Abstract
Auricular vasomotor responses are considered to be signs of clinical conditions including migraine. The mechanisms of auricular vasomotor control are still debatable. This study aimed at investigating perivascular co-transmitters of vasomotor control in the auricle. Another aim was to provide three-dimensional arterial maps of the auricle, as a proxy of periarterial autonomic innervation. Twelve paired human auricles were used to visualize the arteries following Spalteholz clearing and μ-CT-based reconstruction. Perivascular innervation staining was conducted using anti-tyrosine hydroxylase (TH), anti-neuropeptide Y (NPY), anti-vasoactive intestinal peptide (VIP) and anti-choline acetyl transferase (ChAT). The combined Spalteholz technique and μ-CT revealed a highly consistent arrangement of the auricular vasculature. The superficial temporal (STA) and posterior auricular artery (PAA) supply the helical rim arcade and arcade, with the STA mainly forming the superior and the PAA forming the middle and inferior auricular artery. Co-existence of sympathetic NPY+ and TH+ terminals mediating vasoconstriction, and VIP+ and ACh+ indicating cholinergic vasodilatation, was found in the perivascular zone. The presence of both sympathetic vasoconstriction and cholinergic co-innervation for active vasodilatation was shown in the perivascular auricular zone. Assuming that the highly-consistent vasculature gives way to these terminals, this periarterial innervation may be found spread out across the helix.
Similar content being viewed by others
Introduction
The blood supply of the human auricle is known to be provided by the branches of both the superficial temporal artery (STA) and the posterior auricular artery (PAA), both being part of the circulation of the external carotid artery1,2. The predominant mechanism of arterial vasomotor control is based on changes of the sympathetic vascular tone. Vasodilation has been conclusively shown to be the effect of inhibiting the sympathetic vasoconstrictor tone but not due to increased activity of parasympathetic influences3. It has also been postulated that facial nerve parasympathetic fibers may be responsible for the facial autonomic symptomatology on the basis of the trigemino-autonomic reflex (TAC) hypothesis, to the effect that may cause vasodilatation of the ear vasculature4. Prominent trigemino-parasympathetic activations have been reported in case of TAC, along with sympathetic deficits5.
These vasoregulatory mechanisms may have implications for a few signs and symptoms related to the auricle. The red ear syndrome (RES) is known to correlate with migraine, upper cervical disorders and with trigeminal autonomic cephalgia6,7,8,9,10,11,12,13,14,15. In the context of the interconnected trigeminal nerve and facial parasympathetic outflow in the brainstem, the trigeminal-autonomic reflex has been proposed as one potential pathway of RES4. Trigeminovascular activation via the auriculotemporal nerve has also been proposed as an underlying mechanism of action8. The cervical-autonomic reflex was furthermore described as a contributor for the auricular-vascular response in upper cervical disorders via the second and third cervical roots, contributing to ear lobe innervation8. It has been theorized that the RES may result in conjunction with TACs, which may facilitate an imbalanced autonomic system to induce an inhibition of the sympathetic tone of the ear. The sympathetic dysregulation but not the parasympathetic activation is considered to be the predominant mechanism for the RES; therefore, the TAC is thought to play a minor role in isolated cases of RES5. These theories concern the trigemino-autonomic dysregulation in RES, TACs and associated clinical conditions, emphasizing inconsistency regarding the present knowledge on how the human auricle and its vasculature are innervated. Migraine can be associated with RES and is partially provoked by parasympathetic hyper-activation. Consequently, RES may also be a specific sign of parasympathetic activation during migraine16. Detailed information on the perivascular innervation of the human ear with particular focus on the auricular helix is helpful to clarify the underlying mechanisms causing RES, as an insight into autonomic alteration, e.g. as migraine.
