Abstract
Atropa acuminata Royle Ex Lindl (Atropa acuminata) under tremendous threat of extinction in its natural habitat. However, the antimicrobial, antileishmanial and anticancer effects of the plant’s extracts have not been reported yet. In the current study, an attempt has been made to evaluate the pharmacological potential of this plant’s extracts against microbes, Leishmania and cancer. The roots, stems and leaves of Atropa acuminata were ground; then, seven different solvents were used alone and in different ratios to prepare crude extracts, which were screened for pharmacological effects. The aqueous, methanolic and ethanolic extracts of all parts carried a broad spectrum of anti-bacterial activities, while no significant activity was observed with combined solvents. Three types of cytotoxicity assays were performed, i.e., haemolytic, brine shrimp and protein kinase assays. The aqueous extract of all the parts showed significant haemolytic activity while n-hexane extracts of roots showed significant activity against brine shrimp. The acetone extracts strongly inhibited protein kinase while the methanolic extracts exhibited significant cytotoxic activity of roots and stem. The anti-leishmanial assays revealed that the methanolic extract of leaves and roots showed significant activity. These findings suggest that this plant could be a potential source of natural product based drugs.
Similar content being viewed by others
Introduction
According to the World Health Organization (WHO), approximately 80% of the world’s population use medicinal plants for their primary health care, and herbal remedies have been widely used for the treatment of many fatal human diseases in the past decade1,2. Atropa acuminata Royle Ex Lindl (A. acuminata), an important medicinal plant, belongs to family Solanaceae. It is endemic to northern Pakistan, Kashmir and India and is commonly known as Indian Belladonna. In the Himalaya region, it is mostly found in western regions of the subcontinent, starting from Kashmir at the altitude of 1.8–3.6 kilometres (km) to the connecting hills of the Himachal Pradesh up to 2.5 km in altitude. In Northwest Himalaya, it is reported from Kashmir, Muzaffarabad and Chakrata3,4,5.
In traditional medicines, the rhizome and aerial parts of the plant have been used for ages to relieve joint pain, muscle pain, and muscle spasms and to treat arthritis, pancreatitis, peritonitis, scarlet fever, Parkinson’s disease and neuro disorders6,7. Recently, the ethanolic extract of the plant has been reported to possess anti-arthritic activity8. The plant is the best source of tropane alkaloids such as atropine, hyoscyamine and scopolamine, which possess anticholinergic activity and have diversified therapeutic uses in the fields of ophthalmology, cardiology, and gastroenterology9,10. A. acuminata showed strong anti-inflammatory activity in carrageen an-induced inflammation mouse models11. Traditionally, the leaves and roots are used as relaxants, diuretics, mydriatics, narcotics and sedatives12.
The main constituents of A. acuminata. are monoterpenes, sesquiterpenes, phenyl propanoids, flavonoids, saponins and quinine13. A. acuminata also contains tropane alkaloids and highly oxygenated triterpenes14. The analysis of hairy root culture of Atropa through Direct Analysis in Real Time (DART) spectrometric technique revealed that it contains a high amount of alkaloids. High-performance liquid chromatography (HPLC) analysis of different parts of Atropa reveals that each has different alkaloid contents15. The leaves contain an average of 0.4% active alkaloids, whilst the root contains round 0.96%. The alkaloid contents also vary according to the age and developmental stage of the plant. At early ages, it contains low amounts of alkaloids, but the contents increase at the flowering stage16. According to a report, the ethanolic extract from the leaves of the plant contains approximately 188 micro grams of phenolic compounds per ml extract, which is a huge amount compared to other plants in the family17.
Since A. acuminata under tremendous threat of extinction in its natural habitat, the current study was designed to accomplish the in vitro pharmacological evaluation of various organic extracts of the plant by assessing different types of biological activities. This research will draw attention to the biological conservation of the plant as well as increase our understanding of the active ingredients in A. acuminata. However, further research is needed to elucidate the molecular and biochemical mechanisms acting against these microbes, cancer and Leishmania. In addition, this research may also provide a platform for further studies on A. acuminata regarding its pharmacological and clinical application in the treatment of leishmaniasis and cancer.
