Abstract
Unprecedented mass coral bleaching events due to global warming and overall seawater pollution have been observed worldwide over the last decades. Although metals are often considered as toxic substances for corals, some are essential at nanomolar concentrations for physiological processes such as photosynthesis and antioxidant defenses. This study was designed to elucidate, the individual and combined effects of nanomolar seawater enrichment in manganese (Mn) and iron (Fe), on the main physiological traits of Stylophora pistillata, maintained under normal growth and thermal stress conditions. We provide, for the first time, evidence that Mn is a key trace element for coral symbionts, enhancing cellular chlorophyll concentrations, photosynthetic efficiency and gross photosynthetic rates at ambient temperature. Our experiment also highlights the key role of Mn in increasing coral resistance to heat stress-induced bleaching. While Mn-enriched corals did not bleach and did not reduce their rates of photosynthesis and calcification, control corals experienced significant bleaching. On the contrary to Mn, Fe enrichment not only impaired calcification but induced significant bleaching. Such information is an important step towards a better understanding of the response of corals to seawater enrichment in metals. It can also explain, to some extent, species susceptibility to environmental stress.
Similar content being viewed by others
Introduction
Coral reefs are one of the most biodiverse, complex and productive ecosystems on Earth1,2. Over the last decades, rising sea surface temperatures, owing to global warming, have triggered unprecedented mass bleaching events, during which corals lose their symbiotic algae and then undergo nutrient starvation, decreased growth and possible mortality (e.g.)3. Thermal stress-induced bleaching is often due to an over-production of damaging reactive oxygen species (ROS)4. Levels of ROS within the coral tissue are usually regulated by antioxidant enzymes, such as superoxide dismutase (SOD), peroxidases and catalases5,6,7. These enzymes can either neutralize free radicals of ROS by accepting or donating electron(s) to eliminate the unpaired condition of the radical or may directly react with the radicals and destroy them, or make them less reactive8. During thermal stress, ROS exceed the enzymes’ ability to detoxify them, leading to the direct damage of proteins, lipids or DNA4,9.
In addition to global change-related disturbances, coral reefs have also to cope with the local deterioration of the seawater quality, due to increased pollution in inorganic and organic nutrients, particle loads and sedimentation10. Many reefs around the world (e.g. Costa Rica, Panama, Red Sea, Thailand, New Caledonia) are also exposed to elevated metal concentrations11,12,13,14,15,16,17, brought by urban storm water run-off, industrial effluents, mining-operations and atmospheric contaminants18. Experimental studies have emphasized the harmful effect of high concentrations of metals on coral reproduction and early life stages19,20,21,22,23,24. The only three studies which have focused on the combined effects of thermal stress and metal pollution (copper and nickel)12,25,26, have highlighted an increased bleaching susceptibility of adult corals under metal pollution13,26 and a reduced thermal tolerance of coral larvae25.
Although many metals, such as mercury27, copper28 or lead21, are toxic for living organisms, even at nanomolar concentrations, some of them can play key roles in the functionning of photosynthetic organisms (e.g. phytoplankton, plants, algae) (reviewed by29). For example, manganese (Mn) and iron (Fe), whose concentrations in the tropical and subtropical coastal seawaters can reach 10.8 µg L−1 and 2.61 µg L−1, respectively30,31,32,33, are key elements of photosynthetic molecules. Mn is indeed an essential component of the Oxygen Evolving Complex (OEC) of photosystem II, whereas Fe is needed for the structure of the chlorophyll, of the photosystems I and II, the cytochrome b6f complex, and the ferredoxin34,35,36. Both are also cofactors of the antioxidant enzymes, such as superoxide dismutase (MnSOD and FeSOD)37,38, which continuously scavenge ROS within the coral tissue as described above37,38,39,40. With the exception of a single coral study, showing that iron limitation increases coral bleaching susceptibility41, all others have been performed on plants or phytoplankton. They showed that metal limitation impairs the photosynthetic efficiency of photosystem II34,42,43 and enhances the oxidative load, by decreasing antioxidant enzymes’ activity and ROS consumption42.
The aim of this work was to investigate the individual and combined effects of different levels of manganese (Mn) and iron (Fe), on the main physiological traits of the scleractinian coral species Stylophora pistillata, maintained under ambient temperature and thermal stress conditions. The hypotheses were that i) natural, in situ concentrations of Fe and Mn are not a limiting factor for coral productivity under ambient temperature conditions; ii) Fe and Mn can become limiting nutrients for coral productivity due to an increase need of metals for the synthesis of antoxidant enzymes or for chlorophyll repair. We thus hypothesize that an enrichment in Mn and/or Fe can increase the coral resistance to bleaching; iii) we also hypothesize that a combined enrichment in manganese and iron has additive effects compared to an enrichment in either manganese or iron alone.
Results
Multivariate analysis
Principal Coordinate Analysis (PCoA) comparison of coral physiological traits maintained under the eight Mn-Fe-temperature conditions revealed a similar response between duplicated tanks (Fig. 1). PCoA yielded two principal components, PCO1 and PCO2 that explain 51% and 28% of the total variance respectively. The first component (PCO1), is defined by positive correlations between Mn enrichment and photosynthetic (Pg, rETRmax and Fv/Fm, Chl concentrations) or growth parameters (calcification, growth rates) (r > 0.7). PCO1 also demonstrates that Fe enrichment at 32 °C has an opposite effect than manganese. The second component (PCO2) separates corals exposed to ambient and high temperatures and is defined by high weights from the “respiration” parameter (r > 0.7).