This study primarily aimed to investigate the autonomic innervation of the auricular helix with particular focus on the perivascular zone using the co-transmitters predominantly for autonomic nerve supply for vasomotor control to identify the contributing axons in the context of sympathetic and parasympathetic innervation. A second aim was to use the unique approach combining three-dimensional (3D) reconstructions from human auricles cleared with the Spalteholz technique to clarify the consistency of the ear vasculature tree in addition to provide a three-dimensional map of the auricular arterial system. This may be considered as an important pathway for the distribution of sympathetic nerves in the ear in the context of the sympathetic nerves following the facial and ear arteries.
Materials and Methods
While alive, the body donors gave written and informed consent to the donation of their tissues for research and teaching purposes. All tissues were obtained in accordance to the Death and Funeral Act of Vienna (version 2013, section 2, §12,13) and the Saxonian Death and Funeral Act (version 2014, section 8, §18). Ten paired auricles were removed from five human cadavers in a fresh and anatomically unfixed condition (mean age 83.4 ± 10.3 years, 3 females and 2 males) from the Medical University of Vienna. Another two auricles were obtained from a 96-year-old female cadaver from the University of Leipzig. Institutional approval was obtained from both Universities according to this legislation.
Spalteholz technique with consecutive vascular staining and μ-CT
Ten paired auricles were rinsed in isotonic saline prior to injecting red silicone (Elastosil RT 601, Wacker Chemie AG, Burghausen, Germany), according to the procedure shown by Zilinsky et al.2. After casting in resin, the auricles were fixed in 10% (by volume) formaldehyde for three days, then rinsed in tap water and 4% (by volume) hydrogen peroxide for one day each, and then dehydrated in ascending ethanol for five days. Following this, the auricles were transferred into wintergreen oil (methyl ester of salicylic acid, Sigma-Aldrich, Munich, Germany) for two days before being placed in a fresh bath of wintergreen oil, as described by Spalteholz17.
Following the injection procedure, the auricles were scanned using a General Electric phoenix v/tome/x s μ-CT (GE Measurement & Control Solutions, Wunstorf, Germany) with an isovoxel size of 40 (± 2) μm. Segmentation of the region of interest was carried out using Software VG Studio MAX 3.0 (Volumen Graphics GmbH, Heidelberg, Germany), with a threshold-based separation of soft tissue from the vascular injection agent. Larger artifacts were removed manually prior to importing the data as STL files in 3-matic (Materialise GmbH, Gilching, Germany) and then exporting as a 3D-PDF. These files served as a basis to analyze the distribution of the helical arcade and helical rim arcade.
Histology and Immunohistochemistry
Immediately after removal two auricles were immersion-fixed in 4% paraformaldehyde (PFA) with phosphate buffer saline (PBS; 0.1 M; pH 7.4) for six weeks. One ear was cut into radial segments as shown in Fig. 1. Planes were chosen with the external acoustic meatus as an axis. For cryoprotection, segments were immersed in 30% sucrose in PBS with 0.1% sodium azide. 30 µm thick cryosections were made on a cryomicrotome Zeiss Hyrax S30 with freezing unit Zeiss hyrax KS34; sections were collected in PBS with 0.1% sodium azide.
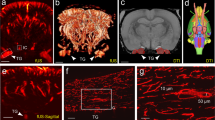
Magnification of a Spalteholz ear showing the area four sections which were used for histology and immunohistochemistry (left), and Anti-choline acetyltransferase (ChAT), anti-vasoactive intestinal peptide (VIP) stainings of the perivascular region indicating cholinergic/parasympathetic innervation as well as well as anti-tyrosine hydroxylase (TH) and anti-neuropeptide Y (NPY) stainings of the perivascular region indicating sympathetic innervation.
For the hematoxylin-eosin staining, sections were mounted on glass slides, dried and processed according to standard procedures18. Prior to immunohistochemical staining, further sections were treated with 60% methanol and 2% H2O2 for 1 hour, followed by a blocking step (blocking solution: PBS-T with 2% bovine serum albumin, 0.3% milk powder and 0.5% donkey serum) for one hour and washed in PBS-T (PBS with 0.05% Tween 20). Slices were incubated with the primary antibodies (diluted in blocking solution) overnight at 4 °C. Primary antibodies are listed in Table 1. After washing in PBS-T, the sections were incubated with biotinylated secondary antibodies (see Table 2) for one hour, followed by incubation with extravidin-peroxidase (Sigma, 1:2000) for one hour and visualization by a nickel-enhanced peroxidase reaction with 3,3′-diaminobenzidine. Sections were mounted onto glass slides and cover slipped with Entellan® in toluene.
