Abstract
The endoplasmic reticulum (ER) forms a complex endomembrane network that reaches into the cellular compartments of a neuron, including dendritic spines. Recent work discloses that the spine ER is a dynamic structure that enters and leaves spines. While evidence exists that ER Ca2+ release is involved in synaptic plasticity, the role of spine ER morphology remains unknown. Combining a new 3D spine generator with 3D Ca2+ modeling, we addressed the relevance of ER positioning on spine-to-dendrite Ca2+ signaling. Our simulations, which account for Ca2+ exchange on the plasma membrane and ER, show that spine ER needs to be present in distinct morphological conformations in order to overcome a barrier between the spine and dendritic shaft. We demonstrate that RyR-carrying spine ER promotes spine-to-dendrite Ca2+ signals in a position-dependent manner. Our simulations indicate that RyR-carrying ER can initiate time-delayed Ca2+ reverberation, depending on the precise position of the spine ER. Upon spine growth, structural reorganization of the ER restores spine-to-dendrite Ca2+ communication, while maintaining aspects of Ca2+ homeostasis in the spine head. Our work emphasizes the relevance of precise positioning of RyR-containing spine ER in regulating the strength and timing of spine Ca2+ signaling, which could play an important role in tuning spine-to-dendrite Ca2+ communication and homeostasis.
Similar content being viewed by others
Introduction
The endoplasmic reticulum (ER) is a multifunctional intracellular organelle, which consists of a complex three-dimensional network of connected endomembrane tubules, stacks and cisternae1,2,3,4. In neurons, the relevance of its strategic positioning is reflected by the fact that it reaches from the nucleus and soma into neurites, i.e., dendrites and axons, and it is frequently found in proximity of excitatory and inhibitory pre- and postsynaptic sides. This observation has coined the term “neuron within a neuron” for neuronal ER morphology5. While its role in synaptic protein synthesis, protein maturation, and transport is still debated, it is best-studied for its ability to release Ca2+ in a receptor-dependent manner, which modulates the capacity of synapses to undergo plastic changes2,6,7,8,9.
The ER consists of a complex, overlapping and partially cell- and region-specific Ca2+ handling machinery, including Ca2+ pumps and transporters2. In hippocampal neurons, for example, inositol trisphosphate receptors (IP3R) are present at high concentrations in dendritic shafts and cell bodies, whereas ryanodine receptors (RyR) are primarily found in dendritic spines and axons10 (see also11). In contrast, Purkinje cells of the cerebellum show high concentrations of IP3R also in dendritic spines12,13. Whether these receptors are evenly distributed along the spine ER compartment or rather clustered at strategic positions remains unknown. More recent work has also established a link between store-operated Ca2+ entry (SOCE), i.e., ORAI-STIM1-mediated Ca2+ signaling, and neuronal ER-mediated plasticity (e.g.14,15). Another major challenge in this field of research is the fact that the ER is a dynamic structure that can rapidly enter and leave pre-existing spines, while changing its position within individual ER-positive spines16,17. Hence, it is conceivable that spine-to-dendrite Ca2+ communication may critically depend on (1) whether or not a spine contains ER, (2) ER Ca2+ receptor composition, and (3) the precise ER morphology and position within a spine.
In order to capture how distinct spine ER properties influence spine-to-dendrite Ca2+ communication, the three-dimensional intracellular architecture must be considered18,19. Therefore, we developed a new spine and ER generator for the simulation framework NeuroBox20 to parametrically design three-dimensional computational domains (Fig. 1a). Existing single-channel models of Na+/Ca2+ exchangers in the plasma membrane, as well as RyR, IP3R and sarco/endoplasmic reticulum Ca2+ ATPases (SERCA) on the ER membrane (see schematic in Fig. 1c) were adapted and integrated in a novel three-dimensional calcium model that is solved by established numerical methods (details provided in Methods). Using this novel framework, we systematically assessed the relevance of selected spine ER properties, i.e., length, width and presence of RyR and IP3R on spine-to-dendrite Ca2+ signaling.
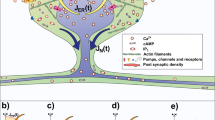
Spine Ca2+ modeling. (a) Schematic illustration of a single spine emerging from a dendrite containing endoplasmic reticulum (ER). Spine ER (ER[spine], green) reaches into the spine compartment. The relevant parameters evaluated in this study are indicated. Spine morphology is based on average values of 21, who used stimulated emission depletion (STED) microscopy to determine parameters such as spine length, spine head size and spine neck width. (b,c) Upon release of Ca2+ ions and inositol trisphosphate (IP3) molecules in the head of the spine, changes in [Ca2+] are determined in the head (yellow), neck (orange) and dendritic region (red), respectively. The model accounts for Ca2+ exchange mechanisms on the plasma membrane (Na+/Ca2+ exchangers (NCX), plasma membrane Ca2+ -ATPases (PMCA)) and on the ER (inositol trisphosphate receptors (IP3R), ryanodine receptors (RyR), and sarco/endoplasmic reticulum Ca2+ -ATPases (SERCA)). (d) Sample 2D triangulation \({\mathscr{S}}=\{{T}_{1},\mathrm{...},{T}_{M}\}\) with fulfilled Delaunay condition, i.e., the unique circumcircle of each Ti, which passes through the three triangular vertices, does not contain any vertices of the grid in its interior. (e) Piecewise linear approximation of a circle with 8 rim vertices as used for the construction of the dendrite, ER and spine structures in the spine generator. (f) Successive circle extrusion with creation of quadrilateral faces enclosing the emerging cylinder barrel during dendrite, ER and spine structure generation. (g) Exemplary triangulation of the planar hole at the left cylinder side as used for closing the encapsulated spine surface geometry. Further details are provided in Tables 1 and 2. See also supplemental movie.
Results
Passive spine ER has no major impact on spine-to-dendrite Ca2+ signaling
To assess the role of spine ER positioning in spine-to-dendrite Ca2+ communication, we first investigated Ca2+ signal propagation in a representative 3D spine model not containing any ER. The simplified morphology of the spine (Fig. 1a) is based on mean values obtained from stimulated emission depletion (STED) live cell microscopy experiments21. Ca2+ ions were released into the spine head with three distinct release profiles, i.e., a 1 ms release and two longer release periods with a 10 ms and 150 ms time constant, respectively. Changes in [Ca2+] were determined in the indicated regions of interest (Fig. 1b), i.e., in the spine head, neck and dendrite.
As shown in Fig. 2 for 1 ms initial Ca2+ release, only a small fraction of Ca2+ reaches the dendritic compartment (<1%; Fig. 2b). When the spine ER is passive, i.e., when it is present as a geometric obstacle, but without any Ca2+ exchange mechanisms that would allow Ca2+ exchange across the ER membrane, nearly identical and near-zero dendritic Ca2+ profiles for all ER lengths are observed (Fig. 2b)22. Similar results were obtained for the longer Ca2+ influx durations (c.f., Supplemental Fig. S1). From these results we conclude that spine-to-dendrite Ca2+ signaling is negligible in the no-spine-ER and the passive-spine-ER setting. Accordingly, precise positioning of a purely passive spine ER compartment has only minor effects on spine-to-dendrite Ca2+ signals in our experimental setting. This observation strengthens the case for active Ca2+ exchange across the spine ER membrane to enable spine-to-dendrite Ca2+ communication.
Effects of passive spine ER on spine-to-dendrite Ca2+ signaling. (a) Schematics of three selected morphological settings. Spine ER length is given in units of µm. (b) Ca2+ profiles for 1 ms initial Ca2+ release into the spine head (cf. Supplemental Fig. S1). Consistent with experimental data, spine-to-dendrite Ca2+ signaling does not occur in our simulations. Presence or length of passive spine ER has only minor effects on Ca2+ dynamics in the spine head and neck. Ca2+ buffering has a major impact in these simulations, thus inactivating spine-to-dendrite Ca2+ communication. (c) Minor effects of the passive spine ER on the Ca2+ profile are shown at higher magnification for the spine neck.