Principal Coordinate Analysis (PCoA) comparison of colony physiological traits maintained during six weeks either at 26 (AT) or 32 °C (HT) to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe). SD, rETRmax, Pg, Chl, Calci, Growth, Fv/Fm and R represent, respectively, the Symbiodinium density, the maximum electron transport rate, the gross photosynthetic rate, the chlorophyll concentration, the calcification rate, the growth rate, the photosynthetic efficiency and the respiration rate.
Effect of thermal stress (32 °C) alone on control corals (not exposed to metal enrichment)
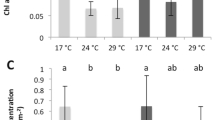
As expected, thermal stress decreased symbiont density, chlorophyll content and Pg by 18%, 11% and 56%, respectively, compared to 26 °C (ANOVA; p < 0.001; Figs 2A,B and 3). No thermal stress effect was observed on the Fv/Fm (ANOVA; p > 0.05; Fig. 4), but reduced rETRmax by 41% (ANOVA; p < 0.001; Fig. 5), respiration rates by 20% in all conditions (ANOVA; p < 0.05; Fig. 3), calcification and growth rates by 59% and 47% respectively (LSD test; p < 0.001; Figs 6 and 7).
Symbiodinium density (A) and total chlorophyll concentration (B) of Stylophora pistillata (mean ± SD, n = 3) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and < 0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Photosynthetic and respiration rates of Stylophora pistillata (mean ± SD, n = 3) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Stylophora pistillata photosynthetic efficiency (Fv/Fm) (mean ± SD, n = 5) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Relative electron transport rates (rETR) of Stylophora pistillata (mean ± SD, n = 5) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Stylophora pistillata calcification rates (mean ± SD, n = 3) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Stylophora pistillata growth rates (mean ± SD, n = 5) exposed during six weeks either at 26 or 32 °C to ambient manganese and iron concentrations (0.06 and <0.22 µg L−1) (Control); to higher manganese concentrations (4.1 µg L−1) (Mn); to higher iron concentrations (3.0 µg L−1) (Fe); and to both higher concentrations in manganese and iron (MnFe).
Combined effect of iron enrichment and thermal stress
At 26 °C, Fe enrichment decreased symbiont density by 11% compared to the control condition (LSD test; p < 0.05; Fig. 2A), but had no effect on chl content, Pg and respiration rates, as well as on the Fv/Fm and rETRmax (ANOVA; p > 0.05; Figs 2B, 3–5). Nevertheless, calcification and growth rates were decreased by ca. 28% compared to the control condition (LSD test; p < 0.01; Figs 6 and 7). No interactive effect between temperature and Fe enrichment was observed (ANOVA; p > 0.05). At 32 °C and under Fe enrichment, thermal stress reduced symbiont density by 35%, Pg rates and rETRmax by 65% and 45% respectively compared to those of Fe corals at 26 °C (ANOVA; p < 0.001; Figs 2A, 3 and 5). As well, thermal stress reduced growth and calcification rates by 40% and 59% respectively compared to those of Fe corals at 26 °C (LSD test; p < 0.001; Figs 6 and 7).
Combined effect of manganese or manganese-iron enrichment and thermal stress
At 26 °C, no effect of Mn or Mn-Fe enrichment was observed on symbiont density (Fig. 2A) and on the calcification and growth rates (LSD test; p > 0.05; Figs 6 and 7). However, both enrichments stimulated chlorophyll concentrations by 30%, as well as Pg and rETRmax by 69% and 29% compared to the controls at 26 °C (ANOVA; p < 0.001; Figs 2B, 3 and 5). Fv/Fm was also enhanced by ca. 40% (LSD test; p < 0.001; Fig. 4).
No interactive effect was observed between temperature and Mn or Mn-Fe enrichment on the tissue parameters, Pg or respiration rates and the Fv/Fm (ANOVA; p > 0.05, Figs 2A,B, 3 and 4). Although chlorophyll concentrations were stimulated by 36% in Mn enriched colonies at 32 °C compared to the ones of control corals at 26 °C (ANOVA; p < 0.001; Fig. 2B), thermal stress reduced the symbiont density by 34% and 13% in Mn and MnFe corals, Pg by 52% and 41% and rETRmax by 39% and 44% compared to those of control corals at 26 °C (ANOVA; p < 0.001; Figs 2A, 3 and 5). However, Pg and rETRmax remained significantly higher (29% and ca. 79%, respectively) than those of control corals submitted to the same thermal stress (ANOVA; p < 0.001; Figs 3 and 5). Finally, a significant interaction was observed between Mn and temperature on calcification and growth rates (ANOVA; p < 0.01, Figs 6 and 7). Both rates were enhanced by thermal stress and Mn addition compared to control corals (LDS test; p < 0.001). Mn enriched colonies at high temperature maintained the same calcification and growth rates than at 26 °C (LSD test; p > 0.05). At 32 °C, Fe however interacted with Mn enrichment as MnFe enriched corals decreased their calcification rates by 21% compared to those of Mn enriched corals under high temperature (LSD test; p < 0.05).
