Abstract
The transcriptional response of cells exposed to proton radiation is not equivalent to the response induced by traditional photon beams. Changes in cellular signalling is most commonly studied using the method Quantitative polymerase chain reaction (qPCR). Stable reference genes must be used to accurately quantify target transcript expression. The study aim was to identify suitable reference genes for normalisation of gene expression levels in normal dermal fibroblasts irradiated with either proton or photon beams. The online tool RefFinder was used to analyse and identify the most stably expressed genes from a panel of 22 gene candidates. To assess the reliability of the identified reference genes, a selection of the most and least stable reference genes was used to normalise target transcripts of interest. Fold change levels varied considerably depending on the used reference gene. The top ranked genes IPO8, PUM1, MRPL19 and PSMC4 produced highly similar target gene expression, while expression using the worst ranked genes, TFRC and HPRT1, was clearly modified due to reference gene instability.
Similar content being viewed by others
Introduction
Proton beam therapy induces an altered stress response in both cancerous and normal cells compared with traditional photon beam therapy1. Previous research indicates that molecular mechanisms such as DNA repair and epigenetic regulation may be affected differently by various radiation qualities2,3,4,5,6. Furthermore, the transcriptional response may also be influenced by position in the proton beam as the linear energy transfer (LET) increases along the proton track7. Therefore, it seems unlikely that the differences in transcriptional response to photon and proton irradiation can be equated by modulating the radiation dose. Potential changes in cellular signalling may lead to alternate outcomes regarding both tumour progression and normal tissue complication development thereby stressing the importance of investigating proton radiobiology.
Currently, quantitative real-time polymerase chain reaction (qPCR) is the most widely used technique for studying differential expression of specific genes following an experimental treatment exposure. Implementation of the qPCR technique has greatly contributed to improve knowledge of cellular responses to various treatment modalities using numerous tumour and normal tissue models8,9,10,11. The comparative CT method is the most widely used strategy for qPCR data analysis12. The method relies on endogenous reference genes to determine relative expression of target transcripts. Expression of ideal reference genes is unaffected by the treatment exposure and stable across the investigated cell types. Genes such as GAPDH and ACTB have traditionally been used as reference genes without much consideration to their stability under the specific conditions of a given experiment13. However, the stability of GAPDH and ACTB have been shown to be affected by a number of experimental exposures and it is unlikely that any universal reference gene exists14. Optimal reference genes for qPCR data analysis have been suggested for multiple tumour cell lines irradiated with particle and photon beams but, to our knowledge, there have not been any reported reference genes for normal cells15.
The present study aimed to identify and evaluate stably expressed reference genes in proton and photon irradiated primary dermal fibroblast cultures. The genes IPO8, MRPL19, PSMC4, and PUM1 performed best in the applied selection algorithms and produced highly similar relative quantification of target transcripts.
Results
In the present study, new qPCR gene expression data for candidate reference genes and target transcripts of interest are presented. The data are derived from material generated in a previous study, where qPCR data for other transcripts were reported. LET values presented here were reported in the previous study7.
Depth-dose profile and LET values
Primary fibroblasts were positioned in the entrance, the middle of the SOBP and the distal edge of the SOBP using three different depth-dose plans to deliver 3.5 Gy(RBE) × 3 fractions in all groups but with increasing LET along the depth-dose profile. Dose-averaged LET values were 1.1, 3.3 and 8.4 keV/µm for protons only, ignoring secondary particles, in the entrance, mid-SOBP and SOBP distal edge groups. The depth-dose profile for cells irradiated with the highest LET proton beam is displayed in Fig. 1.
Depth-dose profile of the proton beam used to irradiate fibroblasts in the SOBP distal edge. The blue line shows the dose deposition and the red shows how the dose-averaged LET increases along the proton track. The blue dot marks the position of the culture flasks. The dotted lines approximately mark the position depth of flasks in the entrance and mid-SOBP groups. Equivalent profiles for the entrance and mid-SOBP groups were scaled to deliver 3.5 Gy(RBE) in their respective positions.
Overview of CT values for candidate reference genes
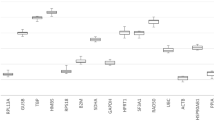
Nine primary dermal fibroblast cultures were used in the treatment and control groups. Descriptive statistics are provided for the CT values of all the included candidate reference genes in Table 1. PMM1 and RPL37A had the smallest coefficient of variation at 1.7%, while ACTB and GAPDH had the highest degree of dispersion with CVs at 3.1% and 3.2%. RPL37A had the smallest CT range with 1.9 cycles from the lowest to the highest CT value across all 45 samples. Mean CT values for all candidates ranged from 17.1 to 27.3, thus covering most of the range for potential target transcripts.