Results
Anti-microbial Activity
Antibacterial assay
Crude extracts of A. acuminata were evaluated against seven bacterial strains. Among them, five were gram negative, and two were gram positive. The minimum inhibitory concentration of each extract is given in Table 1. Among the single solvents, the best activity is shown by the aqueous extract, followed by the methanolic and ethanolic extracts, while no significant activity was seen for polar solvents except chloroform, with higher MICs for each strain. The same pattern was seen for combined solvents with no antagonistic or synergistic effects.
The anti-bacterial activity of 42 extracts from roots, stems, and leaves showed a diverse class of results. However, broad-spectrum results were shown by the aqueous methanol, ethanol and chloroform extract18. The aqueous extract from stems showed a somewhat lower MIC range than that from leaves and roots. This clearly demonstrates the fact that the roots of these plants contain steroidal saponins that are less hydrophilic, but organic polar solvents such as methanol, ethanol and some phenolic solvents have a greater affinity towards them19. Nevertheless, some plants have low levels of steroidal saponins in their leaves, but still, their extract has higher antibacterial activity due to some flavonoids and phenolic compounds20,21. In conclusion, these results revealed that each of the three parts of A. acuminata contains active compounds that have a broad spectrum of antibacterial activities; however, the principal compound isolation and structural elucidation have yet to be achieved.
Anti-Fungal Assay
Anti-fungal activity was determined against four different strains of fungi, i.e., Aspergillus, Fumigatus, Flavous and Mucor. Among the single solvents, the best activity was shown by mostly polar solvents, although in non-polar solvents, no satisfactory anti-fungal activity was observed, except for in chloroform, which exhibited slight activity in combined solvents. The acetone-water extract had the best activity compared to aqueous or acetone extract separately as shown in Table 2, which means that the acetone-water combination enhanced the activity of the extract, although no antagonistic or synergistic effects were seen in the remaining combined solvent systems.
Cytotoxic activity
Haemolytic assay
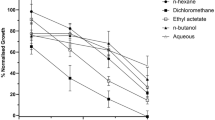
Haemolytic assays were performed for all extracts of each part (root, stem, and leaves). Values above 500 µg/ml were taken as non-significant and represented by a dash, while values lower than 50 µg/ml were considered to indicate strong cytolysis activity22,23. The aqueous extracts carried the lowest IC50 values, while the chloroform extract showed the second strongest activity. Acetone, methanol: ethyl acetate and acetone: water extracts also showed the best activity, as evident from Table 3. The ethyl acetate, methanol, ethanol and methanol: chloroform extracts showed non-significant values; however, good synergistic activity was seen in the methanol: ethyl acetate combined solvent system.
The ethyl acetate presented the best haemolytic activity among the stem extracts, while methanol and acetone showed the second and third highest significant activity. The remaining 11 extracts fell within the significant range (below 500). In comparison with the leaf extracts, no synergism was seen in combined solvent systems, yet the methanolic extract was seen to shift from non-significant (in leaves) to the second most significant one (in the stem), as shown in Table 4.
Among the root extracts, only three, i.e., ethanol, ethanol: n-hexane and acetone: water, revealed higher IC50 values, while the remaining exhibited good cytotoxic activity, of which the best three were methanol, acetone and n-hexane has been shown in Table 5.
Brine shrimp lethality assay
Brine shrimp lethality assays were performed for those extracts having the lowest IC50 values in haemolytic assays24. For this purpose the top three extracts in terms of good IC50 values were taken from each part, i.e., three each from roots stems and leaves. The lethal dose50 (LD50) is the lowest concentration of a drug that has the ability to kill 50% of the cells25. The root methanolic extracts showed the lowest LD50 values while the highest was observed for n-hexane. Similarly, among the stem extracts, the best activity was shown by ethyl acetate followed by aqueous extract, but no significant value was noted for the acetone extract. Among the leaf extracts, the methanolic extract revealed an LD50 value of 61 µg/ml and the acetone extract showed a non-significant value, whereas the chloroform extract showed good activity. Hence, it was observed that overall the methanolic extracts of roots and leaves had the same metabolites, which have potent cytotoxic activity (Table 6).