Microscopy
Tissue sections were imaged using a Keyence research microscope (BZ9000, Keyence, Neu-Isenburg, Germany). Photoshop CS2 (Adobe Systems, Mountain View, CA, USA) was used to process the images with minimal alterations to color, saturation, contrast and background.
Results
Combined Spalteholz technique and μ-CT show the three-dimensional arrangement of the vascular supply of the auricle as being highly consistent
The μ-CT based reconstruction of the Spalteholz auricles has shown one to three distinct arches forming the helical rim arcade with contributions from the auricular branches, commonly with at least one or more continuous and one discontinuous arch, as shown in Fig. 2.
Lateral (A) and posterior view of a Spalteholz ear (B) showing auricular vascularity, and three-dimensional reconstruction using micro computed tomography in the surface topography (C) and isolated vascularity (D). s = superior anterior auricular artery, m = middle anterior auricular artery, i = inferior anterior auricular artery, *superficial temporal artery.
Three auricular branches were found frequently, originating from the superficial temporal artery (STA) and posterior auricular artery (PAA), contributing to the helical rim arcade. These vessels have been named as the superior, middle and inferior anterior auricular arteries. The STA was the main contributor to the superior anterior auricular artery in 7/10 cases (PAA in 3/10 cases) and the PAA was the main contributor to the middle and inferior anterior auricular artery in 9/10 and 10/10 cases, respectively. Similarly, the helical arcade was formed by one continuous arch (8/10 cases) with anastomoses to the helical rim arcade and predominantly superior and middle anterior auricular arteries forming an extended vascular network.
Although some variability exists regarding their feeders, namely the anterior auricular arteries, the helix is rich in supply with a few redundant arches, provided by the PAA and STA. One or more anastomosing arches were observed, and the number in these given experiments might be underestimated.
Combined autonomic innervation in proximity to the helical arcade and rim arcade
A number of vessels were observed microscopically in those planes. Choline acetyl transferase positive (ChAT+) terminals were found in proximity to the vessels with increased density in planes II and III compared to planes II and III (Fig. 1). Vasoactive intestinal peptide positive (VIP+) innervation was observed in all planes without marked differences in density (Fig. 1). Equally, tyrosine hydroxylase positive (TH+) noradrenergic terminals and neuropeptide Y positive (NPY+) endings were observed throughout the helix, especially in planes I, II, and III, with increased density in planes I and III for NPY (Fig. 1).
The Hematoxylin-Eosin stained images gave an overview of the planes being observed (Supplement Fig. 1, left column). In proximity to the laminin-positive vasculature throughout the planes (Supplement Fig. 1, middle column), nerve fiber terminals were seen, indicated by synaptophysin staining (Supplement Fig. 1, right column).
Discussion
This study, for the first time, presents data on the periarterial autonomic innervation of the human auricle in conjunction with a 3D visualization of the vascular tree, showing the proximity of autonomic nerve fibers to the perivascular beds of the helical arteries. It used a combination of the Spalteholz technique and μ-CT to depict auricular vascularity in detail in combination with histology and immunohistochemistry. Though the autonomic innervation of human ear has been investigated with both functional and surgical methodology3,4,5,7,8,9,10,11,12,13,14,15,19,20,21,22,23,24, this was to date not the case for the periarterial autonomic innervation.