Ryanodine receptor-containing ER promotes spine-to-dendrite Ca2+ signaling
We next tested for the role of RyR-containing ER introduced to the spine compartment. Indeed, RyR promoted and even amplified spine-to-dendrite Ca2+ signals in our experimental setting (1 ms initial Ca2+ release illustrated in Fig. 3): For an ER length of 1.5 μm (width 0.036 μm; cf. Fig. 3a and Table 1), an approximately 20fold increase in [Ca2+] was measured in the spine neck (as compared to the purely passive spine ER setting), while up to twice the amount of Ca2+ initially released into the spine head was observed in the dendrite (Fig. 3b; see also supplemental movie). In addition, an increase in spine head [Ca2+] was observed as soon as the ER reached the border between the spine neck and head, while [Ca2+] in the neck and dendrite was comparable under these conditions. RyR-ER at the base of the spine (ER length 0.5 μm) had no apparent effect on spine-to-dendrite Ca2+ communication (Fig. 3b). These simulations indicate that the precise position of RyR-containing ER could have an impact on spine-to-dendrite Ca2+ signaling and Ca2+ signal amplification.
Differential effects of spine ER carrying ryanodine receptor (RyR) and inositol trisphosphate receptor (IP3R) on spine-to-dendrite Ca2+ signaling. (a) Schematics of spine ER morphologies used to investigate the role of RyR- and IP3R-mediated Ca2+ release. Spine ER width is kept constant, while the length is varied between 0–1.5 μm. (b) Ca2+ profiles in response to 1 ms Ca2+ influx into the spine head for the four configurations depicted in (a) in the spine head, neck, and dendrite (from left to right) for RyR-only spine ER. (c) Ca2+ profiles for the four configurations depicted in (a) in the spine head, neck, and dendrite (from left to right) for IP3R-only spine ER. Note that the plateau in the neck profile appears to be small compared to RyR-induced dynamics, as the plateau effect is overshadowed by RyR dynamics in (b) and (d). (d) Ca2+ profiles for the four configurations depicted in (a) for spine ER carrying both RyR and IP3R.
IP3 receptors introduce protracted Ca2+ waves in the spine head and neck
While it has been argued that spine ER may not contain IP3Rs in hippocampal neurons10, our computational approach enabled us to evaluate the behavior of IP3R-only spine ER. Fig. 3c shows a slow rise in [Ca2+] in the head and neck of spines in response to 1 ms Ca2+ influx and simultaneous onset of a 200 ms IP3 release, which became more prominent as the ER reached the spine head. In our simulations, IP3R-only spine ER, similar to a passive ER, does not have the ability to initiate strong spine-to-dendrite communication. Interestingly, although much weaker as compared to RyR-containing ER (cf. Fig. 3b), a small Ca2+ increase was even observed with ER of length 0.5 μm. We attribute this difference between RyR- and IP3R-containing ER to (1) the presence of Ca2+ buffers, which scavenge Ca2+ and limit its reach, while IP3 has a slower decay rate compared to Ca2+, and therefore a longer reach toward the dendrite, and (2) the fact that IP3R is activated at lower [Ca2+] in the presence of IP3. Thus, IP3R-mediated Ca2+ signals are weaker than RyR-Ca2+ signals, but are sensitive to low [Ca2+] even at spine ER positions distant from the synapse.
Combining RyR and IP3R can cause delayed Ca2+ signal reverberation
Based on the results above, we speculated that the slow protracted IP3R-mediated Ca2+ -response could trigger RyR-mediated Ca2+ release from the ER in situations where RyR-only ER is not sufficient to promote spine-to-synapse communication. Thus, IP3R-mediated Ca2+ responses could support RyR-mediated Ca2+ signaling between spines and dendrites.
To test this hypothesis, we repeated our simulations with spine ER containing both RyR and IP3R. ER positions in the neck and head of the spine elicited Ca2+ dynamics that were comparable to the RyR-only simulations (Fig. 3d). When, however, the RyR/IP3R-containing ER was positioned at the base of the spine, an additional protracted Ca2+ response was observed, which propagated back toward the spine head compartment, but dissipated along the way due to Ca2+ buffering. This result is in line with the literature disclosing ER-mediated IP3-dependent protracted Ca2+ signals, which may promote long-term depression of excitatory neurotransmission, e.g.11,23,24.
The precise position of RyR-containing ER may affect the timing of Ca2+ signals
Motivated by the observation that ER positioning affects Ca2+ signals, we next determined the position of RyR spine ER at which spine-to-dendrite communication and Ca2+ signal amplification occurs. Figure 4 shows the transition that occurs for a 1 ms Ca2+ influx when growing the ER beyond a critical length. While no Ca2+ signal can be detected in the dendrite for an ER length of 0.75 μm in the RyR-only case (Fig. 4a,b), spine-to-dendrite communication is detectable for a length of 0.8 μm (Fig. 4a,c). While the exact position of this transition zone depends on the initial Ca2+ release in the spine head (i.e., total number of Ca2+ ions and release current density), the effects of the spine ER within this critical zone are robust. In case of a prolonged, i.e., 150 ms Ca2+ influx, the transition is found between ER lengths of 0.4 μm and 0.45 μm (c.f., Supplemental Fig. S2). Interestingly, a delay in the Ca2+ signal occurs at these transition positions, which can be attributed to the fact that it takes several milliseconds at this transition length for Ca2+ to reach the critical threshold that triggers RyR-mediated Ca2+ release from the ER.
Critical spine ER transition lengths regulate all-or-nothing spine-to-dendrite communication. (a) Schematics of three morphological configurations designed to illustrate Ca2+ signals around critical ER transition lengths in response to 1 ms Ca2+ influx into the spine head. (b) For a RyR-containing ER of length 0.75 μm, no Ca2+ communication to the dendrite is detected. (c) Increasing the RyR-containing ER to a length of 0.8 μm surpasses a critical spine ER length to trigger spine-to-dendrite communication. At this critical length, [Ca2+] in the vicinity of the ER membrane is elevated just above the CICR threshold. This occurs after the initial dampened and dilated (by diffusion) Ca2+ signal, visible in the first milliseconds, propagates to the ER membrane and eventually triggers delayed CICR at roughly 8 ms. In the roughly 5 ms period leading to store release, one can observe the slow elevation of local cytosolic Ca2+ concentration that eventually surpasses the threshold necessary to trigger self-reinforcing Ca2+ release from the ER. (d) Adding IP3R to the ER allows for spine-to-dendrite signals at an ER length for which RyR-only ER is not capable of transmitting a signal to the dendrite (see panel (b) of this figure). The exact position of this transition zone depends on the initial Ca2+ release in the spine head (cf. Supplemental Fig. S2).
Consistent with the results described above, IP3R- and RyR-containing ER at a position, which does not elicit RyR-only responses, triggered the described delayed IP3R-mediated RyR-dependent Ca2+ response (Fig. 4d). These findings suggest that the precise position of RyR-(IP3R)-containing ER (1) enables spine-to-dendrite Ca2+ signaling, (2) amplifies the Ca2+ signal, and may even (3) modulate the exact timing of the Ca2+ signal. Considering that the outcome of plasticity may critically depend on such timing25,26,27, i.e., coincidence detection, this appears to be a relevant observation.
RyR-ER-dependent spine-to-dendrite Ca2+ coupling does not depend on the length of the spine
To test for the role of spine length, we carried out a series of simulations in which a very long spine, i.e., 10 μm spine neck length, was used. All other spine parameters were kept constant. The following major conclusions were drawn from this series of simulations: (1) RyR-ER couples and amplifies Ca2+ signals in dendrites and very long spines, (2) RyR-dependent spine-to-dendrite Ca2+ signal coupling occurs once the ER reaches far enough into the spine (Fig. 5b, position 3), (3) spine ER reaching even further into the spine makes spine head Ca2+ levels increase, (4) at a position that does not show RyR-dependent Ca2+ release from intracellular stores, introducing IP3R establishes a slightly delayed spine-to-dendrite communication (Fig. 5b,c, positions 1, 2). (5) Positioning RyR-only ER at the critical transition length – depending on the initial Ca2+ release in the spine head – has a similar “delaying” effect on Ca2+ signals (cf. Fig. 4).