Protein Kinase inhibition Assay (PK)
A protein kinase inhibition assay was also performed for those extracts that had the lowest IC50 values in the haemolytic assay26. In plate reading three different types of readings were observed, cloudy zones, clear zones or no zones. Clear zones of inhibition were observed for methanol extracts of roots and leaves, cloudy zones for acetone extracts of stem and roots and no zones for aqueous extract of stems. The 13 ± 2 mm and 9 ± 1 (Table 7) zones of inhibition for the methanol extracts of leaves and roots show that they have strong cytotoxic activity.
Anti-leishmanial Assay
An anti-leishmanial assay revealed that the methanolic extract from either leaves or stem was active against both promastigote and amastigote parasites. However, a shift was observed in the IC50 values for both parasites. This shift was calculated in terms of the ratio of the IC50 for promastigotes to the IC50 for amastigotes27. The shift for the methanolic extract from roots was 0.94, while for the root extract, it was 0.6; this means the methanolic extract from the roots is comparatively more active against promastigote than against amastigote and vice versa. The same pattern was seen for acetone extract from roots, chloroform extract from leaves, and ethyl acetate extract from stems; where the SI value for acetone extract from leaves exceeded 1, it means the pattern was the opposite, i.e., more active against amastigote then promastigote. The aqueous and acetone extracts from stems showed no significant activity (Table 8).
Discussion
Antimicrobial Assays
Both the antibacterial assay, the anti-fungal assay showed an opposite pattern of activities among the extracts. For example, the aqueous fraction from all the parts showed a broad spectrum of anti-bacterial activities MIC ranges from 9.1 µg/ml against B. subtillus to 26.3 µg/ml against S. type (Table 1). When tested against fungi the same fraction showed 0 mm (leaves), 1.5 mm (stem) and 0 mm (roots) zones of inhibition against Aspergilus fumigatus while 13 ± 0.4 mm, 5 ± 0.34 mm and 10.71 ± 1 mm zones of inhibitions were noted against Mucor indicus for leaves, stems and roots respectively (Table 2). Conversely, no significant difference was found in antibacterial effects between extracts from roots, stems and leaves except the chloroform extract from the stems that showed no significant effects against S. type and S. aureus. This means that all of the three parts carried almost the same properties for the extracts that showed the best anti-bacterial activity28. However, in the anti-fungal assay, only the chloroform, acetone and ethyl acetate fractions showed a broad spectrum of anti-fungal activity irrespective of the part used29,30. These activities are mainly due to tannins, some non-polar terpenoids or most likely some phenols, flavonoids and hydrophobic saponins. However, their exact identification is still in infancy31,32.
Cytotoxic assays
Cytotoxic assays of the different extracts from A. acuminata revealed the same pattern of activities with different IC50 values except for a few extracts, the IC50 values of which were not in the acceptable range (more than 500 µg/ml). Similarly, some extracts showed the lowest minimum inhibitory concentrations in the haemolytic assay, which led us to subject them to further evaluation.
The IC50 calculation method is more acceptable and accurate, so we used the term IC50 to describe our results33. The comparative analysis of all the extracts from different parts shows that the aqueous extract from leaves showed the lowest IC50 value followed by chloroform extract from the same part, ethyl acetate from the stem and acetone from the roots. This describes the fact that each part contains a different active agent from a class of compounds or different compounds from the same class with different affinities towards the same solvent. However, this may be confirmed by high-performance liquid chromatography (HPLC) or gas chromatography (GC) analysis.
The brine shrimp lethality assays showed a quite simple pattern of results. The methanol extract from roots and leaves showed the lowest LD50, while the results shown by acetone extracts from all the three parts were not satisfactory. The remaining extracts presented different values in each part. Although the selection of all these extracts for brine shrimp assays was made on the basis of their potential activity in the haemolytic assay (see results section), the outcome in the brine shrimp assays appeared quite different. This clearly deviates from the hypothesis that all haemolytic compounds will also destroy the cells of brine shrimp and supports the studies in which the methanol extracts of many plants contain strong killers of brine shrimp34,35.
Protein Kinase Assay
The PK assay showed the same pattern of results for cell killing as the brine shrimp assay except for the acetone extract, which showed inhibition of the enzymes36. The methanol extracts from both roots and leaves showed that these extracts of A. acuminata have a broad spectrum of cytotoxic activities against a variety of cells and can be used in future not only for cancer therapy but also to kill many parasitic insects37.