In this given study, NPY+ nerve endings were observed in the perivascular zones throughout the auricular helix, consistent with animal work25. NPY is known to have a role as a vasoconstrictor25 and has been found as a neurotransmitter after ATP, co-released from sympathetic terminals26. Evidence also exists of co-storage of noradrenalin (NA) as a co-transmitter of NPY to potentiate the contractile response of vascular smooth muscle25, co-localized with TH throughout the arterioles in rats27 and in sympathetic TH+ neuron subpopulations guinea pig ear vessels28. The proposed key role of NPY is to potentiate the vasoconstrictor effects by noradrenaline25,29,30 with NPY+ neuron-induced vasoconstriction having longer effects than NA-induced constriction31. Combined NPY and TH immunostaining showed that they co-exist in perivascular sympathetic nerves13,14,15, as NPY has, to date, only been reported to be secreted by parasympathetic cranial nerve ganglia in glands but not in postganglionic perivascular terminals22. The simultaneous existence of NPY+ and TH+ terminals shown here indicates that the vasoconstriction of the auricular arteries is likely under the influence of sympathetic fibers via NPY and NA. In contrast to this evidence for vasoconstriction, driven by sympathetic innervation, the mechanism for vasodilatation of the ear is more complex. One theory for the ear skin is passive vasodilatation, i.e. the inhibition of the sympathetic vasoconstrictor fibers as opposed to activation of the parasympathetic fibers evoking dilation of the vasculature3.
In the current study, cholinergic co-neurotransmitters in the perivascular zone of the human auricle for active vasodilatation were shown for the first time. Acetylcholine (ACh) and VIP are both involved in early and late vasodilatation response as part of the parasympathetic system32,33. The release of ACh exists at perivascular nerves, with evidence for its function and presence in parasympathetic nerves in other regions32,34,35. The challenge to provide evidence for the latter forms the ambiguity regarding parasympathetic involvement in vasomotor control. While parasympathetic nerves play an active role in brain vasodilatation36, they may have a minute role elsewhere34,37,38,39,40. The lack of suitable markers to specify parasympathetic perivascular fibers adds even more complexity, and a potential reason is also the difficulty in interpreting immunological studies of parasympathetic innervation at the perivascular zone such as VIP41. VIP is a marker for parasympathetic nerves and a proof of parasympathetic contribution4. It has also been reported to be associated with non-cholinergic nerves. Another challenge for determining parasympathetic effects on vasodilatation is the cholinergic vasodilatation mediated by the sympathetic system41,42,43. Up to 15% of the paravertebral sympathetic ganglia have cholinergic neurons44, and cholinergic sympathetic vasodilatator nerves are involved in the cutaneous vasodilatation in humans42,45. The trigemino-facial reflex via the facial nerve parasympathetic fibers may cause vasodilatation of human red ear syndrome4,46, but clear evidence is lacking. Of interest is that the cranial vagus nerve terminals distribute to the ear via an auricular branch using two different pathways; one following the external ear canal and distributes predominantly to the cymba concha area of the auricular skin, and the other hijacking the posterior auricular branch of the facial nerve23,24,47. Though the facial and vagus nerve cholinergic contributions have territories in the human ear, they are unlikely to play a role in perivascular cholinergic fibers except for the greater auricular nerve. The greater auricular nerve with its cervical roots at the C2-C348 may be an efferent arch of auricular vasodilatation response in both the trigeminal-autonomic reflex pathway and upper cervical disorders. This however remains to be substantiated in humans. Another contradiction of passive vasodilatation at the human ear is the difference in vasomotor control of glabrous and non-glabrous skin49. While NA+ fibers innervate the arterioles in glabrous zones of palms, cholinergic perivascular nerve fibers are present in the non-glabrous areas in human skin49,50,51,52,53,54. VIP may play a role in active vasodilatation of non-glabrous human skin42. Moreover, in the non-glabrous skin, as in the ear, VIP and ACh are co-transmitters of cholinergic nerve and they are described as the sympathetic cholinergic active vasodilatator system of the skin55. Based on the co-release of VIP and ACh in the periauricular zone in this study, it can be concluded that sympathetic cholinergic fibers conveying via greater auricular nerve are likely to provide the fibers driving the active vasodilatation of the human ear.