Precisely positioned active ER enables spine-to-dendrite Ca2+ communication in a very long spine. (a) Illustration of the three ER positions used for the simulations in (b) and (c). A very long spine neck (10 μm) was employed to investigate whether neck length is a limiting factor for ER-mediated spine-to-dendrite Ca2+ communication. (b) Simulations for a RyR-only ER at positions 1, 2 and 3 in response to 1 ms Ca2+ influx into the spine head (illustrated in panel (a) of this figure). (c) Addition of IP3R enables spine-to-dendrite Ca2+ communication at ER positions farther away from the synapse. Note the comparable Ca2+ profiles at position 3 between RyR-only and RyR+ IP3R ER.
Role of spine RyR-ER in spine head Ca2+ homeostasis during plasticity
Finally, we tested for the effects of an increase in spine head volume, as seen after the induction of synaptic plasticity (e.g.28,29,30). Considering unchanged Ca2+ entry, we wondered whether changes in ER morphology, i.e., ER position and size, compensate for changes in spine-to-dendrite Ca2+ signaling as the size of the spine head increases. As illustrated in the spine schematics (Fig. 6a), spine head volume was increased by a factor of 2. Depending on the position of the spine ER, this can lead to a loss of spine-to-dendrite communication as well as a considerable decrease in [Ca2+] in the spine head (Fig. 6b,c).
RyR-containing ER compensates increased spine head size. (a) Illustration of the ER morphologies used for the simulations in (b–e). (b,c) Increasing the spine head volume by a factor of 2 disables spine-to-dendrite Ca2+ communication in response to a 1 ms Ca2+ influx into the spine head. The peak [Ca2+] in the spine head is significantly reduced. (d) Increasing the ER length restores spine-to-dendrite communication. However, the original peak [Ca2+] in the head, neck, and dendrite cannot be restored to the original profiles in (b). (e) Introducing a widened terminal segment of the spine ER (“spine-within-spine” morphology) restores the original Ca2+ profiles. (f) For simulations with 10 ms Ca2+ release, the peak amplitude of the Ca2+ signal in the spine head is also restored with the “spine-within-spine” ER morphology. The decay dynamics in the head, however, become sharper in comparison, due to depletion of the intracellular calcium store.
Increasing the length of the ER (length 1.1 μm; width 0.036 μm) or changing the width of the ER (length 1.0 μm; width 0.054 μm) reactivated spine-to-dendrite communication upon an initial 1 ms Ca2+ release in the spine head (Fig. 6d). However, it is not possible to fully restore the original Ca2+ profile, even when the ER is grown all the way close to the Ca2+ entry site (length 1.7 μM, width 0.036 μM; Fig. 6d).
Based on systematic evaluation, we finally determined that a selective volume increase in the ER segment located in the spine head (“spine-within-spine” morphology) leads to the best possible recovery of the Ca2+ profile in the spine head, neck and dendrite under conditions of increased spine head volume (Fig. 6e). For simulations with Ca2+ release of longer duration, the peak amplitude of the Ca2+ signal in the spine head is also restored with the “spine-within-spine” ER morphology. The decay dynamics in the head, however, become sharper in comparison (Fig. 6f). The reason lies in the limited ER Ca2+ store capacity, which rapidly depletes in our experimental setting. In the tested scenarios, dendritic dynamics were restored by morphological reorganization of the spine ER, which did not require a “spine-within-spine” morphology. Hence, complex changes in spine ER morphology seem to be required to restore Ca2+ homeostasis in the spine head, while precise positioning of the ER suffices to restore spine-to-dendrite Ca2+ communication.
Discussion
The present study highlights how functional and structural spine ER properties may affect spine-to-dendrite Ca2+ signaling. While precise positioning of RyR-(IP3R)-containing spine ER has a major impact on spine-to-dendrite Ca2+ communication, affecting both the strength and timing of the signal, growth of the spine neck or the spine head can cause a disruption of Ca2+ signaling. Eventually, comparable Ca2+ profiles can be restored by changes in ER morphology, i.e., position and size. These restoration effects could be demonstrated for Ca2+ entry profiles with different strength/duration. The all-or-none transition points as shown in Figs 4 and 5 are detectable for all tested entry profiles, the transition points, however, shift closer towards or farther away from the spine head, depending on the Ca2+ entry profile. It appears that not the absolute length or volume of the spine neck and head, respectively, determine the nature of spine-to-dendrite Ca2+ communication, but rather the relationship between spine morphology and spine ER morphology (“spine-within-spine” morphology). The presence of RyR and IP3R is important in this context. Once critical distances between the postsynaptic density and the ER are overcome and/or volume ratios between spine head and ER in the spine head are met, the synapse regains its previous spine-to-dendrite Ca2+ communication.
The role of spine ER in synaptic plasticity remains a matter of debate. For example, controversial results exist with respect to the relevance of ER Ca2+ stores in synaptic calcium transients11,28,31,32,33,34,35. This is in part explained by technical limitations in simultaneously visualizing (1) dendritic spine morphology, (2) presence and precise position of spine ER, and (3) in releasing reproducible amounts of Ca2+ while (4) carrying out Ca2+ imaging at high temporal and spatial resolution. Also, it is currently not possible to systematically assess the relevance of individual spine and ER parameters, as they are not easy to manipulate in biologically complex systems. We used a computational approach to compare ER-negative and ER-positive spines and to assess the role of spine ER morphology in spine Ca2+ transients. We show that critical ER lengths can be determined for specific spine geometries, functioning as a binary switch for spine-to-dendrite signaling. While the precise position depends on a given Ca2+ entry profile, beyond this all-or-nothing regulation, ER positioning can also function at the more refined level of timing. Our simulations suggest that the timing of a dendritic Ca2+ signal is determined by ER position and RyR/IP3R configuration. In some cases, even signals reverberating in the spine neck can be detected. In addition, peak amplitudes of Ca2+ transients are affected. Considering the relevance of Ca2+ signaling in synaptic plasticity and coincidence detection, i.e., spike-timing-dependent plasticity25,26,27, our findings imply that precise positioning of the ER could influence the duration, strength and direction of plasticity36,37,38. Naturally, further work (including improvement/development of new experimental techniques) is required to address this interesting hypothesis.
Another intriguing finding of our study concerns the role of spine ER in spine head Ca2+ homeostasis. We provide initial evidence that ER morphologies compensate for changes in spine morphology. This set of simulations also indicates that changes in ER morphology/volume in the area between the spine neck and spine head most effectively modulate Ca2+ signaling. Interestingly, the peak amplitude of the Ca2+ signal in the spine head but not the decay dynamics can be restored, specifically in the case of prolonged Ca2+ influx durations. This observation is attributed to a limited Ca2+ storage capacity, i.e., a rapid depletion of the intracellular store in our simulations, which “sharpens” the Ca2+ kinetics in the spine head. Whether this observation is relevant for ER-containing spines that undergo plasticity needs to be determined. It is also possible that additional molecular mechanisms may account for the limited Ca2+ capacity in order to maintain homeostasis, e.g., changes in SERCA and/or SOCE.