Discussion
While massive bleaching events intensify around the world, there is a need to identify the environmental factors, which can increase the resistance and resilience of corals to thermal-stress induced bleaching. Here, we provide the first evidence that Mn is a key trace element for the coral - Symbiodinium association. It directly enhances symbiont photosynthesis, and indirectly host metabolism, since symbionts translocate most of their photosynthates to the animal host. Mn thus mitigates the negative impact of short-term thermal stress on coral bleaching. We have also observed that seawater enrichment with 3 µg Fe L−1 induced a decrease in calcification rates and counteracts the positive effects of Mn on coral bleaching. Finally, we did not find any link in this study between rates of photosynthesis and calcification, suggesting that inorganic carbon may be a limiting factor for corals under manganese enrichment.
Results obtained in this study first clearly show that Mn is a limiting nutrient for coral photosynthesis, at natural seawater concentrations (0.06 µg Mn L−1) such as those measured outside New Caledonia’s barrier reef30. Photosynthetic efficiency (Fv/Fm, rETRmax) as well as chlorophyll concentrations and gross photosynthetic rates were all enhanced under 4 µg Mn L−1 enrichment, suggesting that corals have high requirements in Mn. Although such Mn stimulation has been previously documented for phytoplankton photosynthesis44, it was unknown for Symbiodinium in hospite. Manganese is involved in several photosynthetic processes, which overall explain the higher rates of carbon fixation observed under normal growth conditions. This metal enters into the composition of the oxygen-evolving complex (OEC) of photosystem II45,46,47, which splits water molecules into electrons, protons and oxygen and shuttles the electrons into the photosystem II reaction center. Mn is also directly involved in chlorophyll synthesis, via the isoprenoid biosynthetic pathway, as demonstrated in phytoplankton and plants48,49,50.
Our experiment also highlights the key role of Mn in increasing coral resistance to heat stress-induced bleaching. At high temperature, Mn-enriched corals presented higher chlorophyll concentrations, photosynthetic and calcification rates than control corals, which experienced significant bleaching, likely due to high oxidative stress5,51. In addition to enter into the composition of photosynthetic molecules, Mn is a key component of anti-oxidant enzymes, such as manganese superoxide dismutase (MnSOD)52. This metallo-enzyme, located in Symbiodinum53,54, is used to eliminate ROS, which are continuously produced through photosynthesis4. SOD thus protects the whole symbiotic association from photosynthetic and thermal stress-induced ROS, and thereby avoids chlorophyll degradation55,56 (as observed in the present study) and likely DNA damage, protein unfolding and lipid peroxidation4,9. Moreover, when Mn replace zinc at the active site of the carbonic anhydrase molecule, this later shows a peroxidase activity with bicarbonate-dependent activity57,58. As such, it can detoxify the H2O2 produced during thermal stress inside the coral cells. Our results therefore show that bleaching is delayed by at least two weeks in presence of manganese but the long-term effect of manganese on the bleaching susceptibility of corals remains to be tested.
Although Mn enrichment allowed corals to maintain maximal rates of photosynthesis and calcification during thermal stress, we did not observe in this study a coupling between photosynthesis and calcification. Following the stimulation of photosynthesis, we could have expected to observe an increase in the rates of short- and/or long-term calcification. Such correlation between photosynthesis and calcification is well known59. Calcification rates of scleractinian corals are indeed enhanced in the light compared to the dark and this process is called light enhanced calcification (reviewed by59). This can be primarily attributed to the photosynthetic activity of the symbionts, which has several impacts, such as the provision of essential molecules for the organic matrix synthesis or the elevation of pH at the site of calcification59,60,61. A link between photosynthesis and calcification has been observed with nickel enrichment, another trace element, which stimulated calcification in Pocillopora damicornis and Acropora muricata directly and indirectly through the stimulation of photosynthesis62. The lack of calcification enhancement in manganese-enriched corals at 26 °C could be explained by a potential limitation in dissolved inorganic carbon (DIC) or a competition for DIC between photosynthesis and calcification, as already observed in previous experiments involving nutrient, iron or cobalt enrichments12,63,64,65. Overall, corals living in a DIC scarce environment may experience DIC limitation and invest a great deal of energy into concentrating carbon at the photosynthesis site66.
Varying effects of iron exposure on corals have been observed in the literature and seem to depend on its concentration in seawater. As iron is involved in metabolic processes such as nitrogen fixation, antioxidant defenses, photosynthetic electron transfer or chlorophyll biosynthesis7,38,56, a complete lack of iron in seawater was shown to induce coral bleaching41. A slight enrichment (0.28 µg L−1) enhanced photosynthesis, but significantly decreased calcification, likely due to a competition between these two processes for dissolved inorganic carbon (DIC65). The highest enrichment used in this study (3 µg L−1), comparable to concentrations measured in some reefs along the New Caledonia’s coast30, not only impaired calcification, but induced significant bleaching. Wells et al.36 showed that the intracellular ROS production within S. pistillata endosymbionts was increased when exposed to Fe-enriched seawater. Such increased ROS level can explain the bleaching observed in this experiment and the subsequent decrease in calcification rate. Indeed, the loss of symbionts induces starvation and decreased energy, which is essential for calcification.