In conclusion, all three parts of A. acuminata showed a diverse range of cytotoxic activities; however, a good correlation was found among the methanolic extracts in all the assays, which demonstrates that the methanolic extract of this contains a potent cytotoxic active agent to be reported upon further analysis.
Anti-leishmanial assay
As plants become a valuable source of new medicinal agents in the absence of vaccines, we calculated the infection kinetics of different extracts of A. acuminata. Remarkable results were obtained for both promastigote and amastigote parasites. Our results showed that the methanol fraction from leaves have IC50 of 53 µg/ml and 77 µg/ml for promastigote and amastigote respectively which is more near to 46 µg/ml and 57 µg/ml for the currently clinically used (Ampoteicine B). When the ratio of promastigote to amastigote IC50 values was calculated we found different efficiencies of different parts of A. acuminata extracts in different solvents ranges from 0.57 to 1.1. As a future perspective, the constituents of different parts of the plant can be characterized to identify active compounds responsible for the positive activity so that it can be used to treat infections caused by parasites.
Conclusion
The current study investigated A. acuminata is a pharmacologically active plant. Many extracts from leaves, stems and roots have broad-spectrum biological activities, but leaves have more potential than other parts. These findings suggest that the plant could be a potential source for infectious diseases caused by microbes and parasites. Cytotoxic results show that this plant extract may show good inhibition of cancerous cells and tumours, so this research provides a baseline for further studies, and further chemical and clinical analyses are needed to investigate the correct structure and mechanism of action of the active agent. Moreover, protection measures concerned with the conservation and cultivation of this medicinally important plant are also needed.
Materials and Methods
Collection of plants and solvent extraction
A. acuminata plant parts (leaves, stems and roots) were collected from the hilltops of Toormang Valley, Dir (Upper), Khyber Pakhtunkhwa, Pakistan. A herbarium specimen with a voucher number (KAA-22) was submitted to the herbarium library of Molecular Systematics and Applied Ethnobotany Lab, Department of Biotechnology, Quaid-i-Azam University, Islamabad, Pakistan, and identified by a taxonomist. The plant parts were dried and ground into fine powder for preparation of extracts. The powders of each part were taken in labeled flasks in the equal concentration of one milligram of powder per three millilitres of solvent (1 mg/3 ml) (25 mg of each part powder in 75 ml of each solvent respectively). Seven different solvents and their combinations were used (Table 9). After adding the powder and solvents, the flasks were tightly closed and sonicated at 60 kHz for 10 minutes to rupture the cells (so that maximum quantity of metabolites will ooze out) and kept for 2–3 days to extract maximum metabolites, that were finally filtered through cellulose filter paper. The extracts were dried in large petri plates and stored in sterilized bottles. A stock solution of each extract was prepared by dissolving 4 mg crude extract in 1 ml absolute DMSO (Dimethyl Sulfoxide) for further activities.
Evaluation of Anti-microbial Activity
Antibacterial assay
Anti-bacterial tests were performed for each extract against seven bacterial strains i.e. Bacillus subtillus (ATCC 6051-U), Escherichia coli (ATCC 33559), klebsiella pneumoniae (ATCC BAA-1705), Micrococus luteus (ATCC 12698), Pseudomonas aeruginosa (ATCC BAA-2110), Staphylococcus aureus (ATCC 35844), and Salmonella typhi (ATTC 39926) (Table 1). The broth dilution method was used to assess antibacterial activity. In brief, the strains were cultured on LB (Luria Bertani) broth (is the most widely used medium for bacterial growth, in liquid form, it is known as LB broth) medium for 12 hours followed by cold shock at 5–3 °C. Then, each culture was diluted, and to test the extracts against bacterial culture, 7.5 µl of crude extract was taken from 4 mg/ml DMSO stock extract in 96-well plates and serially diluted four times with DMSO to obtain 4 different concentrations. After making the dilutions, 195 µl of inoculum was added to each well38. Cefotaxime sodium was taken as a positive control in the same concentration as the extract, while 5 µl of DMSO was taken as a negative control. Readings were taken two times on a micro plate reader (ELx 800 BioTek) at a wavelength of 630 nm. The first or R1 reading was taken just after a half hour of incubation, while the second reading or R2 was taken 22 hours after R1. The percent growth inhibition was calculated using the formula39:
Where
R2 = Sample reading after 22 hrs.