It is worth noting in this context that spine ER can assume peculiar morphological conformations that may resemble the herein described “spine-within-spine” ER morphology. The spine apparatus organelle is found in a subset of dendritic spines, consisting of stacked ER, which is typically located in the spine neck and head39,40,41,42. While its role in local protein synthesis is still debated, a link has been established between the spine apparatus and intracellular Ca2+ stores9,28,43,44,45. Indeed, using the actin-binding protein synaptopodin, which is a marker and essential component of the spine apparatus46,47, evidence has been provided that synaptopodin-associated Ca2+ transients from intracellular stores modulate the ability of neurons to express synaptic plasticity28,48,49,50. Similar to spine ER, the spine apparatus appears to be a dynamic structure that leaves and enters individual spines28 and changes its size, i.e., stack number51. Moreover, evidence has been provided that the spine apparatus is part of a Ca2+-dependent negative-feedback mechanism mediating homeostatic synaptic plasticity51 and that the size of spine apparatuses can change under pathological conditions such as systemic inflammation52 or experimentally induced seizures53. Apparently, our current findings motivate a rigorous study of the importance of stacked, membrane-infolded ER architectures (potentially to increase a surface to volume ratio). We are confident that these future studies will shed new and important light on the relevance of ER conformation in Ca2+ wave segregation and propagation and may thus provide new insight into the functional significance of ER derivatives/specializations, such as spine apparatuses39,41,42, cisternal organelles54,55,56,57,58,59,60, or subsurface organelles in dendrites and cell bodies61,62,63.
While we addressed basic principles of spine ER reorganization using simplified morphologies in this study, it will now be important to also employ more complex, i.e., realistic spine and ER morphologies based on super-resolution microscopy and/or serial electron microscopy. These models should also consider complex synaptic activity, i.e., plasticity-inducing AMPA-R-, NMDA-R- and mGluR-mediated Ca2+ signals. Yet, the results of the present study show that the major conclusions are robust across various Ca2+ release profiles. Since more complex effects may arise, e.g., from local depletion of intracellular Ca2+ stores and ORAI-STIM1-mediated SOCE, it will be important to also integrate these findings into models that also account for dendritic, somatic and axonal ER configurations. It is well established that space-time integration of Ca2+ signals originating at multiple spines plays an important role in Ca2+ signaling toward the soma and nucleus64,65. Hence, it is conceivable that the precise nature of the timing and the waveform of synaptically induced Ca2+ signals are relevant not only for spine-to-dendrite communication, but also for inter-synaptic and synapse-to-nucleus communication. Thus, spine ER positioning along entire dendritic branches and within multiple synaptic spines must be considered. The nature of structure/function interplay demands an inclusion of the three-dimensional intracellular architecture in order to capture the ways in which cellular organization can influence biochemical (and potentially electrical66) signals. The parametric geometry design approach developed for this study was included in the simulation toolbox NeuroBox20. This modular framework could be extended for future studies on more complex surface/volume/distance law models that integrate and test for the relevance of complex activity patterns on spine, dendrite and somatic ER morphologies in Ca2+ homeostasis and synapse-to-nucleus communication.
Methods
All necessary components were implemented in the simulation toolbox NeuroBox20.
NeuroBox spine generator
NeuroBox is a simulation toolbox that combines models of electrical and biochemical signaling on one- to three-dimensional computational domains. NeuroBox allows the definition of model equations, typically formulated as ordinary and partial differential equations, of the cellular computational domain and specification of the mathematical discretization methods and solvers67. Built with VRL-Studio68, NeuroBox offers user interface workflow canvases to control the simulation workflow and all biological and numerical parameters.
A novel spine generator using a parametric design approach was developed and implemented in NeuroBox, that allowed us to systematically vary the morphology of a spine (as well as the endoplasmic reticulum) and study its influence on the intracellular Ca2+ dynamics (see Sec. “model equations”–“membrane transport mechanisms”). The resulting partial differential equations with membrane mechanisms on the endoplasmic and plasma membrane were solved using a finite volume discretization and a parallel iterative solver (see Sec. “numerical methods”).
In the numerical simulation framework UG 467, a computational domain \({\rm{\Omega }}\in {{\mathbb{R}}}^{n}\), n ∈ {1, 2, 3}, is represented by a piecewise linear approximation Ωh (“grid”). All grid-related data structures and algorithms are implemented in the UG 4 core library lib_ grid which also constitutes the basis for the UG 4 plugin and cross-platform meshing software ProMesh69,70. lib_ grid features state-of-the-art grid generation data structures and algorithms which were incorporated in a consecutive workflow to automatically construct 3D grid variations of a dendritic segment including the ER and spine with corresponding spine ER. To this end, the spine generator utilizes the lib_ grid functionality for basic geometric element initialization and manipulation, i.e., insertion/deletion of vertices, edges, faces and volumes, as well as translation and scaling. Furthermore, composite functions for creating and extruding simple geometric objects like circles, as well as sophisticated grid generation algorithms for constrained Delaunay tetrahedrization71,72 are accessed in the ProMesh plugin. A Delaunay tetrahedrization \({\mathscr{S}}=\{{T}_{1},\mathrm{...},{T}_{M}\}\) is a special kind of tetrahedrization in which every tetrahedron Ti complies with the Delaunay condition, i.e., the unique circumsphere of each Ti, which passes through the four tetrahedral vertices, does not contain any vertices of the grid in its interior (Fig. 1d). This leads to high-quality grids which avoid tetrahedra with particularly acute or obtuse interior angles73, an essential grid property for accurate approximation and fast solution in numerical simulation74,75,76.
The user can specify 10 characteristic geometric parameters to specify the individual morphology of the spine grid output (Table 1). The generated grids are written to the native UG 4 file format UGX and can be viewed and modified using the GUI version of ProMesh. The fundamental workflow can be summarized as follows:
-
1.
The exterior dendrite and interior ER structures are constructed around the origin (0.0, 0.0, 0.0) using the ProMesh create-circle approximation with a chosen default resolution of 8 rim vertices and user-specified radii (Fig. 1e).
-
2.
The dendritic and ER circles, respectively, are then successively extruded along the z-axis while at the same time creating quadrilateral faces enclosing the emerging cylinder barrels using user-specified lengths (Fig. 1f).
-
3.
The previous process is interrupted by an in-between extrusion step for creating a measurement zone of user-specified spine neck length around the spine ER placed at the user-specified z-coordinate for the spine position.
-
4.
At the z-coordinate for the spine position, spine ER and neck are generated by a circular remeshing of the local ER and dendritic surface grid, and subsequent extrusion along the y-axis, creating quadrilateral faces enclosing the emerging cylinder barrels using specified lengths.
-
5.
The spine head is placed on top of the neck by continued extrusion along the y-axis. Subsequently, the cylindrical spine head vertices are projected to spherical coordinates around the head barycenter using the head radius.
-
6.
The remaining planar holes at the dendrite and ER cylinder top and bottom are triangulated to close the encapsulated surface geometry (Fig. 1g).
-
7.
Surface elements are selected automatically by their coordinates in order to be assigned to individual subsets for access during numerical simulation.
-
8.
Given the piecewise linear closed and encapsulated surface geometry, the corresponding volume grid is generated using constrained Delaunay tetrahedrization allowing for an individual subset assignment of tetrahedral elements which are separated by lower dimensional subsets.
Model equations
Three-dimensional spatio-temporal Ca2+ and inositol trisphosphate (IP3) dynamics in the intracellular space are modeled by a system of diffusion-reaction equations described in the following. The boundary conditions for this partial differential equation system are specified by Ca2+ - and IP3-dependent flux boundary conditions described in Sec. “Membrane transport mechanisms”.
The model considers the quantities calcium (cytosolic (cc) and endoplasmic (ce)), calbindin-D28k (b), and IP3 (p), which is required to model IP3 receptors embedded in the endoplasmic membrane. Mobility in the cytosol/ER is described by the diffusion equation
where u(x, t) stands for the the four quantities mentioned above. The diffusion constants D are defined using data from77,78.
The interaction between cytosolic Ca2+ and calbindin-D28k (CalB) is described by
The rate constants \({\kappa }_{b}^{-}\) and \({\kappa }_{b}^{+}\) are given in Table 2. While CalB has four distinct high-affinity Ca2+-binding sites79, we currently treat it as though it had only one, at the same time quadrupling its concentration in our model. This amounts to assuming that all four binding sites are essentially equal and binding is non-cooperative (though data by80,81 indicate this might not neccessarily be the case).
The equations for cytosolic Ca2+ and CalB are thus given by
in the cytosolic domain, where the concentration of the CalB-Ca2+ compound is expressed by the difference of the total concentration of CalB present in the cytosol (btot) and free CalB, the former of which is assumed to be constant in space and time (this amounts to the assumption that free and Ca2+ -binding CalB have the same diffusive properties). All parameters are listed in Table 2.