Two interesting interactions between iron and manganese enrichment were observed in this study. A synergetic interaction between Mn and Fe enrichments, which stimulated the photosynthetic efficiency, has been recorded. This observation suggests that Symbiodinium photosynthesis is more dependent on the quantity of available electron brought by the O2 evolving complex (Mn-dependent) than by molecules ensuring the transfer of these electrons (Fe-dependent), since an enrichment only in Fe does not modify the rates of photosynthesis. In addition, there was a strong negative effect of Fe on coral calcification, since Fe enrichment partially inhibited the positive effect of manganese on calcification rates under thermal stress.
In summary, our paper highlights the importance of taking into account seawater metal concentrations to explain the heat-stress induced changes in coral physiology. Overall, Mn appeared as a limiting nutrient for coral photosynthesis and antioxidant capacity. Mn enrichment thus increased rates of photosynthesis and calcification, and decreased the bleaching susceptibility of S. pistillata (Fig. 8). According to our results, high Mn concentrations in seawater can potentially explain the between-reef variability in bleaching susceptibility. Different uptake or assimilation rates of Mn between coral species can also explain species-specific bleaching. For example, reefs in New Caledonia, which have the specificity to be located in Mn-enriched waters, experienced their first massive bleaching event only in February 2016. However, the degree heating weeks (DHW) had yet already exceeded 8 °C-Weeks (threshold values normally leading to widespread bleaching and mortality) several times in the past (in 1980, 1996, 2005) without causing coral bleaching (https://coralreefwatch.noaa.gov/satellite/vs/melanesia.php#Amedee_NewCaledonia). More in-depth studies are however needed to confirm or rule out this hypothesis. In addition, this study has explored short-term exposure time to Mn enrichment, and/or to thermal stress. More studies are needed to refine the role of Mn enrichment in the mitigation of coral bleaching. Indeed, responses of corals are highly dynamic and tend to change with increasing accumulation of stress; the beneficial role of Mn on symbiont’s photosynthesis may therefore change with the length or the amplitude of the stress. Otherwise, it has also to be noticed that metal pollution is never based on a unique metal enrichment, and we clearly showed that metal interactions have to be considered when assessing the response of corals to thermal stress. Fe enrichment significantly impaired coral calcification, through processes that remain to be investigated. Finally, in the future, reefs will also experience increased ocean acidification, which not only will impact corals directly, but also indirectly through changes in the metal water chemistry67. Additional experiments, simultaneously testing the effects of acidification and warming on manganese uptake rates are thus needed to provide a better understanding of the metal role in the limitation of coral bleaching phenomenon.
Schematic diagram summarizing the effects of a manganese enrichment on adult coral physiology under ambient temperature or thermal stress. Mn increases coral resistance to heat stress-induced bleaching by boosting all physiological parameters compared to control corals, which experienced significant bleaching likely due to oxidative stress. The fact that (1) Mn enters into the composition of the oxygen-evolving complex (OEC) of photosystem II, (2) Mn is directly involved in chlorophyll synthesis and (3) Mn is also a key component of anti-oxidant enzymes, such as Mn superoxide dismutase (MnSOD) (used to eliminate ROS during thermal stress), justify the stimulating properties of this metal.
Methods
Coral collection and experimental setup
Effects of manganese and iron enrichment alone or together associated with a thermal stress on coral physiology were assessed on the scleractinian coral, Stylophora pistillata, originating from the Red Sea and grown in aquaria at the Centre Scientifique de Monaco. To perform this experiment, 384 apex (2-cm long) (i.e. microcolonies) were sampled from six large mother colonies. Microcolonies were then hung on nylon wires, evenly distributed in 16 tanks (25 L) before the three weeks recovering period under controlled conditions. To improve recovery, corals received nauplii of Artemia salina twice a week. Tanks were supplied with filtered seawater pumped from 40 meters depth, renewed at a rate of 12 L h−1. Temperature (26 or 32 ± 0.2 °C) was controlled all along the experiment by heaters connected to Elli-Well PC 902/T controllers. Corals was illuminated by eight 400 W metal halide lamps (HPIT, Philips) at an irradiance of 200 ± 10 µmol photons m−2 s−1 (photoperiod was 12 h:12 h light:dark). Once a week, light was controlled by a LI-COR data logger (LI- 1000) connected to a spherical quantum sensor (LI-193).
After the recovery, eight experimental conditions in a cross factor design (with manganese, iron and temperature as factors) were tested in duplicate. In other words, 4 metal conditions under two temperatures (26 °C and 32 °C) were achieved in duplicate: 1) control (C), with 0.06 ± 0.05 μg Mn L−1 68 and < 0.22 μg Fe L−1 65, 2) manganese enrichment (4.1 ± 0.75 μg Mn L−1), 3) iron enrichment (3.00 ± 0.2 µg Fe L−1) and 4) manganese-iron enrichment at the two above concentrations (MnFe) (Supplementary Fig. S1 and Table 1). Concentrations were chosen to be ecologically relevant and correspond to the highest concentration measured along the coast of New-Caledonia30.