The STA and the PAA both originate from the external carotid artery and form important vessels of the viscerocranium. The sympathetic fibers for both arteries originate from the C2 to T1 (first thoracic segment)56, which is consistent with RES, which has been reported in migraine cases and upper cervical disorders, including C2 and greater auricular nerve anti-dromic vasodilatation studies8,48. For the trigemino-cervical complex the nociceptive afferents reside in the caudal region of the trigeminal nucleus caudalis and extend into the dorsal horns of C1 and C257. The convergence of the trigeminal system with C2 and C3 can also explain the underlying mechanism of the non-cervical but cranially originated nociceptive disorders on auricular flashing episodes. The highly consistent three-dimensional distribution of the ear arteries was observed for the helical rim arcade and the helical arcade shown here is in line with previous anatomical and surgical studies on the blood supply1,2,58.
Auricular electrostimulation as a neuromodulation modality has been reported to alleviate pain syndromes including migraine59. The underlying mechanism of auricular electrostimulation is mainly attributed to the stimulation of the auricular branch of the vagus nerve in the context of auricular electrode placement59. As a consequence, the interactions between the trigeminal spinal nucleus and nucleus tractus solitarius are considered to play a key role in modulating the trigemino-autonomic dysregulation of both migraine and TACs. While the exact mechanism and contributing neural networks and reflex arches still need to be investigated in more detail, peri-arterial autonomic innervation of the auricles should be considered while interpreting the contributing autonomic pathways for auricular electrostimulation studies in the context of presented results.
A number of limitations exist for the given study. First, the sample size was small. Further advancements of the Spalteholz method exist, including CLARITY for three-dimensional histological assessment60, but have been untested to date for specimens such as full auricles. The number of anastomoses may have been underestimated as a consequence of blood clotting. Further differentiation of the origin of the perivascular cholinergic nerves and further studies to clarify the origin of these nerves are also pending.
The present study focused on the autonomic innervation of the periarterial tree of the human auricle and our results did not exclude any potential contributions of sensory nerve endings for vasodilation, such as the trigeminal nerve. The trigeminal nerve or free nerve endings may potentially contribute to auricular vasodilatation with the secretion of substance P and calcitonin gene-related peptide (CGRP) in humans61,62. Of relevance to migraine, a study reported elevated levels of CGRP in the jugular vein but not in the cubital vein during a migraine attack, which can be considered as a sign for local effects63,64. Another study, however, failed confirming such change in both veins65.
It is worth noting that substance P can induce both vasodilation and plasma protein extravasation, whereas the CGRP solely induces vasodilation, without known plasma extravasation effects in humans66. It can therefore be theorized that CGRP would be more likely to contribute auricular vasodilation and RES in migraine if any contributions may exist via trigeminal nerve and/or free nerve endings. To date, the presence of CGRP and its role as a vasodilatator has been reported in human skin, meningeal vascular smooth muscle cells and rat ear skin67,68,69,70. Further studies are needed to investigate this role on human auricular vasomotor control with interactions of autonomic vasomotor neurotransmitters, which have been demonstrated in the given study.
Conclusions
This study gave evidence for ChAT+ and VIP+ nerve terminals along with NPY+ and TH+ terminals at the human auricular helix, indicating the innervation of active vasoconstriction and -dilatation. These terminals were found in the perivascular zone, formed by terminal branches of the superficial temporal artery and posterior auricular artery. The 3D reconstruction from μ-CT of ears showed a highly consistent pattern of anterior auricular branches, potentially forming the pathways for the perivascular autonomic nerve system.
References
Zilinsky, I. et al. The arterial blood supply of the helical rim and the earlobe-based advancement flap (ELBAF): a new strategy for reconstructions of helical rim defects. J Plast Reconstr Aesthet Surg 68, 56–62, https://doi.org/10.1016/j.bjps.2014.08.062 (2015).
Zilinsky, I. et al. Reevaluation of the arterial blood supply of the auricle. J Anat 230, 315–324, https://doi.org/10.1111/joa.12550 (2017).