Identification of the most stable reference genes
The online tool RefFinder was used to generate an overall ranking of the most stable reference genes based on the scores from the Genorm, NormFinder, BestKeeper and ΔCt algorithms (Fig. 2). The genes PUM1, CALM2, and MRPL19 had the best overall scores. The algorithms Genorm, NormFinder and ΔCt produced similar results, while BestKeeper identified other genes as ideal for normalization. NormFinder found the combination of PUM1 and CALM2 optimal for normalization, these were identified as best and third best by the ΔCt method and Genorm ranked them fourth and sixth. Genorm identified IPO8, MRPL19 and PSMC4 as the most stably expressed genes across all samples. NormFinder ranked them third, fifth and sixth most stable, while the ΔCt method ranked them fourth, fifth, and sixth. BestKeeper identified RPL37A and PMM1 as optimal reference genes. All of the algorithms identified TFRC as the least stable reference gene with HPRT1 as the second least stable. The most frequently used reference gene GAPDH was ranked third least stable of the 22 included candidates.
Ranking of the 22 reference gene candidates. The most stable genes, as identified by the RefFinder overall scoring, are shown left to right on the x-axis. The curves show the achieved rank in the four algorithms ΔCt method, Genorm, NormFinder and Bestkeeper. Nine primary dermal fibroblast cultures were used in each of the four treatment groups and in the untreated control group.
Assessing the impact of reference gene selection
It is an inherent problem in identification of optimal reference genes that selected reference genes cannot be clearly validated. Instead, reliability can be evaluated by determining how fold changes of target transcripts are influenced by different reference genes. Here, BTG2, IL1b and PDGFB were chosen as target transcripts as they are involved in critical molecular mechanisms such as regulation of cell proliferation, inflammatory response, angiogenesis, and differentiation. Fold changes were calculated for target transcripts by normalisation with the reference genes IPO8, PMM1, MRPL19, PSMC4, PUM1 and CALM2 and by using the average of TFRC and HPRT1. IPO8, PMM1, MRPL19, PSMC4, PUM1 and CALM2 were chosen as they were recommended by the different algorithms in RefFinder, thus not selected by the overall ranking. The average of TFRC and HPRT1 is also included in the evaluation as the genes performed worst in all the algorithms and thereby provides a basis for comparison. Using IPO8, MRPL19, PSMC4 or PUM1 as reference genes produced similar fold changes for all target genes within respective treatment groups. PMM1 and CALM2 generated smaller fold changes than when TFRC and HPRT1 were applied (Fig. 3).
Estimated median fold change levels of the genes BTG2, IL1b and PDGFB. Median fold changes are shown for the four radiation treated groups relative to controls with 95% confidence intervals. The same 12 cell lines were used in each group (n = 12). Six of the best performing reference genes and the average of the two worst performing reference genes were used to calculate median fold change to assess the impact of using different reference genes.
It is also of great interest to investigate how differences between treatment groups are affected by normalisation with different reference genes. The influence of radiation quality on target transcript regulation is determined by performing a one-way Analysis of Variance (ANOVA) with a random patient effect to test for no difference in estimated median fold change between treatment groups for BTG2, IL1b, and PDGFB. The differences in fold changes between groups were quantified by post-hoc, pairwise comparisons. CALM2 and PUM1 produced the smallest and biggest differences between treatment groups for all three target transcripts. For instance, BTG2 fold change was more strongly induced in the SOBP distal edge group than in the entrance group no matter what reference gene was used. Expression was increased by 26% (95%-CI: 9–45%, p < 0.0001) when CALM2 was used as reference and 99% (14–248%, p = 0.016) when PUM1 was used as reference.
Discussion
Three of the four selection algorithms employed in the RefFinder tool generally ranked IPO8, MRPL19, PSMC4, PUM1, and CALM2 as the most stable. However, BestKeeper identified PMM1 and RPL37A as the most stable genes. Those two genes also had narrow CT ranges across all samples and the lowest Coefficients of Variation. Rydbirk et al. also reported that BestKeeper produced a ranking that deviated from the other algorithms16. A major drawback of using these selection algorithms is that they cannot determine whether the most stable genes are actually sufficiently stable for accurate analysis. To limit the effects of using an inappropriate reference gene, the average CT of 3 or more reference genes should generally be employed as a normalization factor17.
