotherapy are shown in Fig. 3. In pre-neoadjuvant chemotherapy specimens, c-MYC amplification and copy number gain were not associated with disease-free survival of the patients (P = 0.116, P = 0.098; adj. P = 0.348, adj. P = 0.294, respectively), and a high Shannon index using a cutoff value obtained by ROC analyses was significantly associated with poor disease-free survival (P = 0.014; adj. P = 0.042). In post-neoadjuvant chemotherapy specimens, a high Shannon index showed a significant association with poor disease-free survival (P = 0.006; adj. P = 0.018) and c-MYC amplification and copy number gain were not associated with disease-free survival of the patients (P = 0.117, P = 0.113; adj. P = 0.351, adj. P = 0.339, respectively).
Kaplan-Meier survival analyses based on c-MYC copy number variation (CNV) before and after neoadjuvant chemotherapy. In the pre-neoadjuvant chemotherapy (NAC) samples, c-MYC amplification (A) and copy number gain (B) were not associated with disease-free survival of the patients, while a high Shannon index for c-MYC CNV (C) shows a significant association with poor disease-free survival. Similarly, in the post-NAC samples, c-MYC amplification (D) and copy number gain (E) were not associated with disease-free survival, and a high Shannon index (F) shows a significant association with poor disease-free survival.
Upon subgroup analysis according to hormone receptor status, high Shannon indices before and after neoadjuvant chemotherapy was associated with adverse clinical outcome only in hormone receptor-positive group (P = 0.004, P = 0.027, respectively; Fig. 4A,B) and not in hormone receptor-negative subgroup (P = 0.961, P = 0.411, respectively; Fig. 4C,D). In subgroup analysis by molecular subtype, high Shannon indices in pre-neoadjuvant chemotherapy samples were associated with poor disease-free survival (P = 0.012) in luminal B subtype, but those in post-neoadjuvant chemotherapy samples did not show an association with survival of the patients in any subtypes.
Kaplan-Meier survival analyses based on Shannon index for c-MYC copy number variation before and after neoadjuvant chemotherapy in subgroups according to hormone receptor status. In hormone receptor-positive subgroup, high Shannon indices before (A) and after (B) neoadjuvant chemotherapy (NAC) are associated with poor disease-free survival. On the contrary, in hormone receptor-negative subgroup, Shannon indices before (C) and after (D) NAC do not show association with patient survival.
In subgroup analyses according to chemo-responsiveness, a high Shannon index after neoadjuvant chemotherapy revealed an association with poor disease-free survival in the chemo-resistant group (P = 0.026; Fig. 5A), and it was not associated with survival in the chemo-sensitive group (P = 0.121; Fig. 5B).
Kaplan-Meier survival analyses based on Shannon index for c-MYC copy number variation after neoadjuvant chemotherapy in subgroups according to chemo-responsiveness. (A) In chemo-resistant group, high Shannon index after neoadjuvant chemotherapy (NAC) shows a significant association with poor disease-free survival. (B) In chemo-sensitive group, it is not associated with disease-free survival of the patient.
Besides Shannon index for c-MYC CNV, high clinical N stage, pre- and post-neoadjuvant chemotherapy negative ER and PR status, and high Ki-67 index were poor prognostic indicators (Supplementary Table S3). In multivariate analyses, pre- and post-neoadjuvant chemotherapy high Shannon indices were revealed as adverse independent prognostic factors along with high N stage and negative hormone receptor status (Table 3).