Fox, R. H., Goldsmith, R. & Kidd, D. J. Cutaneous vasomotor control in the human head, neck and upper chest. J Physiol 161, 298–312 (1962).
Goadsby, P. J. & Lipton, R. B. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain 120(Pt 1), 193–209 (1997).
Lambru, G., Miller, S. & Matharu, M. S. The red ear syndrome. J Headache Pain 14, 83, https://doi.org/10.1186/1129-2377-14-83 (2013).
Lance, J. W. The red ear syndrome. Neurology 47, 617–620 (1996).
Hirsch, A. R. Red ear syndrome. Neurology 49, 1190 (1997).
Raieli, V. et al. Red ear syndrome and migraine: report of eight cases. Headache 42, 147–151 (2002).
Boes, C. J., Swanson, J. W. & Dodick, D. W. Chronic paroxysmal hemicrania presenting as otalgia with a sensation of external acoustic meatus obstruction: two cases and a pathophysiologic hypothesis. Headache 38, 787–791 (1998).
Forderreuther, S. & Straube, A. [A rare headache syndrome. SUNCT syndrome, hemicrania continua and red ear syndrome]. Nervenarzt 70, 754–758 (1999).
Dodick, D. W. Extratrigeminal episodic paroxysmal hemicrania. Further clinical evidence of functionally relevant brain stem connections. Headache 38, 794–798 (1998).
Donnet, A. & Valade, D. The red ear syndrome. J Neurol Neurosurg Psychiatry 75, 1077 (2004).
Lundberg, J. M., Terenius, L., Hokfelt, T. & Goldstein, M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett 42, 167–172 (1983).
Lundberg, J. M. et al. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116, 477–480, https://doi.org/10.1111/j.1748-1716.1982.tb07171.x (1982).
Uddman, R., Ekblad, E., Edvinsson, L., Hakanson, R. & Sundler, F. Neuropeptide Y-like immunoreactivity in perivascular nerve fibres of the guinea-pig. Regul Pept 10, 243–257 (1985).
Goadsby, P. J., Knight, Y. E. & Hoskin, K. L. Stimulation of the greater occipital nerve increases metabolic activity in the trigeminal nucleus caudalis and cervical dorsal horn of the cat. Pain 73, 23–28 (1997).
Spalteholz, W. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. (S. Hirzel, 1914).
Mulisch, M. W., U. Romeis - Mikroskopische Technik. (Springer Spektrum, 2010).
Coffa, P. F. & Kotecha, N. Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea-pig small intestine. J Auton Nerv Syst 77, 125–132 (1999).
Freidin, M. & Kessler, J. A. Cytokine regulation of substance P expression in sympathetic neurons. Proc Natl Acad Sci USA 88, 3200–3203 (1991).
Grasby, D. J., Morris, J. L. & Segal, S. S. Heterogeneity of vascular innervation in hamster cheek pouch and retractor muscle. J Vasc Res 36, 465–476, https://doi.org/10.1159/000025689 (1999).
Leblanc, G. G. & Landis, S. C. Target specificity of neuropeptide Y-immunoreactive cranial parasympathetic neurons. J Neurosci 8, 146–155 (1988).
Mulazimoglu, S., Flury, R., Kapila, S. & Linder, T. Effects of a sensory branch to the posterior external ear canal: coughing, pain, Ramsay Hunt’s syndrome and Hitselberger’s sign. J Laryngol Otol 131, 329–333, https://doi.org/10.1017/S0022215117000160 (2017).
Schuknecht, H. F. Pathology of the Ear. (University Press, 1974).
Sundler, F., Hakanson, R., Ekblad, E., Uddman, R. & Wahlestedt, C. Neuropeptide Y in the peripheral adrenergic and enteric nervous systems. Int Rev Cytol 102, 243–269 (1986).
Burnstock, G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol 49, 1–30, https://doi.org/10.1146/annurev.pharmtox.052808.102215 (2009).