It is evident in the present study that the choice of reference gene can affect fold change levels of target transcripts to such an extent that it will influence the biological interpretation. This is observed when comparing fold change levels of IL1b when normalised with either the average of TFRC and HPRT1 or CALM2. TFRC and HPRT1 normalized fold change levels always show a substantial upregulation of IL1b, while CALM2 normalized expression is either unaffected, up- or downregulated, thus resulting in two different conclusions on how IL1b is regulated in fibroblasts following radiation exposure. Similar observations can be made for PDGFB. While BTG2 is conclusively upregulated by irradiation no matter what reference gene is used, the extent of upregulation is strongly affected by choice of reference gene.
Interestingly, the average CT of PUM1 and CALM2 were identified by NormFinder as the optimal normalization factor although they produced highly varying fold change levels in the present study. Actually, normalization with either PUM1 or CALM2 resulted in the biggest and smallest increase of BTG2 median fold change between the entrance and SOBP distal edge treatment groups with 99% and 26%, respectively. Critically, when using any of the two genes for normalization, a significant increase in BTG2 expression could be identified in the SOBP distal edge group compared with the entrance group, thus not drastically altering the conclusion on how different LET proton beams influence regulation of BTG2.
Sharungbam et al. have previously reported RPLP0, UBC, PPIA, TBP and PSMC4 to be the most stable reference genes from a panel of 32 candidates15. These were identified using three tumour cell lines treated with photon, proton and carbon-ion irradiation. Except for UBC, the other genes were included in the candidate panel in the present study and only PSMC4 was ranked among the best performing reference genes. This discrepancy may have multiple explanations. The potentially substantial cytogenetic differences between established tumour cell lines and primary normal cell lines could alone lead to identification of different optimal reference gene sets. Furthermore, carbon-ion irradiation used in the study by Sharungbam et al. appears to have a unique impact on molecular processes which will affect gene expression thereby leading to identification of reference genes suitable for photon, proton, and carbon-ion irradiation18. Finally, the differences in composition of the candidate gene panels could affect the outcome. The Genorm algorithm, also used by Sharungbam et al., employs stepwise exclusion of the least stable candidate gene based on the stability score M. For a specific candidate gene, M is the average pairwise variation with all other candidate genes17. The composition of the candidate gene panel can especially impact selection of optimal reference genes if any panel genes are co-expressed19.
In the present study, it was demonstrated that identifying appropriate reference genes for comparative CT analysis is critical in order to obtain robust results. Choice of reference genes may affect the quantification of target transcript fold change levels. IPO8, MRPL19, PSMC4 and PUM1 were among the most stably expressed genes in dermal fibroblasts exposed to different radiation qualities as identified by the most commonly used selection algorithms. The identified genes comprise a suitable normalization factor in the comparative CT method and the genes are suggested as candidates for future proton radiobiology research where qPCR is employed to determine differential gene expression in non-malignant cells.
Materials and Methods
Primary dermal fibroblast cultures
The primary dermal fibroblast cultures used in the present study were available from a biobank previously generated at the Aarhus University Hospital20. Fibroblast cultures were established using skin biopsies taken from the forearm of patients about to undergo treatment for head and neck cancer. At the time, patients provided informed consent for their samples to be used in research. The use of the fibroblast biobank for this study was approved by the Danish National Committee of Medical Ethics (case no. 1-10-72-170-15) and the study is performed in accordance with their guidelines.
Cell culturing
AmnioMAX-C100 Basal Medium supplemented with 7.5% AmnioMAX-C100 Supplement, 7.5% HyCloneTM fetal bovine serum, 1% penicillin–streptomycin and 1% L-Glutamine 200 mM (ThermoFisher Scientific, Waltham, MA, USA) was used for all cell culturing. Incubation was performed at 37 °C with 5% CO2 and around 90% relative humidity. Cultures were passaged twice before approximately 200,000 cells were seeded in T25 flasks and cultured for 3 days. Thereafter, growth medium was changed and cultures were incubated for another 24 hours. The culture flasks had to be irradiated in a vertical position hence the flasks were filled with medium immediately prior to delivery of the first of three fractions. The flasks were kept vertical and filled with medium until the cells were harvested 2 days later. Surface adherence, growth rate and lethality of the fibroblasts were previously verified to be identical in the vertical position compared with the same fibroblasts kept under standard culturing conditions.