R1 = Sample reading at 0 hrs.
C2 = Control reading after 22 hrs.
C1 = Control reading at 0 hrs.
Antifungal assay (MIC, MFC)
Old cultures of all selected fungal strains i.e. Aspergilus fumigatus (ATCC 1022), Candida albicans (ATCC MP-8), Mucor indicus (ATCC 90364), Aspergillus flavus (ATCC 16883) were re-cultured on tryptic soy broth (TSB) medium for 24 hours to obtain active and fresh cultures. After 24 hours, the refreshed cultures were spread on Sabouraud dextrose agar (SDA) medium using a sterilized bent glass rod. The disc diffusion method was used to check the antifungal effect of the crude extracts at the concentration of 20 mg/ml, and discs were placed on their respective labelled plate for 50 to 70 hours at 25 to 30 °C. Clotrimazole (4 mg/ml) stock was used as a positive control.
Anti-leishmanial Activity
Anti-Promastigote Activity
For this assay, a culture of Leishmania major (KMU 25) was sub cultured in RPMI 1640 medium (Bio-Rad) with 10% foetal bovine serum (FBS) added. Then, the culture was incubated for 6 days, subjected to light microscopy and counted using a haemocytometer at 2.5 × 106 cells/ml density. The anti-promastigote assay was performed using an MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)-based micro-assay as a detector of cell sustainability40. Ten microlitres of each crude extract from 20 μg/ml stocks and 100 μL from a culture containing 2.5 × 105 cells per ml were added to 96-well cell culture plates. The plates were then incubated at 24 °C for three days. DMSO was used as negative control, and amphotericin was taken as positive control. Then, 10 μl of MTT reagent was added to each well, and the plates were re-incubated under the same conditions for at least 3 hours. Then, 100 μl of a solution containing 50% isopropanol and 10% SDS (sodium dodecyl sulphate) was added in the same pattern and the incubation step repeated for a half hour. Then, readings were taken at 540 nm using (ELX 800, BIOTEK) a micro-titre plate reader. The percent viability of cells was calculated using the formula:
Where Ae is the absorbance of the plant extract, Ac is the absorbance of the control and Ab is the absorbance of the blank. Efficient fractions were selected on the basis of % mortality for further processing. The IC50 (% inhibitory concentration) for each extract was calculated on the basis of threefold serial dilution41.
Anti-amastigote Activity
The Amastigote culture was donated by Dr. Yaseen Yousafzai, laboratory of Nano-technology Quaid-i-Azam University Islamabad Pakistan, preserved in M119 medium (pH 5). One millilitre of the leishmanial culture was centrifuged for 10 minutes at 3000 rpm, and the pellet was removed and washed with PBS at least three times. The pellet was then transferred into 20 ml of medium and incubated at 33 °C. 15 μl of each crude extract from the 4 mg/ml stock was added to the first 14 wells in a row order from left to right and then serially diluted in the consecutive rows to obtain 4 different concentrations of each extract, i.e., 65 µg/ml, 130 µg/ml, 261 µg/ml and 546 µg/ml. Then, 100 μL of the culture containing 2.5 × 106 cells/ml promastigotes was added to each well. The plates were kept in an incubator for 70–74 hours at room temperature. DMSO was used as a negative control and amphotericin as used as a positive control. After incubation, 10 μL of MTT reagent was added to each well and re-incubated for 3 hours under the same conditions. The rest of the procedure was the same as for promastigotes41.
Evaluation of in vitro protein kinase inhibition, cytotoxic and toxic effects
Three assays were performed to assess the protein kinase inhibition, cytotoxic and toxic effects of A. acuminata respectively: protein kinase enzyme inhibition assay, haemolytic assay, and brine shrimp assay.
Protein kinase enzyme inhibition assay
To evaluate the protein kinase inhibition of the crude extract the method of Hua et al., 2005, was used with some modifications42. Streptomyces coelicolor was used as a test organism, the protein kinase of which has several biochemical and physiological similarities with that of eukaryotes43. Two types of media, i.e., tryptic soy broth (TSB) medium and ISP4 (inorganic salt starch agar) medium, were used. A Streptomyces coelicolor culture was prepared in TSB medium and then paper discs were dipped in the crude extract at 20 mg/ml. Finally, the plates were incubated at 30 °C for 50 to 70 hours and then readings were taken with the help of a magnifying glass.