Discussion
In locally-advanced breast cancer, neoadjuvant chemotherapy is considered standard treatment. The degree of response to neoadjuvant chemotherapy reflects chemo-sensitivity of a tumor to a certain chemotherapeutic agent and is associated with patient outcome; thus, patients who achieve pathologic complete response to neoadjuvant chemotherapy show excellent disease-free and overall survival21,22. With regards to intratumoral heterogeneity, a genetically homogenous population is expected to achieve complete response more frequently than genetically heterogeneous tumors. In residual disease after neoadjuvant chemotherapy, the partially-responsive tumors would remain as genetically homogeneous tumors due to a population bottleneck effect and reconstitution by the resistant clones while the resistant tumors would show no change in genetic diversity even after chemotherapy23. Thus, in this study, we tried to find the relationship between chemo-responsiveness and change in diversity index after neoadjuvant chemotherapy. To evaluate chemo-responsiveness, we adopted the Miller-Payne regression grading system24, which well matches the design of this study as it is based on the reduction in the proportion of tumor cellularity compared to pre-treatment primary tumor samples. We found that the Shannon index for c-MYC CNV generally decreased in the post-neoadjuvant chemotherapy samples compared to the pre-neoadjuvant chemotherapy samples in the whole group. In subgroup analyses according to chemo-responsiveness, the chemo-sensitive group showed a more significant decrease in Shannon index after neoadjuvant chemotherapy, whereas there was no difference in the Shannon indices between pre- and post-neoadjuvant chemotherapy samples in the chemo-resistant group. Thus, our study supports the current paradigm that a chemo-sensitive tumor is reconstituted by a relatively homogeneous tumor cell population and shows low genetic diversity after chemotherapy. More importantly, we showed that the change in Shannon index after neoadjuvant chemotherapy is indicative of chemo-responsiveness.
Previously, Almendro et al. analyzed diversity indices using probes for 8q24, 10p13, 16p13.3, and 20q13.31 in pre- and post-neoadjuvant chemotherapy samples of breast cancer patients; they showed that genetic diversity did not change after chemotherapy17. Chemotherapeutic agents cause DNA damage and cell cycle arrest, leading to genomic instability. Combined effects of decreased genetic diversity from selection pressure and increased genomic instability from genotoxicity of chemotherapeutic agents can result in no change in diversity index after chemotherapy. However, in their study, the number of cases was limited to only 43 cases, and the patients did not receive uniform treatment with some patients even receiving trastuzumab in combination with other chemotherapeutic agents. Moreover, they adopted clinical response over pathologic response in evaluation of chemoresponse. Generally, tumor cellularity decreases after chemotherapy; however, this decrease in tumor cellularity does not always result in a decrease in tumor size25. The clinical response to neoadjuvant chemotherapy evaluated by imaging studies such as MRI does not equate to the pathological response26,27. Only by pathologic examination of the residual tumors in surgically-resected specimens can a tumor’s chemo-sensitivity be accurately evaluated. For this reason, direct comparison of the results of the aforementioned study by Almendro et al. and ours is not feasible. A further large-scale study in an evenly-treated population is warranted to validate the results of this study.
In this study, we revealed the prognostic significance of high Shannon index for c-MYC CNV before and after neoadjuvant chemotherapy. The adverse prognostic value of high Shannon index prior to neoadjuvant chemotherapy is in line with our previous study14 which showed the prognostic value of high Shannon indices for gene CNV using c-MYC and FGFR1 in treatment-naïve breast cancer patients. We believe this is the first study demonstrating the prognostic significance of post-neoadjuvant chemotherapy Shannon index using gene CNV in breast cancer. High Shannon index after neoadjuvant chemotherapy was proven an independent prognostic factor even after adjusting for Miller-Payne regression grade and post-neoadjuvant chemotherapy Ki-67 index, which are known to be associated with chemo-responsiveness and tumor recurrence after neoadjuvant chemotherapy24,28,29. Furthermore, the prognostic significance of high Shannon index after neoadjuvant chemotherapy was also found in the chemo-resistant subgroup.
In the post-neoadjuvant chemotherapy samples, high Shannon indices calculated with c-MYC CNV were associated with high Ki-67 index. As post-treatment Ki-67 is known to be higher in non-responders than responders28, the close relationship between Shannon index and Ki-67 index after neoadjuvant chemotherapy may suggest that a high Shannon index after neoadjuvant chemotherapy represents chemo-resistance. However, there was no statistical difference in Shannon indices after neoadjuvant chemotherapy between chemo-resistant and chemo-sensitive groups. Moreover, Shannon indices prior to and after neoadjuvant chemotherapy was commonly associated with adverse features of breast cancer including high histologic grade and high Ki-67 index, and they were correlated with each other. Thus, it seems that increased Shannon index after neoadjuvant chemotherapy not only represents chemo-resistance but also the intrinsic aggressiveness of the tumor, thereby encompassing poor prognostic impact in breast cancer patients who receive neoadjuvant chemotherapy.