Exponential IP3 decay towards a basal IP3 concentration pr in the cytosolic space is modeled by a reaction term that is added to the IP3 diffusion equation, leading to the diffusion-reaction equation
for IP3 in the cytosolic domain. Endoplasmic Ca2+ dynamics are modeled by simple diffusion
in the endoplasmic domain.
Membrane transport mechanisms
In order to study the influence of intracellular organization on Ca2+ signals, we include Ca2+ exchange mechanisms on the endoplasmic membrane (ERM) and the plasma membrane (PM). IP3 receptors (IP3R), ryanodine receptors (RyR), sarco/endoplasmic reticulum Ca2+ -ATPase pumps (SERCA) as well as a leakage term are modeled to describe the bi-directional exchange of Ca2+ across the ER membrane. For the plasma membrane we consider plasma membrane Ca2+ -ATPase pumps (PMCA), Na+/Ca2+ exchangers (NCX) and a leakage term. This amounts to the flux equations
where jI is the IP3R flux density, jR the RyR flux density, jS the SERCA flux density and jl,e the leakage flux density on the ERM, and jP, jN and jl,p the flux densities of PMCA, NCX, and leakage flux density of the PM, respectively. Homogeneous distributions of all exchange mechanisms were assumed, as experimental data on precise numbers and spatio-temporal distribution of these receptors within individual spines are not available.
IP3R channels
The flux density jI (number of ions per membrane area and time) through the ER membrane is calculated by
where ρI is the density of IP3 receptors in the ER membrane, \({p}_{I}^{o}\) is the open state probability of a single channel, and II the single channel Ca2+ current.
The single channel current model is based on82, where experimental data are fitted by a Michaelis-Menten equation, and is quasi-linear in the physiologically relevant range for luminal Ca2+ concentrations (and below). Thus, we chose
with a reference concentration \({c}_{e}^{{\rm{ref}}}\) well inside the admissible range.
For the open state probability, we used the model from83:
with kinetic parameters d1, d2, d3 and d5 (see Table 2).
RyR channels
Similar to the IP3R channels, the Ca2+ flux density generated by ryanodine receptor channels at the ER membrane is given by an expression of the form
where ρR is the density of RyR in the ER membrane, \({p}_{R}^{o}\) is the open state probability of a single channel, and IR the single channel Ca2+ current.
Using the approach from84, we describe the single channel ionic current by
where the reference current \({I}_{R}^{{\rm{ref}}}\) is approximated from data presented in85.
The open probability for RyR channels is taken from84 and can be calculated as the sum of the two open states o1 and o2 in the system of ordinary differential equations
with the kinetic constants \({k}_{a}^{\pm }\), \({k}_{b}^{\pm }\) and \({k}_{c}^{\pm }\) (see Table 2), that can be solved independently for every point on the surface of the ER membrane.
SERCA pumps
The current from sarco/endoplasmic reticulum Ca2+ -ATPase pumps is described by a model from86, which was adapted for the three-dimensional case, and gives rise to the Ca2+ flux density
The model reflects the dependence of the Ca2+ current not only on the cytosolic concentration but also on the endoplasmic saturation. Parameter specifications can be found in Table 2.
PMCA pump
Using the model presented by87, we model the plasma membrane Ca2+-ATPase current as a second-order Hill-equation
All parameters are listed in Table 2.
NCX pump
For the Na+/Ca2+ exchanger current, we assume a constant Na+concentration at the plasma membrane, following the first-order Hill-equation used in87:
All parameters are listed in Table 2.
Leakage
Both the ERM and the PM allow a leakage flux not accounted for by the above transport mechanisms. These leakage fluxes are calibrated to ensure zero membrane net flux in the equilibrium state for all simulated ions and agents. Leakage flux densities are modeled by
where co is the extracellular Ca2+ concentration, which is assumed to be constant throughout all simulations.
Calcium release and IP3 production
Calcium release is modeled as a Neumann boundary condition for the cytosolic Ca2+ concentration, i.e., a time-dependent influx density function defined at the postsynaptic membrane. The 1 ms release is modeled by a linearly decreasing Ca2+ pulse of 1 ms duration starting at an initial maximal specific current density \({j}_{c}^{{\rm{rls}}}\). The 10 ms calcium release profile was modeled as a decaying exponential influx
with a decay constant τrls = 10 ms. The prolonged calcium release was modeled by an NMDA receptor model that defined a flux with time constant τNMDAR = 150 ms:
where ρNMDAR is the density of NMDARs in the postsynaptic membrane, \({p}_{{\rm{\max }}}^{o}\) is the maximal single-channel open probability, and the single-channel ionic current INMDAR is given by the Goldman-Hodgkin-Katz expression
The product \({\rho }_{{\rm{NMDAR}}}\,{p}_{{\rm{\max }}}^{o}\) was calibrated such that the maximal expected number of open channels in the spine was one88. The permeability pNMDAR was set to a value that results in a single-channel current in accordance with data published by Jahr and Stevens89. We supposed a constant membrane potential value Vm = −70 mV and used the fact that approximately 10% of the current through NMDARs is carried by Ca2+ at 2 mM extracellular Ca2+ concentrations89. We took into account the Mg2+ block that reduces the channel conductance by a factor of about twenty90 at physiological 0.7 mM extracellular Mg2+ concentrations91.
The production of IP3 is also modeled as an “influx” (as it is produced at the plasma membrane) decaying linearly over the course of 200 ms from a specific current density \({j}_{p}^{{\rm{rls}}}\). Since we only simulate on a portion of the dendrite at the base of a single spine, we do not capture diffusion across the dendritic boundaries of our geometry. While this is irrelevant for fast-buffered calcium, IP3 can diffuse farther and is eventually constrained by the geometric boundaries. Over time this would lead to unrealistically high accumulation of IP3. To adjust for this effect, IP3 production was reduced compared to92.
Values for all model parameters are gathered in Table 2.
Numerical Methods
For numerical simulations, the four equations are discretized in space using a finite volumes method. Current densities, both synaptic and across the ER and plasma membranes, can be incorporated into the reaction-diffusion process very naturally and easily this way. We show how this is achieved using the cytosolic Ca2+ Eq. (3) as an example: It is reformulated (using the divergence theorem) to an integral version
where B is a control volume that will be specified shortly, and n∂B is the outward normal on the boundary of B. For control volumes located at the ER membrane, some portion of its boundary will coincide with the ER membrane. Since there is no diffusive flux density Dc∇cc across the ER membrane, we can simply substitute it by the ER flux density jERM as given in (7) in the boundary integral for this portion of the boundary. The same applies to the plasma membrane and the synapse area. The diffusive flux is set to zero on the rest of the cytosolic domain boundary. If we denote the cytosolic boundary by Γ, its ER/plasma membrane and synaptic parts by ΓERM, ΓPM and Γsyn, respectively, this yields the following equation:
Control volumes are constructed as a Voronoi-like dual tesselation of the original tetrahedral mesh by connecting the mid-points of edges, faces and volumes through planar facets. Equation (27) must hold for all control volumes, giving rise to one equation per control volume.
Time discretization is realized using a backwards Euler scheme, i.e., for each point in time t, the term \(\frac{\partial {c}_{c}}{\partial t}\) in (27) is replaced by the discretized term \(\frac{{c}_{c}(t)-{c}_{c}(t-\tau )}{\tau }\) and all quantities on the right-hand side are evaluated at time t. Here, τ is the time step size of the time discretization.
By limiting the function space to the space of continuous functions that are linear on all volumes of the original mesh, the integrals in Eq. (27) can be evaluated efficiently. Moreover, the solution can be represented by one degree of freedom per volume, so there is one equation for each degree of freedom. The system of equations arising from this procedure is nonlinear (due to the nonlinear reaction term and, more importantly, the highly nonlinear transport terms across the membranes) and is therefore linearized by a Newton iteration.