Haemolytic assay
For haemolytic activity RBCs were isolated from a human. One millilitre of blood was taken and transferred to an EDTA tube followed by centrifugation for 5 minutes at 14000–15000 rpm. The supernatant was then decanted and 800 µl from the remaining pellet (blood) was transferred to 40 ml of phosphate-buffered saline (PBS) and centrifuged for 10 minutes at 2000 rpm. Then, the supernatant was removed and the procedure was repeated three times. Finally, 50 µl was taken from four different stock concentrations i.e., 60 mg/ml, 20 mg/ml, 6 mg/ml, 4 mg/ml and 0.6 mg/ml, of each crude extract. The finally washed RBC pellet was dissolved in PBS, and 150 µl of this suspension was added to the crude extract and incubated for 60 minutes at 37 °C. The suspension was then centrifuged at 2500 rpm for 10 minutes; 100 µl of supernatant from each tube was transferred to a 96-well plate, and cell lysis was checked at 540 nm wavelength using a micro plate reader (ELx 800 BioTek). Triton X-100 (0.5%) was used as positive control, and PBS and DMSO were used as blank controls. The percentage of RBC lysis was calculated using the following formula:
Toxicity assay against brine shrimp
Toxicity assay of A. acuminata against brine shrimp was performed by a method previously described44. Seawater was prepared by dissolving 17 g of sea salt in 500 ml of distilled water and filter sterilizing. A compartmentalized rectangular dish made of Teflon was filled with seawater; eggs (Ocean Star Inc., USA) of shrimp (Artemia salina) were poured in its large compartment, and the compartment was covered with aluminium foil, while the small compartment was kept bear with a lamp shining on it. These settings were done in an incubator, which was closed and left for 24 hours at 24 °C to 25 °C. After 24 hours, due to the light movement of newly hatched larvae or nauplii was observed from the large compartment to the small compartment, from where they were collected carefully through a dropper into a beaker containing seawater and a small amount of yeast. Different quantities from different stock solutions of each crude extract were poured into a 96-well plate, and 100 ml seawater was added to obtain different concentrations of each extract. Finally, 10–15 nauplii were shifted to each well in the micro plate and observed under a 3 × magnifying glass. The final volume of the mixture was kept at 200 µl in each well by adding further seawater, and it was incubated at 24 °C for 24 hours. The larvae were again picked and counted under a 3 × magnifying glass to check the number of live and dead shrimp in each well. DMSO was taken as a blank control, and the lethal dose (LD50) of the crude extracts for brine shrimp was calculated by applying the formula:
Where
D is the number of dead cells
L1 is the number of live cells before treatment
L2 is the number of live cells after treatment.
Change history
23 September 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Farnsworth, N. R., Akerele, O., Bingel, A. S., Soejarto, D. D. & Guo, Z. Medicinal plants in therapy. Bulletin of the world health organization 63, 965 (1985).
Khan, S. U. et al. Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicology and industrial health 32, 154–161 (2016).
Kaul, M. K. Medicinal plants of Kashmir and Ladakh: temperate and cold arid Himalaya. (Indus publishing, 1997).
Mehraj, G. et al. Patterns of alien plant diversity in the urban landscapes of global biodiversity hotspots: a case study from the Himalayas. Biodiversity and Conservation 27, 1055–1072 (2018).
Mehraj, G., Khuroo, A. A., Muzafar, I., Rashid, I. & Malik, A. H. An updated taxonomic inventory of flora of Srinagar City (Kashmir Himalaya) India, using herbarium reconstruction approach. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 1–7 (2017).
Kahn, A. et al. Prevention of Airnray Obstructions during Sleep in Infants with Breath-Holding Spells by Means of Oral Belladonna: A Prospective Double-Blind Crossover Evaluation. Sleep 14, 432–438 (1991).
King, J. Anisotropine methylbromide for relief of gastrointestinal spasm: double-blind crossover comparison study with belladonna alkaloids and phenobarbital. Current therapeutic research, clinical and experimental 8, 535 (1966).