In paired analyses of pre- and post-neoadjuvant chemotherapy breast cancer samples, a significant number of cases showed alterations in c-MYC amplification or copy number gain status (14.3% for amplification; 33.6% for copy number gain) with most cases showing positive to negative conversion. Therefore, it is probable that only the tumor cell populations with c-MYC amplification or copy number gain underwent complete response to chemotherapy, and the residual tumor showed no amplification or copy number gain. However, there were some cases that showed negative to positive conversion as well. Interestingly, all the cases that changed from a non-amplified to amplified status belonged to the chemo-resistant group, and c-MYC amplification after neoadjuvant chemotherapy was associated with high ypT stage. It is impossible for a tumor to have acquired c-MYC amplification or copy number gain during neoadjuvant chemotherapy if it responded to treatment. However, if the tumor were resistant to chemotherapy, it may be possible to acquire c-MYC amplification or copy number gain during neoadjuvant chemotherapy. Also, re-diversification may have occurred during the period from the end of neoadjuvant chemotherapy to surgery. Perhaps a more likely explanation may be that there existed primary intratumoral heterogeneity in c-MYC amplification or copy number gain in the treatment-naïve tumors, and sampling bias of the pre-neoadjuvant chemotherapy biopsy specimens caused the negative to positive conversion after neoadjuvant chemotherapy.
In conclusion, Shannon index for c-MYC CNV decreased after neoadjuvant chemotherapy especially in the chemo-sensitive group, and a post-treatment high Shannon index was found to be an independent poor prognostic indicator, suggesting that the change in Shannon index after neoadjuvant chemotherapy reflects chemo-responsiveness and that Shannon index after neoadjuvant chemotherapy can be used as a prognostic factor in patients with breast cancer who receive neoadjuvant chemotherapy.
Methods
Patients and tissue samples
This study was a retrospective one that included 144 patients with clinical stage II (n = 65) or III (n = 79) breast cancer. The patients received breast conserving surgery/mastectomy at Seoul National University Bundang Hospital between October 2004 and December 2012 following anthracycline/anthracycline and taxane-based neoadjuvant chemotherapy and had residual disease in the surgical resection specimen. Pre-chemotherapeutic core needle biopsy was performed prior to neoadjuvant chemotherapy, and all cases were diagnosed with invasive breast cancer. Surgery was performed 3–4 weeks after the last cycle of chemotherapy. For each patient, a pair of formalin-fixed and paraffin-embedded tumor samples from a pre-chemotherapy biopsy specimen and a post-chemotherapy resection specimen was retrieved. Pre-neoadjuvant chemotherapy biopsy specimen was not available in 25 patients whose biopsy was performed at an outside hospital or whose samples were too small for further study. As a result, comparison of pre- and post-neoadjuvant chemotherapy samples was performed in 119 patients. Clinicopathologic information including age, sex, initial clinical T stage and N stage, chemotherapeutic regimen, number of neoadjuvant chemotherapy cycles, pathologic T stage and N stage after neoadjuvant chemotherapy, histologic subtype, histologic grade, and lymphovascular invasion was obtained from the medical records and H&E-stained sections. Pathologic response to neoadjuvant chemotherapy was evaluated by Miller-Payne regression grading system24. Of the 144 post-neoadjuvant chemotherapy samples, 12 (8.3%) cases belonged to Miller-Payne regression grade 1, 39 (27.1%) to grade 2, 91 (63.2%) to grade 3, and the rest 2 (1.4%) to grade 4. Miller-Payne regression grades 1 and 2 were regarded as chemo-resistant, non-responder group, and Miller-Payne regression grades 3 and 4 were regarded as chemo-sensitive, responder group. The study was approved by the institutional review board of Seoul National University Bundang Hospital (protocol # B-1601/332-304), which waived the requirement for informed consent. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Fluorescence in situ hybridization
Commercially available LSI c-MYC SpectrumOrange probe (8q24.12-q24.13) (Abbott Molecular, Downers Grove, IL, USA) was used for fluorescence in situ hybridization (FISH) of c-MYC. 4-μm-thick deparaffinized tumor tissue sections were incubated in pretreatment solution (Abbott Molecular) at 80 °C for 30 min first then in protease solution (Abbott Molecular) for 20 min at 37 °C. tDen-Hyb-2 hybridization buffer (InSitus Biotechnologies, Albuquerque, NM) was used for dilution of the probes. DNA denaturation was achieved by incubating the probes and the tissue sections in HYBriteTM (Abbott Molecular) for 5 min at 73 °C and hybridization at 37 °C for 16 hours. Post-hybridization washes were performed as per manufacturer’s instructions. Slides were mounted in 40, 6-diamidino-2-phenylindole/anti-fade, and a fluorescence microscope was used to view them.