Proton scanning beam and Cobalt-60 photon irradiation
Irradiation was performed at the Institute of Nuclear Physics – Polish Academy of Sciences, Krakow, Poland. To deliver the radiation dose, three culture flasks per fraction were placed vertically in a water-filled polymethylmethacrylate (PMMA) phantom. All cell cultures were irradiated following the same radiation regimen of 3.5 Gy(RBE) × 3 fractions with 24-hour intervals and using a RBE of 1.1 to scale proton doses. The IBA ProteusPLUS gantry with a Pencil Beam Scanning (PBS) dedicated nozzle was used for all proton irradiations. Fibroblast cultures were placed in 3 different positions of the proton depth-dose profile. They were placed in the entrance, SOBP centre or at the SOBP distal edge of different depth-dose profiles in order to deliver the same dose in each group. X-ray imaging and a Markus Ion Chamber were used to position the phantom and verify the depth-dose profiles. Total uncertainty in dose delivery was lower than 2.5%. Cobalt-60 beam irradiation was delivered using the Theratron 780E system with a 0.307 Gy/min dose rate. Additional set-up details are previously reported elsewhere7.
RNA purification and qPCR
Cell lysis and RNA purification were performed with the miRNeasy mini kit (Qiagen, Hilden, Germany). Cells were lysed with the Quiazol Lysis Reagent 2 hours after the last fraction was delivered. Residual genomic DNA was enzymatically digested in the purification process. QubitTM 3.0 Fluorometer with Qubit RNA Broad-Range Assay Kit was used to determine the RNA concentration in samples. The High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific) was used to generate cDNA from approximately 2 µg of RNA according to the manufacturer’s instructions. Transcripts were quantified using Taqman Gene Expression Assays (ThermoFisher Scientific) except for the CALM2 and RPL37A transcripts, where probes and primers from DNA technology were used (DNA Technology, Risskov, Denmark). Taqman assays spanning exon-exon junctions were selected when available to minimise risk of genomic DNA contamination. The 7900HT Fast Real-Time PCR System was used to perform qPCR (ThermoFisher Scientific).
Identifying optimal reference genes and statistical analysis
Twenty-two potentially suitable reference genes commonly used for qPCR data normalization were chosen as candidates (Table 1). The online tool RefFinder (currently available at: http://150.216.56.64/referencegene.php) was used to evaluate the expression stability of reference gene candidates by applying the four algorithms: Genorm17, NormFinder19, BestKeeper21 and the ΔCt method22. RefFinder also produced an overall performance ranking of the candidate genes based on the scores obtained in the four algorithms. The signalling pathways and functions of the included candidate genes were examined to limit the risk of co-dependent expression, which potentially could skew the selection of optimal reference genes in some of the applied algorithms.
Regulation of target genes in response to irradiation is described here as fold changes. Fold changes of the three target genes BTG2 (Hs00198887_m1), IL1b (Hs01555410_m1), and PDGFB (Hs00966522_m1) were produced using selected reference genes to assess how they perform in normalisation. The comparative CT method was used to calculate fold changes12:
Log-transformation was performed on fold changes to approach a normal distribution. Analysis of Variance (ANOVA) with a random patient effect was performed to test for no difference between treatment groups. Pairwise comparisons were used to identify differences in median fold changes between groups. Stata software, version 14.2, StataCorp (College Station, Texas, USA) was used for data analysis.
Monte-Carlo simulations
Particle transport simulations were performed to obtain detailed spatial distribution of dose and LET. For this purpose, the Monte-Carlo particle transport code SHIELD-HIT12A code version 0.7.2. was used23. To obtain satisfactory statistical convergence of the results this code was run in parallel on 200 CPU cores on Prometheus supercomputer, on each core, trajectories of 106 primary protons and all secondary particles were transported. Simulated geometry consisted of a simplified model of flasks filled with medium, cells and water phantom. The initial scanning proton beam spot weights, positions, and initial kinetic energies were obtained from the accelerator control file. Output parameters were scored on a rectangular mesh, with 1 × 1 cm² cross-section and 1 mm resolution in the beam axis direction.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Durante, M. New challenges in high-energy particle radiobiology. British Journal of Radiology 87 (2014).
Girdhani, S., Lamont, C., Hahnfeldt, P., Abdollahi, A. & Hlatky, L. Proton irradiation suppresses angiogenic genes and impairs cell invasion and tumor growth. Radiat. Res. 178, 33–45 (2012).
Goetz, W., Morgan, M. N. M. & Baulch, J. E. The Effect of Radiation Quality on Genomic DNA Methylation Profiles in Irradiated Human Cell Lines. Radiat. Res. 175, 575–587 (2011).