Two peristaltic pumps (ISMATEC) ensured the metal enrichments by continuously supplying the experimental tanks with 15 mL h−1 of a solution of stable manganese (MnCl2, Humeau, France) or stable iron (FeCl2, Humeau, France). In order to avoid any concentration gradient in the tank and to ensure a good seawater brewing, tanks were equipped with a submersible pump (Aquarium system, micro-jet MC 320, Mentor, OH, USA). After three weeks under metal enrichment, temperature was gradually increased during one week up to 32 °C (1 °C per day) in two tanks of each metal condition and maintained for an additional two weeks without stopping metal enrichment. Photosynthetic efficiency, growth rates, calcification rates, and tissue parameters (zooxanthellae density, chlorophyll and protein concentration) were measured at the beginning of the experiment, after three weeks of metal enrichment and at the end of the two weeks of thermal stress.
Metals enrichment were realized with their divalent forms, Fe2+ and Mn2+, because they are the most soluble form of these metals and are considered as the most bioavailable69,70. While Mn2+ remains largely bioavailable in the time due to its very long half-life time71, the half-life of the Fe2+ ions is only of few minutes at 30 °C72,73. Thus, in order to be sure that the concentrations in the tanks match with the concentrations desired, seawater samples have been realized in each tank to measure metal concentrations. Samples were filtered using a 0.2 µm filter and were acidified with 1% of ultrapure concentrated nitritic acid (HNO3−), before sending for analysis (see supplementary materials for values).
Concentrations of Mn and Fe were determined by sector field inductively coupled plasma-mass spectrometry (SF-ICP-MS) using a Thermo Element 2 instrument. Mass spectra were collected in medium resolution and MeOH was added to enhance ionization. Based on repeat measurements, as well as external and internal standards analytical uncertainty was estimated to be better than 5%. All reagents used were analytical grade quality or better.
Photosynthetic parameters
All the photosynthetic parameters were measured as described in Biscéré et al.62. Thus, photosynthetic efficiency (Fv/Fm) of the coral symbiont and the relative electron transport rate (rETR) of their Photosystem II (PSII) were firstly measured early in the morning with a DIVING-PAM fluorometer (Walz, Germany) (n = 5 for each tank). Then, immediately after, respiration (R) and gross photosynthesis rates (Pg) were assessed on three apex from each tank under their own living conditions (temperature, metal enrichment, light). Finally, samples were frozen at −20 °C to further estimate their Symbiodinium density and chlorophyll concentrations. Respiration and gross photosynthesis rates, as well as Symbiodinium density and chlorophyll concentration were normalized per unit surface area (cm²). This one was estimated by weighting apex before and after dipping in hot paraffin. The weight of paraffin gave the surface area thanks to a linear regression of known surfaces against paraffin weight74.
Growth parameters
As for photosynthetic parameters, methods to measure growth and calcification rates are fully described in Biscéré et al.62. Long-term calcification was assessed using the buoyant weight technique75, when short-term calcification rates were estimated using the alkalinity anomaly technique76. Five microcolonies in each tank were weekly weighted all along the experiment to measure the long-term calcification, which was calculated as the daily change in dry weight and expressed in mg g−1 d−1. However, only three microcolonies were used to estimate short-term calcification rates at each time because data being expressed as µmol CaCO3 cm−2 d−1, samples needed to be dried immediately after to measure their surface area.
Statistical analysis
Principal Coordinate Analysis (PCoA) was performed on a multidimensional similarity matrix based on the Euclidian distance between all experimental conditions, using normalized parameters (Fv/Fm, ETR, growth rates, calcification rates, photosynthetic and respiration rates, symbiodinium and chlorophyll concentrations). PCoA was optimized with vector overlays of raw Pearson correlations (limited to r > 0.6). All similarity analyses were performed using PRIMER 6 statistical software77.
Three-way ANOVAs were used to test the effects of manganese and iron concentrations, thermal stress and their interactions on the 8 coral physiological parameters assessed. All the statistical results are given in the Supplementary Table 1. Data were first tested for normality and homoscedasticity using Shapiro Wilk’s test and Bartlett’s test respectively. For normality requirements, all data were transformed according to the Box-Cox transformation78, except for respiration rates, for which the square root transformation was applied. When the ANOVA determined significant differences between factors, a post-hoc pair-wise Least Significant Difference (LSD) test was performed to assign differences to specific factors. Individual differences revealed by the LSD test are represented by letters on the figures. All data are expressed as mean ± SD. All tests were performed using R software.
References
Moberg, F. & Folke, C. Ecological goods and services of coral reef ecosystems. Ecological Economics 29, 215–233 (1999).
Hoegh-Guldberg, O. et al. Coral reefs and rapidclimate change: impacts, risks and implications for tropical societies. In IOP Conference Series: Earth and Environmental Science 302004 (2009).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377 (2017).
Weis, V. M. Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. Journal of Experimental Biology 211, 3059–3066 (2008).
Muller-Parker, G., D’Elia, C. F. & Cook, C. B. Interactions between corals and their symbiotic algae. Coral Reefs in the Anthropocene, 99–116, https://doi.org/10.1007/978-94-017-7249-5_5 (2015).