The number of c-MYC signals per cell was counted in 100 tumor cells. c-MYC copy number gain was defined as an average copy number ≥3.0, c-MYC amplification as a copy number ≥6.0, and c-MYC copy number loss as a copy number < 1.6. To express diversity in c-MYC copy number per cell as a numerical value, we adopted the Shannon index, a diversity measure used in ecology, which estimates the number and distribution of species in a population. It is calculated as H’ = −∑ pi ln(pi), where pi equals the frequency of species i in the population30. A species, in this study, represents the tumor cells with the same copy number of c-MYC and pi represents the proportion of tumor cells with the same copy number of c-MYC. The Shannon index appears as any non-negative value with a high value indicating high diversity.
Immunohistochemical analyses of standard biomarkers
Expression of ER, PR, HER2, Ki-67, and p53 was evaluated at the time of diagnosis or during the study by immunohistochemistry (IHC). For ER and PR, positive expression was defined as nuclear staining in 1% or more tumor cells, and the expression level was recorded in 10% increments. HER2 considered positive when IHC revealed strong positive expression of 3+ or when gene amplification was observed in FISH for HER2 IHC 2+ cases. P53 overexpression was defined as positive staining in 10% or more tumor cells. Positive staining in 20% or more tumor cells was considered to have high Ki-67 proliferation index.
Classification of breast cancer subtypes was done in accordance with the 2011 St. Gallen Expert Consensus31: luminal A (ER+ and/or PR+, HER2−, Ki-67 < 14%), luminal B (ER+ and/or PR +, HER2−, Ki-67 ≥ 14%; ER+ and/or PR+, HER2+), HER2 + (ER−, PR−, HER2+), and triple-negative subtype (ER−, PR−, HER2−).
Statistical analysis
Statistical package, SPSS version 21.0 for Windows (IBM Corp., ARMONK, NY) was used for statistical analysis. Pearson’s chi-square test was used to compare categorical variables between two groups. Continuous variables between two independent groups were compared with independent sample t-test. Paired sample t-test was used to compare continuous variables between pre- and post-neoadjuvant chemotherapy samples. Cut-off values for Shannon index that maximized the sum of sensitivity and specificity in outcome prediction were determined from receiver operating characteristic (ROC) analysis. Kaplan-Meier survival curves were drawn for survival analysis, and the difference was analyzed by log rank test. Multivariate analysis was performed using the Cox proportional hazards regression model with a backward stepwise selection method. Hazard ratios (HR) as well as 95% confidence intervals (CI) were calculated for the statistically significant variables. When needed, corrections for multiple testing were performed with Bonferroni method and adjusted (adj.) P values were presented. P values less than 0.05 were considered significant with all reported P values being two-sided.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet 38, 468–473 (2006).
Merlo, L. M. et al. A comprehensive survey of clonal diversity measures in Barrett’s esophagus as biomarkers of progression to esophageal adenocarcinoma. Cancer Prev Res (Phila) 3, 1388–1397 (2010).