For the results we present here, the emerging linearized problems were solved using a Bi-CGSTAB93 linear solver preconditioned by an incomplete LU decomposition. Computations were facilitated by a domain decomposition parallelization approach and carried out using the UG 4 framework67 on the JURECA computer system at the Jülich Supercomputing Centre94.
References
Porter, K. Observations on a submicroscopic basophilic component of cytoplasm. J Exp Med 97, 727–750 (1953).
Verkhratsky, A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 85, 201–279, https://doi.org/10.1152/physrev.00004.2004, http://physrev.physiology.org/content/85/1/201.full.pdf (2004).
Stutzmann, G. E. & Mattson, M. P. Endoplasmic reticulum Ca2+ handling in excitable cells in health and disease. Pharmacol. Rev. 63, 700–727, https://doi.org/10.1124/pr.110.003814., http://pharmrev.aspetjournals.org/content/63/3/700.full.pdf (2011).
Friedman, J. & Voeltz, G. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21, 709–717 (2011).
Berridge, M. Neuronal calcium signaling. Neuron 21, 13–26 (1998).
Segal, M. & Korkotian, E. Endoplasmic reticulum calcium stores in dendritic spines. Front. Neuroanat. 8, 64, http://journal.frontiersin.org/article/10.3389/fnana.2014.00064, https://doi.org/10.3389/fnana.2014.00064 (2014).
Maggio, N.&Vlachos, A. Synaptic plasticity at the interface of health and disease: New insights on the role of endoplasmic reticulum intracellular calcium stores. Neurosci. 281, 135–146, http://www.sciencedirect.com/science/article/pii/S0306452214007921, https://doi.org/10.1016/j.neuroscience.2014.09.041 (2014).
Finch, E. A., Tanaka, K. & Augustine, G. J. Calcium as a trigger for cerebellar long-term synaptic depression. The Cerebellum 11, 706–717, https://doi.org/10.1007/s12311-011-0314-x (2012).
Jedlicka, P. & Deller, T. Understanding the role of synaptopodin and the spine apparatus in Hebbian synaptic plasticity – New perspectives and the need for computational modeling. Neurobiol. Learn. Mem. 138, 21–30, http://www.sciencedirect.com/science/article/pii/S107474271630123X, https://doi.org/10.1016/j.nlm.2016.07.023. MCCS 2017 (2017).
Sharp, A. et al. Differential immunohistochemical localization of inositol 1, 4, 5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 13, 3051–3063, http://www.jneurosci.org/content/13/7/3051.full.pdf (1993).
Holbro, N., Grunditz, Å. & Oertner, T. G. Differential distribution of endoplasmic reticulum controls metabotropic signaling and plasticity at hippocampal synapses. Proc. Natl. Acad. Sci. 106, 15055–15060, https://doi.org/10.1073/pnas.0905110106, http://www.pnas.org/content/106/35/15055.full.pdf (2009).
Goto, J.-I. & Mikoshiba, K. Inositol 1, 4, 5-trisphosphate receptor-mediated calcium release in Purkinje cells: from molecular mechanism to behavior. The Cerebellum 10, 820–833, https://doi.org/10.1007/s12311-011-0270-5 (2011).
Rose, C. R. & Konnerth, A. Stores not just for storage. Neuron 31, 519–522, http://www.sciencedirect.com/science/article/pii/S0896627301004020, https://doi.org/10.1016/S0896-6273(01)00402-0 (2001).
Dittmer, P. J., Wild, A. R., Dell’Acqua, M. L. & Sather, W. A. STIM1 Ca2+ sensor control of L-type Ca2+-channel-dependent dendritic spine structural plasticity and nuclear signaling. Cell Reports 19, 321–334, http://www.sciencedirect.com/science/article/pii/S2211124717304175, https://doi.org/10.1016/j.celrep.2017.03.056 (2017).
Tshuva, R. Y., Korkotian, E. & Segal, M. ORAI1-dependent synaptic plasticity in rat hippocampal neurons. Neurobiol. Learn. Mem. 140, 1–10, http://www.sciencedirect.com/science/article/pii/S1074742716303483, https://doi.org/10.1016/j.nlm.2016.12.024 (2017).
Toresson, H. & Grant, S. G. N. Dynamic distribution of endoplasmic reticulum in hippocampal neuron dendritic spines. Eur. J. Neurosci. 22, 1793–1798, https://doi.org/10.1111/j.1460-9568.2005.04342.x (2005).
Ng, A. N. & Toresson, H. Endoplasmic reticulum dynamics in hippocampal dendritic spines induced by agonists of type I metabotropic glutamate but not by muscarinic acetylcholine receptors. Synap. 65, 351–355, https://doi.org/10.1002/syn.20887 (2011).
Biess, A., Korkotian, E. & Holcman, D. Diffusion in a dendritic spine: The role of geometry. Phys. Rev. E 76, 021922, https://doi.org/10.1103/Phys-RevE.76.021922 (2007).
Yasuda, R. Biophysics of biochemical signaling in dendritic spines: Implications in synaptic plasticity. Biophys. J. 113, 2152–2159, http://www.sciencedirect.com/science/article/pii/S000634951730855X, https://doi.org/10.1016/j.bpj.2017.07.029 (2017).
Breit, M. et al. Anatomically detailed and large-scale simulations studying synapse loss and synchrony using NeuroBox. Front. Neuroanat. 10, 8, https://doi.org/10.3389/fnana.2016.00008 (2016).
Tønnesen, J., Katona, G., Rózsa, B. & Nägerl, U. V. Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 17, 678–685, https://doi.org/10.1038/nn.3682 (2014).
Sabatini, B. L., Oertner, T. G. & Svoboda, K. The life cycle of Ca2+ ions in dendritic spines. Neuron 33, 439–452, http://www.sciencedirect.com/science/article/pii/S0896627302005731, https://doi.org/10.1016/S0896-6273(02)00573-1 (2002).
Reyes, M. & Stanton, P. Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J Neurosci 16, 5951–5960 (1996).
Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M. M. & Kato, K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nat. 408, 584–588 (2000).
Feldman, D. E. The spike-timing dependence of plasticity. Neuron 75, 556–571 (2012).
Markram, H., Gerstner, W. & Sjöström, P. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci 3, https://doi.org/10.3389/fnsyn.2011.00004 (2011).
Dan, Y. & Poo, M. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 86, 1033–1048, https://doi.org/10.1152/physrev.00030.2005 (2006).
Vlachos, A. et al. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci. 29, 1017–1033, https://doi.org/10.1523/JNEUROSCI.5528-08.2009, http://www.jneurosci.org/content/29/4/1017.full.pdf (2009).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nat. 429, 761–766 (2004).
Bosch, M. et al. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459, https://doi.org/10.1016/j.neuron.2014.03.021 (2014).
Sabatini, B., Maravall, M. & Svoboda, K. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 11, 349–356, https://www.ncbi.nlm.nih.gov/pubmed/?term=sabatini+bl+2001 (2001).
Kovalchuk, Y., Eilers, J., Lisman, J. & Konnerth, A. NMDA receptor-mediated subthreshold Ca(2+) signals in spines of hippocampal neurons. J Neurosci. 20, 1791–1799 https://www.ncbi.nlm.nih.gov/pubmed/?term=kovalchuk+2000+calcium (2000).
Mainen, Z., Malinow, R. & Svoboda, K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nat. 399, 151–155, https://www.ncbi.nlm.nih.gov/pubmed/10335844 (1999).
Emptage, N. Calcium on the up: supralinear calcium signaling in central neurons. Neuron 24, 495–497 https://www.ncbi.nlm.nih.gov/pubmed/10595500 (1999).
Emptage, N. Roles of calcium stores and store-operated channels in plasticity of dendritic spines. Neurosci. 22, 477–485, https://www.ncbi.nlm.nih.gov/pubmed/26511041, https://doi.org/10.1177/1073858415613277 (2016).
Abraham, W. & Bear, M. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19, 126–130 (1996).
Abraham, W. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9, 387 (2008).