Cunning, R. & Baker, A. C. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nature Climate Change 3, 259–262 (2013).
Shcolnick, S. & Keren, N. Metal Homeostasis in Cyanobacteria and Chloroplasts. Balancing Benefits and Risks to the Photosynthetic Apparatus. Plant Physiology 141, 805–810 (2006).
Lü, J.-M., Lin, P. H., Yao, Q. & Chen, C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Journal of Cellular and Molecular Medicine 14, 840–860 (2010).
Halliwell, B. Free Radicals in Biology and Medicine. Plant Physiology 141, 312–322 (2006).
Cooper, T. F., Gilmour, J. P. & Fabricius, K. E. Bioindicators of changes in water quality on coral reefs: Review and recommendations for monitoring programmes. Coral Reefs 28, 589–606 (2009).
Ali, Aa. M., Hamed, M. A. & Abd El-Azim, H. Heavy metals distribution in the coral reef ecosystems of the Northern Red Sea. Helgoland Marine Research 65, 67–80 (2011).
Biscéré, T. et al. Responses of two scleractinian corals to cobalt pollution and ocean acidification. PLoS One 10, 1–18 (2015).
Biscéré, T. et al. Nickel and ocean warming affect scleractinian coral growth. Marine Pollution Bulletin 120, 250–258 (2017).
Fujita, M. et al. Heavy metal contamination of coastal lagoon sediments: Fongafale Islet, Funafuti Atoll, Tuvalu. Chemosphere 95, 628–634 (2014).
Guzmán, H. M. & Jiménez, C. E. Contamination of coral reefs by heavy metals along the Caribbean coast of Central America (Costa Rica and Panama). Marine Pollution Bulletin 24, 554–561 (1992).
Tanaka, K. et al. Metal contents of Porites corals from Khang Khao Island, Gulf of Thailand: Anthropogenic input of river runoff into a coral reef from urbanized areas, Bangkok. Applied Geochemistry 37, 79–86 (2013).
Whitall, D. et al. Organic and metal contamination in marine surface sediments of Guánica Bay, Puerto Rico. Marine Pollution Bulletin 80, 293–301 (2014).
Reichelt, A. J. & Jones, G. B. Trace metals as tracers of dredging activity in cleveland bay-field and laboratory studies. Marine and Freshwater Research 45, 1237–1257 (1994).
Gissi, F., Stauber, J., Reichelt-Brushett, A., Harrison, P. L. & Jolley, D. F. Inhibition in fertilisation of coral gametes following exposure to nickel and copper. Ecotoxicology and Environmental Safety 145, 32–41 (2017).
Reichelt-Brushett, A. J. & Harrison, P. L. The effect of copper on the settlement success of larvae from the scleractinian coral Acropora tenuis. Marine Pollution Bulletin 41, 385–391 (2000).
Reichelt-Brushett, A. J. & Harrison, P. L. The effect of selected trace metals on the fertilization success of several scleractinian coral species. Coral Reefs 24, 524–534 (2005).
Reichelt-Brushett, A. J. & Harrison, P. L. Development of a sublethal test to determine the effects of copper and lead on scleractinian coral larvae. Archives of Environmental Contamination and Toxicology 47, 40–55 (2004).
Reichelt-Brushett, A. J. & Hudspith, M. The effects of metals of emerging concern on the fertilization success of gametes of the tropical scleractinian coral Platygyra daedalea. Chemosphere 150, 398–406 (2016).
Leigh-Smith, J., Reichelt-Brushett, A. & Rose, A. L. The characterization of iron (III) in seawater and related toxicity to early life stages of scleractinian corals. Environmental Toxicology and Chemistry 37, 1104–1114 (2018).
Negri, A. P. & Hoogenboom, M. O. Water contamination reduces the tolerance of coral larvae to thermal stress. Plos One 6, (2011).
Fonseca, J. et al. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia harttii. Aquatic Toxicology 190, 121–132 (2017).
Bastidas, C. & García, E. M. Sublethal effects of mercury and its distribution in the coral Porites astreoides. Marine Ecology Progress Series 267, 133–143 (2004).
Sabdono, A. Heavy metal levels and their potential toxic effect on coral Galaxea fascicularis from Java Sea, Indonesia. Research Journal of Environmental Sciences 3, 96–102 (2009).
Dalcorso, G., Manara, A., Piasentin, S. & Furini, A. Nutrient metal elements in plants. Metallomics 6, 1770–1788 (2014).
Moreton, B. M., Fernandez, J. M. & Dolbecq, M. B. D. Development of a field preconcentration/elution unit for routine determination of dissolved metal concentrations by ICP-OES in marine waters: Application for monitoring of the New Caledonia Lagoon. Geostandards and Geoanalytical Research 33, 205–218 (2009).
Srichandan, S. et al. Distribution of trace metals in surface seawater and zooplankton of the Bay of Bengal, off Rushikulya estuary, East Coast of India. Marine Pollution Bulletin 111, 468–475 (2016).
Mokhtar, M. B., Praveena, S. M., Aris, A. Z., Yong, O. C. & Lim, A. P. Trace metal (Cd, Cu, Fe, Mn, Ni and Zn) accumulation in Scleractinian corals: A record for Sabah, Borneo. Marine Pollution Bulletin 64, 2556–2563 (2012).