Fleming, B. P., Gibbins, I. L., Morris, J. L. & Gannon, B. J. Noradrenergic and peptidergic innervation of the extrinsic vessels and microcirculation of the rat cremaster muscle. Microvasc Res 38, 255–268 (1989).
Morris, J. L., Anderson, R. L. & Gibbins, I. L. Neuropeptide Y immunoreactivity in cutaneous sympathetic and sensory neurons during development of the guinea pig. J Comp Neurol 437, 321–334 (2001).
Edvinsson, L., Ekblad, E., Hakanson, R. & Wahlestedt, C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol 83, 519–525 (1984).
Wahlestedt, C., Edvinsson, L., Ekblad, E. & Hakanson, R. Neuropeptide Y potentiates noradrenaline-evoked vasoconstriction: mode of action. J Pharmacol Exp Ther 234, 735–741 (1985).
Hodges, G. J., Jackson, D. N., Mattar, L., Johnson, J. M. & Shoemaker, J. K. Neuropeptide Y and neurovascular control in skeletal muscle and skin. Am J Physiol Regul Integr Comp Physiol 297, R546–555, https://doi.org/10.1152/ajpregu.00157.2009 (2009).
Shastry, S., Minson, C. T., Wilson, S. A., Dietz, N. M. & Joyner, M. J. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol (1985) 88, 467–472 (2000).
Lundberg, J. M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 48, 113–178 (1996).
Storkebaum, E. & Carmeliet, P. Paracrine control of vascular innervation in health and disease. Acta Physiol (Oxf) 203, 61–86, https://doi.org/10.1111/j.1748-1716.2011.02333.x (2011).
van Zwieten, P. A. & Doods, H. N. Muscarinic receptors and drugs in cardiovascular medicine. Cardiovasc Drugs Ther 9, 159–167 (1995).
M.J., C. The Cerebral Circulation. (Morgan & Claypool Life Sciences, 2010).
Bevan, J. A. & Brayden, J. E. Nonadrenergic neural vasodilator mechanisms. Circ Res 60, 309–326 (1987).
Boysen, N. C., Dragon, D. N. & Talman, W. T. Parasympathetic tonic dilatory influences on cerebral vessels. Auton Neurosci 147, 101–104, https://doi.org/10.1016/j.autneu.2009.01.009 (2009).
Hill, C. E., Phillips, J. K. & Sandow, S. L. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev 21, 1–60 (2001).
Ogawa, F. et al. Effect of nitric oxide synthase inhibitor on increase in nasal mucosal blood flow induced by sensory and parasympathetic nerve stimulation in rats. Ann Otol Rhinol Laryngol 119, 424–430 (2010).
Westcott, E. B. & Segal, S. S. Perivascular innervation: a multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 20, 217–238, https://doi.org/10.1111/micc.12035 (2013).
Bennett, L. A., Johnson, J. M., Stephens, D. P., Saad, A. R. & Kellogg, D. L. Jr. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552, 223–232, https://doi.org/10.1113/jphysiol.2003.042135 (2003).
Uvnas, B. Cholinergic vasodilator nerves. Fed Proc 25, 1618–1622 (1966).
Mathias, C. J. & Bannister, R. Autonomic failure: a textbook of clinical disorders of the autonomic nervous system. Fifth edition edn, (2013).
Kellogg, D. L. Jr. et al. Cholinergic mechanisms of cutaneous active vasodilation during heat stress in cystic fibrosis. J Appl Physiol (1985) 103, 963–968, https://doi.org/10.1152/japplphysiol.00278.2007 (2007).
Goadsby, P. J. & Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 117(Pt 3), 427–434 (1994).
Lang, J. Clinical Anatomy of the Head Neurocranium · Orbit · Craniocervical Regions. (Springer-Verlag 1983).
Holton, P. Antidromic vasodilatation in the isolated perfused ear of the rabbit. J Physiol 131, 176–185 (1956).