Hulme, S. R., Jones, O. D. & Abraham, W. C. Emerging roles of metaplasticity in behaviour and disease. Trends Neurosci 36, 353–362, https://doi.org/10.1016/j.tins.2013.03.007 (2013).
Gray, E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. J. Anat. 93, 420–433, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1244535/ (1959).
Hanus, C. & Ehlers, M. D. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic 9, 1437–1445, https://doi.org/10.1111/j.1600-0854.2008.00775.x (2008).
Spacek, J. Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat. Embryol. 171, 235–243 (1985).
Spacek, J. & Harris, K. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 17, 190–203 (1997).
Fifkova, E., Markham, J. & Delay, R. Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res 266, 163–168 (1983).
Segal, M., Vlachos, A. & Korkotian, E. The spine apparatus, synaptopodin, and dendritic spine plasticity. The Neurosci. 16, 125–131, https://doi.org/10.1177/1073858409355829 PMID: 20400711 (2010).
Korkotian, E. & Segal, M. Synaptopodin regulates release of calcium from stores in dendritic spines of cultured hippocampal neurons. The J. Physiol. 589, 5987–5995, https://doi.org/10.1113/jphysiol.2011.217315 (2011).
Mundel, P. et al. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. The J. Cell Biol. 139, 193–204, https://doi.org/10.1083/jcb.139.1.193. http://jcb.rupress.org/content/139/1/193.full.pdf (1997).
Deller, T., Merten, T., Roth, S. U., Mundel, P. & Frotscher, M. Actin-associated protein synaptopodin in the rat hippocampal formation: Localization in the spine neck and close association with the spine apparatus of principal neurons. The J. Comp. Neurol. 418, 164–181 (2000).
Deller, T. et al. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc. Natl. Acad. Sci. 100, 10494–10499, https://doi.org/10.1073/pnas.1832384100, http://www.pnas.org/content/100/18/10494.full.pdf (2003).
Jedlicka, P. et al. Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle. Hippocampus 19, 130–140, https://doi.org/10.1002/hipo.20489 (2009).
Korkotian, E., Frotscher, M. & Segal, M. Synaptopodin regulates spine plasticity: mediation by calcium stores. J. Neurosci. 34, 11641–11651, https://doi.org/10.1523/JNEUROSCI.0381-14.2014, http://www.jneurosci.org/content/34/35/11641.full.pdf (2014).
Vlachos, A. et al. Synaptopodin regulates denervation-induced homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. 110, 8242–8247, https://doi.org/10.1073/pnas.1213677110. http://www.pnas.org/content/110/20/8242.full.pdf (2013).
Strehl, A. et al. Systemic inflammation is associated with a reduction in synaptopodin expression in the mouse hippocampus. Exp. Neurol. 261, 230–235, http://www.sciencedirect.com/science/article/pii/S0014488614001484, https://doi.org/10.1016/j.expneurol.2014.04.033 (2014).
Lenz, M., Ben Shimon, M., Deller, T., Vlachos, A. & Maggio, N. Pilocarpine-induced status epilepticus is associated with changes in the actin-modulating protein synaptopodin and alterations in long-term potentiation in the mouse hippocampus. Neural Plast. 2017, 7, https://doi.org/10.1155/2017/2652560 (2017).
Palay, S., Sotelo, C., Peters, A. & Orkand, P. The axon hillock and the initial segment. J Cell Biol 38, 193–201 (1968).
Somogyi, P. & Hamori, J. A quantitative electron microscopic study of the Purkinje cell axon initial segment. Neurosci. 1, 361–365 (1976).
Kosaka, T. The axon initial segment as a synaptic site: ultrastructure and synaptology of the initial segment of the pyramidal cell in the rat hippocampus (CA3 region). J Neurocytol 9, 861–882 (1980).
Lindsey, J. & Ellisman, M. The neuronal endomembrane system. III. the origins of the axoplasmic reticulum and discrete axonal cisternae at the axon hillock. J Neurosci 5, 3135–3144 (1985).
Bas Orth, C., Schultz, C., Muller, C., Frotscher, M. & Deller, T. Loss of the cisternal organelle in the axon initial segment of cortical neurons in synaptopodin-deficient mice. J Comp Neurol 504, 441–449 (2007).
Rasband, M. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11, 552–562 (2010).
Schlüter, A. et al. Structural plasticity of synaptopodin in the axon initial segment during visual cortex development. Cereb. Cortex 27, 4662–4675, https://doi.org/10.1093/cercor/bhx208 (2017).
Rosenbluth, J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol 13, 405–421 (1962).
Takahashi, K. & Wood, R. Subsurface cisterns in the Purkinje cells of cerebellum of Syrian hamster. Z Zellforsch Mikrosk Anat 110, 311–320 (1970).
Gallart-Palau, X. et al. Neuregulin-1 is concentrated in the postsynaptic subsurface cistern of C-bouton inputs to a-motoneurons and altered during motoneuron diseases. FASEB 28, 3618–3662 (2014).
Wittmann, M. et al. Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling. J Neurosci 29, 14687–14700 (2009).
Lee, K. F., Soares, C., Thivierge, J.-P. & Béïque, J.-C. Correlated synaptic inputs drive dendritic calcium amplification and cooperative plasticity during clustered synapse development. Neuron 89, 784–799 http://www.sciencedirect.com/science/article/pii/S0896627316000131, https://doi.org/10.1016/j.neuron.2016.01.012 (2016).
Cartailler, J., Kwon, T., Yuste, R. & Holcman, D. Deconvolution of voltage sensor time series and electro-diffusion modeling reveal the role of spine geometry in controlling synaptic strength. Neuron http://www.sciencedirect.com/science/article/pii/S089662731830059X, https://doi.org/10.1016/j.neuron.2018.01.034 (2018).
Vogel, A., Reiter, S., Rupp, M., Nägel, A. & Wittum, G. UG 4: A novel flexible software system for simulating PDE based models on high performance computers. Comput. Vis. Sci. 16, 165–179, https://doi.org/10.1007/s00791-014-0232-9 (2013).
Hoffer, M., Poliwoda, C. & Wittum, G. Visual reflection library: a framework for declarative GUI programming on the Java platform. Comput. Vis. Sci. 16, 181–192, https://doi.org/10.1007/s00791-014-0230-y (2013).
Reiter, S. ProMesh – Meshing of unstructured grids in 1, 2, and 3 dimensions, http://promesh3d.com (2012).
Reiter, S. Effiziente Algorithmen und Datenstrukturen für die Realisierung von adaptiven, hierarchischen Gittern auf massiv parallelen Systemen. Phd. thesis, Goethe-Universität Frankfurt am Main (2014).
Shewchuk, J. Constrained Delaunay tetrahedralizations and provably good boundary recovery. In Eleventh International Meshing Roundtable, 193–204 (2002).
Si, H. TetGen, a Delaunay-based quality tetrahedral mesh generator. ACM Trans. Math. Softw. 41, 11:1–11:36, https://doi.org/10.1145/2629697 (2015).
Rajan, V. T. Optimality of the delaunay triangulation in rd. Discret. & computational geometry 12, 189–202, https://doi.org/10.1007/BF02574375 (1994).
Deuflhard, P. & Weiser, M. Numerische Mathematik 3 - Adaptive Lösung partieller Differentialgleichungen (De Gruyter, 2011).
Xu, J. & Zikatanov, L. A monotone finite element scheme for convection-diffusion equations. Math. Comput. 68, 1429–1446 (1999).
Shewchuk, J. What is a good linear element? Interpolation, conditioning, and quality measures. In Eleventh International Meshing Roundtable, 115–126 (2002).
Allbritton, N., Meyer, T. & Stryer, L. Range of messenger action of calcium ion and inositol 1, 4, 5-trisphosphate. Sci. 258, 1812–1815, https://doi.org/10.1126/science.1465619, http://www.sciencemag.org/content/258/5089/1812.full.pdf (1992).