Chan, I., Hung, J., Peng, S., Tseng, L. & Ho, T. Comparison of metal accumulation in the azooxanthellate scleractinian coral (Tubastraea coccinea) from different polluted environments. Marine Pollution Bulletin 85, 648–658 (2014).
Greene, R. M., Geider, R. J. & Falkowski, P. G. Effect of iron limitation on photosynthesis in a marine diatom. Limnology and Oceanography 36, 1772–1782 (1991).
Geider, R. J. & La Roche, J. The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosynthesis Research 39, 275–301 (1994).
Wells, M., Shick, J. M. & Long, P. Effects of Trace Metal Limitation on Oxidative Stress in Zooxanthellae and Its Role in Coral Bleaching. University of Maine Office of Research and Sponsored Programs: Grant Reports Paper 291 (2011).
Richier, S. Symbiosis-induced adaptation to oxidative stress. Journal of Experimental Biology 208, 277–285 (2005).
Wolfe-Simon, F., Grzebyk, D., Schofield, O. & Falkowski, P. G. The role and evolution of superoxide dismutases in algae. Journal of Phycology 41, 453–465 (2005).
Twining, B. S. & Baines, S. B. The Trace Metal Composition of Marine Phytoplankton. Annual Review of Marine Science 5, 191–215 (2013).
Rodriguez, I. B., Lin, S., Ho, J. & Ho, T. Y. Effects of trace metal concentrations on the growth of the coral endosymbiont Symbiodinium kawagutii. Frontiers in Microbiology 7, 1–10 (2016).
Shick, J. M. et al. Responses to iron limitation in two colonies of Stylophora pistillata exposed to high temperature: Implications for coral bleaching. Limnology and Oceanography 56, 813–828 (2011).
Peers, G. & Price, N. M. A role for manganese in superoxide dismutases and growth of iron-deficient diatoms. Limnology and Oceanography 49, 1774–1783 (2004).
Shi, T., Sun, Y. & Falkowski, P. G. Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS101. Environmental Microbiology 9, 2945–2956 (2007).
Cao, C., Sun, S., Wang, X., Liu, W. & Liang, Y. Effects of manganese on the growth, photosystem II and SOD activity of the dinoflagellate Amphidinium sp. Journal of Applied Phycology 23, 1039–1043 (2011).
Ghanotakis, D. F. & Yocum, C. F. Photosystem II and the oxygen evolving complex. Annual review of plant physiology and plant molecular biology 41, 255–276 (1990).
Diner, B. A. & Babcock, G. T. Structure, dynamics, and energy conversion efficiency in photosystem II. In Oxygenic Photosynthesis: The Light Reactions (eds Ort, D. R. & Yocum, C. F.) 503, 213–247 (Kluwer Academic, 1996).
Hill, R. & Ralph, P. J. Impact of bleaching stress on the function of the oxygen evolving complex of zooxanthellae from scleractinian corals. Journal of Phycology 44, 299–310 (2008).
Wilkinson, R. E. & Ohki, K. Influence of manganese deficiency and toxicity on isoprenoid syntheses. Plant physiology 87, 841–846 (1988).
Lidon, F. C., Barreiro, M. G. & Ramalho, J. C. Manganese accumulation in rice: implications for photosynthetic functioning. Journal of Plant Physiology 161, 1235–1244 (2004).
Millaleo, R., Reyes-Diaz, M., Ivanov, A., Mora, M. & Alberdi, M. Manganese as essential and toxic element for plants: transport, accumulation and resistance Mechanisms. Journal of soil science and plant nutrition 10, 470–481 (2010).
Hill, R., Brown, C. M., DeZeeuw, K., Campbell, D. A. & Ralph, P. J. Increased rate of D1 repair in coral symbionts during bleaching is insufficient to counter accelerated photo-inactivation. Limnology and Oceanography 56, 139–146 (2011).
Krueger, T. et al. Differential coral bleaching-Contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comparative Biochemistry and Physiology -Part A: Molecular and Integrative Physiology 190, 15–25 (2015).
Lin, S. et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 350, 691–694 (2015).
Zhang, H., Zhuang, Y., Gill, J. & Lin, S. Proof that dinoflagellate spliced leader (DinoSL) is a useful hook for fishing dinoflagellate transcripts from mixed microbial samples: Symbiodinium kawagutii as a case study. Protist 164, 510–527 (2013).
Macfie, S. M. & Taylor, G. J. The effects of excess manganese on photosynthetic rate and concentration of chlorophyll in Triticum aestivum grown in solution culture. Physiologia Plantarum 85, 467–475 (1992).
Downs, C. A. et al. Oxidative stress and seasonl coral bleaching. Free Radical Biology and Medicine 33, 533–543 (2002).
Okrasa, K. & Kazlauskas, R. J. Manganese-substituted carbonic anhydrase as a new peroxidase. Chemistry - A European Journal 12, 1587–1596 (2006).
Lionetto, M. G., Caricato, R., Giordano, M. E. & Schettino, T. The complex relationship between metals and carbonic anhydrase: New insights and perspectives. International Journal of Molecular Sciences 17 (2016).