Fontana, A. O. et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother. Oncol. 116, 374–380 (2015).
Narang, H., Kumar, A., Bhat, N., Pandey, B. N. & Ghosh, A. Effect of Proton and Gamma Irradiation on Human Lung Carcinoma Cells: Gene Expression, Cell Cycle, Cell Death, Epithelial-Mesenchymal Transition and Cancer-Stem Cell trait as Biological End Points. Mutat. Res. Mol. Mech. Mutagen. 780, 35–46 (2015).
Niemantsverdriet, M. et al. High and low LET radiation differentially induce normal tissue damage signals. Int. J. Radiat. Oncol. Biol. Phys. 83, 1291–1297 (2012).
Nielsen, S. et al. Differential gene expression in primary fibroblasts induced by proton and cobalt-60 beam irradiation. Acta Oncol. 56, 1406–1412 (2017).
Alsner, J., Rødningen, O. K. & Overgaard, J. Differential gene expression before and after ionizing radiation of subcutaneous fibroblasts identifies breast cancer patients resistant to radiation-induced fibrosis. Radiother. Oncol. 83, 261–266 (2007).
Sørensen, B. S. et al. The usability of a 15-gene hypoxia classifier as a universal hypoxia profile in various cancer cell types. Radiother. Oncol. 116, 346–351 (2015).
Gilder, A. S. et al. The Urokinase Receptor Induces a Mesenchymal Gene Expression Signature in Glioblastoma Cells and Promotes Tumor Cell Survival in Neurospheres. Sci. Rep. 8, 2982 (2018).
Bandrés, E. et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer 5 (2006).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008).
Sharan, R. N., Vaiphei, S. T., Nongrum, S., Keppen, J. & Ksoo, M. Consensus reference gene(s) for gene expression studies in human cancers: end of the tunnel visible? Cellular Oncology 38, 419–431 (2015).
Bustin, S. A. Absolute quantification of mrna using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25, 169–193 (2000).
Sharungbam, G. D. et al. Identification of stable endogenous control genes for transcriptional profiling of photon, proton and carbon-ion irradiated cells. Radiat. Oncol. 7, 70 (2012).
Rydbirk, R. et al. Assessment of brain reference genes for RT-qPCR studies in neurodegenerative diseases. Sci. Rep. 6, 37116 (2016).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 34–1 (2002).
Hamada, N. Recent Insights into the Biological Action of Heavy-Ion Radiation. J. Radiat. Res. 50, 1–9 (2009).
Andersen, C., Jensen, J. & Orntoft, T. Normalization of Real Time Quantitative Reverse Transcription PCR Data: Model Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 64, 5245 (2004).
Andreassen, C. N., Overgaard, J. & Alsner, J. Independent prospective validation of a predictive test for risk of radiation induced fibrosis based on the gene expression pattern in fibroblasts irradiated in vitro. Radiother. Oncol. 108, 469–472 (2013).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515 (2004).
Silver, N., Best, S., Jiang, J. & Thein, S. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 33 (2006).
Bassler, N. et al. SHIELD-HIT12A - A Monte Carlo particle transport program for ion therapy research. J. Phys. Conf. Ser. 489 (2014).
Acknowledgements
The authors want to acknowledge the contributions made by laboratory technicians Lene Bjerrre Borup and Alice Baden and staff members of the Cyclotron Centre Bronowice. This study was financially supported by the Danish Cancer Society, under grant numbers R98-A6344-14-S24 and R125-A7753-15-S37, and Dansk Kræftforsknings fond (2015). PL-Grid Infrastructure provided computational resources.
Author information
Authors and Affiliations
Contributions
S.N., N.B. and B.S.S. conceived and designed the experiments. S.N. performed the experimental work. N.B. and L.G. performed Monte Carlo simulations and provided dose and LET values. J.S. and P.O. provided proton beam time and assisted in building experimental set-up. C.A. established the employed biobank. S.N., B.S.S. and J.A. performed statistical analyses. The manuscript was prepared by S.N. under supervision from B.S.S. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nielsen, S., Bassler, N., Grzanka, L. et al. Optimal reference genes for normalization of qPCR gene expression data from proton and photon irradiated dermal fibroblasts. Sci Rep 8, 12688 (2018). https://doi.org/10.1038/s41598-018-30946-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30946-0
This article is cited by
-
Validation of reference genes for use in untreated bovine fibroblasts
Scientific Reports (2021)
-
Disparities in Cardiac Care of Women: Current Data and Possible Solutions
Current Treatment Options in Cardiovascular Medicine (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.