Turner, N. C. & Reis-Filho, J. S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol 13, e178–185 (2012).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366, 883–892 (2012).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Yates, L. R. et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nature medicine 21, 751–759 (2015).
Hernandez, L. et al. Genomic and mutational profiling of ductal carcinomas in situ and matched adjacent invasive breast cancers reveals intra-tumour genetic heterogeneity and clonal selection. J Pathol 227, 42–52 (2012).
Mroz, E. A. et al. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer 119, 3034–3042 (2013).
Morris, L. G. et al. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget 7, 10051–10063 (2016).
Park, S. Y., Gonen, M., Kim, H. J., Michor, F. & Polyak, K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest 120, 636–644 (2010).
Almendro, V. et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer research 74, 1338–1348 (2014).
Yang, F. et al. Intratumor heterogeneity predicts metastasis of triple-negative breast cancer. Carcinogenesis 38, 900–909 (2017).
Chung, Y. R. et al. Diversity index as a novel prognostic factor in breast cancer. Oncotarget 8, 97114–97126 (2017).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Findlay, J. M. et al. Differential clonal evolution in oesophageal cancers in response to neo-adjuvant chemotherapy. Nature communications 7, 11111 (2016).
Almendro, V. et al. Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell reports 6, 514–527 (2014).
Deming, S. L., Nass, S. J., Dickson, R. B. & Trock, B. J. C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. British journal of cancer 83, 1688–1695 (2000).
Adelaide, J. et al. Integrated profiling of basal and luminal breast cancers. Cancer research 67, 11565–11575 (2007).
Huppi, K., Pitt, J. J., Wahlberg, B. M. & Caplen, N. J. The 8q24 gene desert: an oasis of non-coding transcriptional activity. Frontiers in genetics 3, 69 (2012).
Chollet, P. et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. British journal of cancer 86, 1041–1046 (2002).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Navin, N. E. Tumor evolution in response to chemotherapy: phenotype versus genotype. Cell reports 6, 417–419 (2014).
Ogston, K. N. et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12, 320–327 (2003).
Choi, W. J. et al. Evaluation of the Tumor Response After Neoadjuvant Chemotherapy in Breast Cancer Patients: Correlation Between Dynamic Contrast-enhanced Magnetic Resonance Imaging and Pathologic Tumor Cellularity. Clinical breast cancer (2017).
Bouzon, A. et al. Diagnostic accuracy of MRI to evaluate tumour response and residual tumour size after neoadjuvant chemotherapy in breast cancer patients. Radiology and oncology 50, 73–79 (2016).
Burcombe, R. J. et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. British journal of cancer 92, 147–155 (2005).
Nishimura, R., Osako, T., Okumura, Y., Hayashi, M. & Arima, N. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast cancer 17, 269–275 (2010).
Guarneri, V. et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Annals of oncology: official journal of the European Society for Medical Oncology 20, 1193–1198 (2009).
Shannon, C. E. The mathematical theory of communication (Reprinted). M D Comput 14, 306–317 (1997).
Goldhirsch, A. et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Annals of oncology: official journal of the European Society for Medical Oncology 22, 1736–1747 (2011).
Acknowledgements
This study was funded by a grant from the Basic Science Research program through the National Research Foundation of Korea (NRF) from the Ministry of Science, ICT and Future planning (Grant No. NRF-2015R1A2A2A01007907) to Park S.Y. The authors thank Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Author information
Authors and Affiliations
Contributions
Y.R.C. participated in the interpretation and analysis of data and drafted the manuscript. H.J.K. carried out the experiments and participated in the interpretation of the data. M.K. and S.A. participated in the acquisition and interpretation of pathologic data. S.Y.P. conceived of the study, participated in its design, and was responsible for preparation of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, Y.R., Kim, H.J., Kim, M. et al. Clinical implications of changes in the diversity of c-MYC copy number variation after neoadjuvant chemotherapy in breast cancer. Sci Rep 8, 16668 (2018). https://doi.org/10.1038/s41598-018-35072-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-35072-5
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.