Nisar, A. et al. Modulation of T-helper cytokines and inflammatory mediators by Atropa accuminata. Royle in adjuvant induced arthritic tissues. Journal of ethnopharmacology 162, 215–224 (2015).
Cardillo, A. B., Rodriguez Talou, J. & Giulietti, A. M. Establishment, Culture, and Scale-up of Brugmansia candida Hairy Roots for the Production of Tropane Alkaloids. Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, Second Edition, 173–186 (2016).
Kursinszki, L., Hank, H., László, I. & Szőke, É. Simultaneous analysis of hyoscyamine, scopolamine, 6β-hydroxyhyoscyamine and apoatropine in Solanaceous hairy roots by reversed-phase high-performance liquid chromatography. Journal of chromatography A 1091, 32–39 (2005).
Majid, R., Zargar, M. & Ahmad, L. Evaluation of anti-inflammatory potential of Atropa acuminata in carrageenan induced inflammation in rats. Journal of Medicinal Plants Research 6, 5586–5592 (2012).
Chopra, R., Nayar, S. & Chopra, I. Glossary of Indian medicinal plants (including the supplement). Council of Scientific and Industrial Research. New Delhi, 2–79 (1986).
Butt, M. A. et al. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. Journal of ethnopharmacology 168, 164–181 (2015).
Mehmood, A. et al. Highly oxygenated triterpenes from the roots of Atropa acuminata. Natural product letters 16, 371–376 (2002).
Ashtiania, F. & Sefidkonb, F. Tropane alkaloids of Atropa belladonna L. and Atropa acuminata Royle ex Miers plants. Journal of Medicinal Plants Research 5, 6515–6522 (2011).
Dräger, B. & Schaal, A. Tropinone reduction in Atropa belladonna root cultures. Phytochemistry 35, 1441–1447 (1994).
Nisar, A., Malik, A. H. & Zargar, M. A. Atropa acuminata Royle Ex Lindl. blunts production of pro-inflammatory mediators eicosanoids., leukotrienes, cytokines in vitro and in vivo models of acute inflammatory responses. Journal of ethnopharmacology 147, 584–594 (2013).
Ashraf, Z., Muhammad, A., Imran, M. & Tareq, A. H. In vitro antibacterial and antifungal activity of methanol, chloroform and aqueous extracts of Origanum vulgare and their comparative analysis. International Journal of Organic Chemistry 1, 257 (2011).
Ferro, E., Alvarenga, N., Ibarrola, D., Hellión-Ibarrola, M. & Ravelo, A. A new steroidal saponin from Solanum sisymbriifolium roots. Fitoterapia 76, 577–579 (2005).
Eleazu, C., Eleazu, K., Awa, E. & Chukwuma, S. Comparative study of the phytochemical composition of the leaves of five Nigerian medicinal plants. Journal of Biotechnology and Pharmaceutical Research 3, 42–46 (2012).
Pereira, A. P. et al. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 12, 1153–1162 (2007).
Que, Z. et al. Dihydroartemisin inhibits glioma invasiveness via a ROS to P53 to beta-catenin signaling. Pharmacological research 119, 72–88, https://doi.org/10.1016/j.phrs.2017.01.014 (2017).
Ullah, I., Shinwari, Z. & Khalil, A. Investigation of the Cytotoxic and Antileishmanial Effects of Fagonia indica L. Extract and Extract Mediated Silver Nanoparticles (Agnps). Vol. 49 (2017).
Jimenez, P. C. et al. Biological activity in extracts of ascidians (Tunicata, Ascidiacea) from the northeastern Brazilian coast. Journal of Experimental Marine Biology and Ecology 287, 93–101 (2003).
Ahmed, M. Acute toxicity (Lethal Dose 50 Calculation) of herbal drug Somina in rats and mice. Pharmacology & Pharmacy 6, 185 (2015).
Zivanovic, A., Pastro, N. J., Fromont, J., Thomson, M. & Skropeta, D. Kinase Inhibitory, haemolytic and cytotoxic activity of three deep-water sponges from North Western Australia and their fatty acid composition. (2011).
Kayser, O., Kiderlen, A., Bertels, S. & Siems, K. Antileishmanial activities of aphidicolin and its semisynthetic derivatives. Antimicrobial agents and chemotherapy 45, 288–292 (2001).