Schmidt, H., Stiefel, K. M., Racay, P., Schwaller, B. & Eilers, J. Mutational analysis of dendritic Ca2+ kinetics in rodent Purkinje cells: role of parvalbumin and calbindin D28k. The J. Physiol. 551, 13–32, https://doi.org/10.1113/jphysiol.2002.035824, http://jp.physoc.org/content/551/1/13.full.pdf+html (2003).
Veenstra, T. D., Johnson, K. L., Tomlinson, A. J., Naylor, S. & Kumar, R. Determination of calcium-binding sites in rat brain calbindin D28k by electrospray ionization mass spectrometry. Biochem. 36, 3535–3542, https://doi.org/10.1021/bi9628329 PMID: 9132004 (1997).
Nägerl, U. V., Novo, D., Mody, I. & Vergara, J. L. Binding kinetics of calbindin-D28k determined by flash photolysis of caged Ca2+. Biophys. J. 79, 3009–3018, http://linkinghub.elsevier.com/retrieve/pii/S0006349500765374, https://doi.org/10.1016/S0006-3495(00)76537-4, http://www.jbc.org/content/277/19/16662.full.pdf (2000).
Berggård, T. et al. Calbindin D28k exhibits properties characteristic of a Ca2+ sensor. J. Biol. Chem. 277, 16662–16672, https://doi.org/10.1074/jbc.M200415200, http://www.jbc.org/content/277/19/16662.full.pdf+html (2002).
Bezprozvanny, I. & Ehrlich, B. E. Inositol (1, 4, 5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. The J. Gen. Physiol. 104, 821–856, https://doi.org/10.1085/jgp.104.5.821, http: //jgp.rupress.org/content/104/5/821.full.pdf+html (1994).
De Young, G. W. & Keizer, J. A single-pool inositol 1, 4, 5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. 89, 9895–9899 http://www.pnas.org/content/89/20/9895.full.pdf+html (1992).
Keizer, J. & Levine, L. Ryanodine receptor adaptation and Ca2+-induced Ca2+ release-dependent Ca2+ oscillations. Biophys. J. 71, 3477–3487, https://doi.org/10.1016/S0006-3495(96)79543-7, http://download.cell.com/biophysj/pdf/PIIS0006349596795437.pdf (1996).
Tinker, A., Lindsay, A. R. G. & Williams, A. J. Cation conduction in the calcium release channel of the cardiac sarcoplasmic reticulum under physiological and pathophysiological conditions. Cardiovasc. Res. 27, 1820–1825, https://doi.org/10.1093/cvr/27.10.1820, http://cardiovascres.oxfordjournals.org/content/27/10/1820.full.pdf (1993).
Sneyd, J. et al. A model of calcium waves in pancreatic and parotid acinar cells. Biophys. J. 85, 1392–1405, http://www.sciencedirect.com/science/article/pii/S000634950374572X, https://doi.org/10.1016/S0006-3495(03)74572-X (2003).
Graupner, M. A theory of plasma membrane calcium pump function and its consequences for presynaptic calcium dynamics. Diploma thesis, Technische Universität Dresden (2003).
Nimchinsky, E. A., Yasuda, R., Oertner, T. G. & Svoboda, K. The number of glutamate receptors opened by synaptic stimulation in single hippocampal spines. J. Neurosci. 24, 2054–2064, https://doi.org/10.1523/JNEUROSCI.5066-03.2004, http://www.jneurosci.org/content/24/8/2054.full.pdf (2004).
Jahr, C. E. & Stevens, C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc. Natl. Acad. Sci. 90, 11573–11577, https://doi.org/10.1073/pnas.90.24.11573, http://www.pnas.org/content/90/24/11573.full.pdf (1993).
Jahr, C. & Stevens, C. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J. Neurosci. 10, 3178–3182, https://doi.org/10.1523/JNEUROSCI.10-09-03178.1990, http://www.jneurosci.org/content/10/9/3178.full.pdf (1990).
Saris, N.-E. L., Mervaala, E., Karppanen, H., Khawaja, J. A. & Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chimica Acta 294, 1–26, http://www.sciencedirect.com/science/article/pii/S0009898199002582, https://doi.org/10.1016/S0009-8981(99)00258-2 (2000).
Fink, C. C. et al. An image-based model of calcium waves in differentiated neuroblastoma cells. Biophys. J. 79, 163–183, http://www.sciencedirect.com/science/article/pii/S0006349500762813, https://doi.org/10.1016/S0006-3495(00)76281-3 (2000).
van der Vorst, H. A. B-CGSTAB: A fast and smoothly converging variant of Bi-CG for the solution of nonsymmetric linear systems. SIAM J. on Sci. Stat. Comput. 13, 631–644, https://doi.org/10.1137/0913035. (1992).
Jülich Supercomputing Centre. JURECA: General-purpose supercomputer at Jülich Supercomputing Centre. J. large-scale research facilities 2, https://doi.org/10.17815/jlsrf-2-121 (2016).
Luzzi, V., Sims, C. E., Soughayer, J. S. & Allbritton, N. L. The physiologic concentration of inositol 1, 4, 5-trisphosphate in the oocytes of Xenopus laevis. J. Biol. Chem. 273, 28657–28662, https://doi.org/10.1074/jbc.273.44.28657, http://www.jbc.org/content/273/44/28657.full.pdf+html (1998).
Müller, A. et al. Endogenous Ca2+ buffer concentration and Ca2+ microdomains in hippocampal neurons. The J. Neurosci. 25, 558–565, https://doi.org/10.1523/JNEUROSCI.3799-04.2005, http://www.jneurosci.org/content/25/3/558.full.pdf+html (2005).
Wang, S. S., Alousi, A. A. & Thompson, S. H. The lifetime of inositol 1, 4, 5-trisphosphate in single cells. The J. Gen. Physiol. 105, 149–171, https://doi.org/10.1085/jgp.105.1.149, http://jgp.rupress.org/content/105/1/149.full.pdf+html (1995).
Means, S. et al. Reaction diffusion modeling of calcium dynamics with realistic ER geometry. Biophys. J. 91, 537–557, http://linkinghub.elsevier.com/retrieve/pii/S0006349506717532 (2006).
Chiu, V. C. K. & Haynes, D. H. Rapid kinetic studies of active Ca2+ transport in sarcoplasmic reticulum. J. Membr. Biol. 56, 219–239, https://doi.org/10.1007/BF01869478 (1980).
Elwess, N. L., Filoteo, A. G., Enyedi, A. & Penniston, J. T. Plasma membrane Ca2+ pump isoforms 2a and 2b are unusually responsive to calmodulin and Ca2+. J. Biol. Chem. 272, 17981–17986, https://doi.org/10.1074/jbc.272.29.17981, http://www.jbc.org/content/272/29/17981.full.pdf+html (1997).
Acknowledgements
The authors gratefully acknowledge the computing time granted by the John von Neumann Institute for Computing (NIC) and provided on the supercomputer JURECA at Jülich Supercomputing Centre (JSC). This work was supported by Deutsche Forschungsgemeinschaft (CRC974 to AV), BMBF (Collaborative Research in Computational Neuroscience 01GQ1410B to GQ), and a joint NIH/BMBF Collaborative Research Grant to AV and GQ (R01MH118930-01).
Author information
Authors and Affiliations
Contributions
The study was conceived and supervised by A.V. and G.Q. The model was developed by M.B. and G.Q. Simulations were carried out by M.B. and M.K. M.K. and M.S. developed the spine generator. The manuscript was written by A.V. and G.Q. with the help of M.B. and M.S. A.V., G.Q. and M.B. were involved in data interpretation and critically revising the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Breit, M., Kessler, M., Stepniewski, M. et al. Spine-to-Dendrite Calcium Modeling Discloses Relevance for Precise Positioning of Ryanodine Receptor-Containing Spine Endoplasmic Reticulum. Sci Rep 8, 15624 (2018). https://doi.org/10.1038/s41598-018-33343-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33343-9
Keywords
This article is cited by
-
NeuroBox: computational mathematics in multiscale neuroscience
Computing and Visualization in Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.