Gattuso, J.-P., Allemand, D. & Frankignoulle, M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. American Zoologist 39, 160–183 (1999).
Allemand, D., Tambutté, É., Zoccola, D. & Tambutté, S. Coral calcification, cells to reefs. In Coral reefs: an ecosystem in transition 119–150 (2011).
Tremblay, P., Gori, A., Maguer, J. F., Hoogenboom, M. & Ferrier-Pagès, C. Heterotrophy promotes the re-establishment of photosynthate translocation in a symbiotic coral after heat stress. Scientific Reports 6, 1–14 (2016).
Biscéré, T. et al. Enhancement of coral calcification via the interplay of nickel and urease. Aquatic Toxicology 200, 247–256 (2018).
Stambler, N., Popper, N., Dubinsky, Z. & Stimson, J. S. Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pacific Science 45, 299–307 (1991).
Marubini, F. & Davies, P. S. Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Marine Biology 127, 319–328 (1996).
Ferrier-Pagès, C., Schoelzke, V., Jaubert, J., Muscatine, L. & Hoegh-Guldberg, O. Response of a scleractinian coral, Stylophora pistillata, to iron and nitrate enrichment. Journal of Experimental Marine Biology and Ecology 259, 249–261 (2001).
Tansik, A. L., Fitt, W. K. & Hopkinson, B. M. Inorganic carbon is scarce for symbionts in scleractinian corals. Limnology and Oceanography 62, 2045–2055 (2017).
Millero, F., Woosley, R., DiTrolio, B. & Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 22, 72–85 (2009).
Morley, N. H., Burton, J. D., Tankere, S. P. C. & Martin, J. M. Distribution and behaviour of some dissolved trace metals in the western Mediterranean Sea. Deep-Sea Research Part II: Topical Studies in Oceanography 44, 675–691 (1997).
Campbell, P. G. C., Tessier, A. & Turner, D. R. Metal speciation and bioavailability in aquatic systems. IUPAC Series on analytical and physical chemistry of environmental systems. Wiley, Chichester (1995).
Fairbrother, A., Wenstel, R., Sappington, K. & Wood, W. Framework for Metals Risk Assessment. Ecotoxicology and Environmental Safety 68, 145–227 (2007).
Morgan, J. Chemical equilibria and kinetic properties of manganese in natural water. Principles and applications of water chemistry 561–623 (1967).
Santana-Casiano, J. M., González-Dávila, M. & Millero, F. J. Oxidation of nanomolar levels of Fe(II) with oxygen in natural waters. Environmental science & technology 39, 2073–2079 (2005).
Sunda, W. G. Bioavailability and bioaccumulation of iron in the sea. The biogeochemistry of iron in seawater 41–84 (2001).
Stimson, J. & Kinzie, R. A. The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. Journal of Experimental Marine Biology and Ecology 153, 63–74 (1991).
Davies, S. P. Short-term growth measurements of corals using an accurate buoyant weighing technique. Marine Biology 101, 389–395 (1989).
Chisholm, J. R. M. & Gattuso, J.-P. Validation of the alkalinity anomaly technique for investigating calcification of photosynthesis in coral reef communities. Limnology and Oceanography 36, 1232–1239 (1991).
Clarke, K. R. & Warwick, R. M. Change in marine communities: an approach to statistical analysis and interpretation 2nd edition. PRIMER-E:Plymouth (2001).
Box, G. E. P. & Cox, D. R. An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological) 26, 211–252 (1964).
Acknowledgements
T. Biscéré was beneficiary of a PhD grant (CIFRE No. 2015/0301,France) supported by the Koniambo Nickel SAS and Ginger Soproner companies in New Caledonia. Part of this work was also funded by the CNRT Nickel (DYNAMINE project). We wish to thank Magali Boussion and Cécile Rottier of the Centre Scientifique de Monaco (France) for her assistance during all the experiment. We are especially grateful to Christophe Menkes for his data and fruitful comments on temperature variations in New Caledonian lagoon.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: T.B., F.H. and C.F.P. Performed the experiments: T.B. Analysed the data: T.B., A.G. and F.H. Contributed reagents/materials/analysis tools: F.H., C.F.P. and T.P. Wrote the paper: T.B., F.H. and C.F.P.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biscéré, T., Ferrier-Pagès, C., Gilbert, A. et al. Evidence for mitigation of coral bleaching by manganese. Sci Rep 8, 16789 (2018). https://doi.org/10.1038/s41598-018-34994-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34994-4
Keywords
This article is cited by
-
What are the toxicity thresholds of chemical pollutants for tropical reef-building corals? A systematic review
Environmental Evidence (2023)
-
Long-term exposure to an extreme environment induces species-specific responses in corals’ photosynthesis and respiration rates
Marine Biology (2022)
-
Determinants of Tubastraea coccinea invasion and likelihood of further expansion in the northern Gulf of Mexico
Marine Biodiversity (2020)
-
Endosymbiotic dinoflagellates pump iron: differences in iron and other trace metal needs among the Symbiodiniaceae
Coral Reefs (2020)
-
Copper enrichment reduces thermal tolerance of the highly resistant Red Sea coral Stylophora pistillata
Coral Reefs (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.