Jancso, G. Neurogenic inflammation in health and disease. First edition edn.
Charkoudian, N. & Johnson, J. M. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol (1985) 87, 381–385 (1999).
Fox, R. H. & Edholm, O. G. Nervous control of the cutaneous circulation. Br Med Bull 19, 110–114 (1963).
Johnson, J. M. Nonthermoregulatory control of human skin blood flow. J Appl Physiol (1985) 61, 1613–1622 (1986).
Rowell, L. B. Reflex control of the cutaneous vasculature. J Invest Dermatol 69, 154–166 (1977).
Johnson, J. M. & D.W., P. Cardiovascular adjustments to heat stress. (American Physiological Society, 1996).
Wong, B. J. & Hollowed, C. G. Current concepts of active vasodilation in human skin. Temperature (Austin) 4, 41–59, https://doi.org/10.1080/23328940.2016.1200203 (2017).
Berguer, R. Function and surgery of the carotid and vertebral arteries (2013).
Elahi, F. & Reddy, C. Neuromodulation of the great auricular nerve for persistent post-traumatic headache. Pain Physician 17, E531–536 (2014).
Erdmann, D. et al. The helical arcade: anatomic basis for survival in near-total ear avulsion. J Craniofac Surg 20, 245–248, https://doi.org/10.1097/SCS.0b013e318184343a (2009).
Straube, A., Ellrich, J., Eren, O., Blum, B. & Ruscheweyh, R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain 16, 543, https://doi.org/10.1186/s10194-015-0543-3 (2015).
Morawski, M. et al. Developing 3D microscopy with CLARITY on human brain tissue: Towards a tool for informing and validating MRI-based histology. Neuroimage, https://doi.org/10.1016/j.neuroimage.2017.11.060 (2017).
Eftekhari, S. et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 169, 683–696, https://doi.org/10.1016/j.neuroscience.2010.05.016 (2010).
Hou, M. et al. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 909, 112–120 (2001).
Goadsby, P. J., Edvinsson, L. & Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28, 183–187, https://doi.org/10.1002/ana.410280213 (1990).
Iyengar, S., Ossipov, M. H. & Johnson, K. W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158, 543–559, https://doi.org/10.1097/j.pain.0000000000000831 (2017).
Tvedskov, J. F. et al. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol 58, 561–568, https://doi.org/10.1002/ana.20605 (2005).
Weidner, C. et al. Acute effects of substance P and calcitonin gene-related peptide in human skin–a microdialysis study. J Invest Dermatol 115, 1015–1020, https://doi.org/10.1046/j.1523-1747.2000.00142.x (2000).
Asghar, M. S., Becerra, L., Larsson, H. B., Borsook, D. & Ashina, M. Calcitonin Gene-Related Peptide Modulates Heat Nociception in the Human Brain - An fMRI Study in Healthy Volunteers. PLoS One 11, e0150334, https://doi.org/10.1371/journal.pone.0150334 (2016).
Brain, S. D., Williams, T. J., Tippins, J. R., Morris, H. R. & MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 313, 54–56 (1985).
Grant, A. D., Gerard, N. P. & Brain, S. D. Evidence of a role for NK1 and CGRP receptors in mediating neurogenic vasodilatation in the mouse ear. Br J Pharmacol 135, 356–362, https://doi.org/10.1038/sj.bjp.0704485 (2002).
Miller, S. et al. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 328, 165–183, https://doi.org/10.1016/j.neuroscience.2016.04.046 (2016).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study, analyzed the data: Y.O.C., N.H. data acquisition, critical revision for content: Y.O.C., C.S., C.J., M.M., M.C.S., M.W., N.H. critically revised the manuscript: Y.O.C., S.C. and N.H. wrote the paper: Y.O.C., N.H.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cakmak, Y.O., Cotofana, S., Jäger, C. et al. Peri-arterial Autonomic Innervation of the Human Ear. Sci Rep 8, 11469 (2018). https://doi.org/10.1038/s41598-018-29839-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29839-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.