Ruiz-Bustos, E. et al. Antibacterial and antifungal activities of some Mexican medicinal plants. Journal of medicinal food 12, 1398–1402 (2009).
Anushia, C., Sampathkumar, P. & Ramkumar, L. Antibacterial and antioxidant activities in Cassia auriculata. Glob J Pharmacol 3, 127–130 (2009).
Rehman, A., Rehman, A. & Ahmad, I. Antibacterial, antifungal, and insecticidal potentials of Oxalis corniculata and its isolated compounds. International journal of analytical chemistry 2015 (2015).
Mondello, F., De Bernardis, F., Girolamo, A., Salvatore, G. & Cassone, A. In vitro and in vivo activity of tea tree oil against azole-susceptible and-resistant human pathogenic yeasts. Journal of Antimicrobial Chemotherapy 51, 1223–1229 (2003).
Savoia, D. Plant-derived antimicrobial compounds: alternatives to antibiotics. Future microbiology 7, 979–990 (2012).
Kumar, P., Singh, V., Hira, S., Manna, P. & Kumar, P. In vitro Cytotoxicity, Apoptotic and Hemolysis Assay of Kalsilite‐Based Glass Ceramics for Dental Veneering Application. International Journal of Applied Ceramic Technology 13, 78–87 (2016).
Rahman, S. et al. Antimicrobial activity and brine shrimp toxicity of methanolic whole plant extract of Boerhavia repens L.(Family: Nyctaginaceae). Int. J. Phytopharm 4, 135–139 (2014).
Sahgal, G. et al. Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (Linn.) Jacq. seed methanolic extract. Pharmacognosy research 2, 215 (2010).
Olaokun, O. O., McGaw, L. J., Eloff, J. N. & Naidoo, V. Evaluation of the inhibition of carbohydrate hydrolysing enzymes, antioxidant activity and polyphenolic content of extracts of ten African Ficus species (Moraceae) used traditionally to treat diabetes. BMC Complementary and Alternative medicine 13, 94 (2013).
Karpouhtsis, I. et al. Insecticidal and genotoxic activities of oregano essential oils. Journal of Agricultural and Food Chemistry 46, 1111–1115 (1998).
Mustafa, N. R., De Winter, W., Van Iren, F. & Verpoorte, R. Initiation, growth and cryopreservation of plant cell suspension cultures. Nature protocols 6, 715 (2011).
Rahman, M. & Bari, M. Antibacterial Activity of Cell Suspension Cultures of Castor (Ricinus communis L. cv. Roktima). (2013).
Śladowski, D., Steer, S., Clothier, R. & Balls, M. Use of a filtration plate in the MTT assay. Toxicology in vitro 8, 739–741 (1994).
Williams, C. et al. Hydrosoluble formazan XTT: its application to natural products drug discovery for Leishmania. Journal of microbiological methods 55, 813–816 (2003).
Hua, Y. et al. Screening the active constituents of Chinese medicinal herbs as potent inhibitors of Cdc25 tyrosine phosphatase, an activator of the mitosis-inducing p34cdc2 kinase. Journal of Zhejiang University Science B 6, 656–663 (2005).
Petříčková, K. & Petříček, M. Eukaryotic-type protein kinases in Streptomyces coelicolor: variations on a common theme. Microbiology 149, 1609–1621 (2003).
McLaughlin, J. L., Chang, C.-J. & Smith, D. Bench-top bioassays for the discovery of bioactive natural products: an update. Studies in natural products chemistry 9, 383–409 (1991).
Acknowledgements
We are grateful to Higher Education Commission (HEC), Pakistan for providing financial support to the current study.
Author information
Authors and Affiliations
Contributions
K.R. and Z.K.S. initiated and designed the research, K.R. performed the experiments, K.R., S.U.K., D.F., S.F., S.K., I.U., S.I.A., S.M., A.J.K., W.U.K., M.H.U.K. and M.J. analyzed the data and wrote the manuscript, M.A., M.N., S.F. and Z.K.S. revised and edited the manuscript and also provided advice on the experiments.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, K., Khan, S.U., Fahad, S. et al. In vitro biological screening of a critically endangered medicinal plant, Atropa acuminata Royle Ex Lindl of north western Himalaya. Sci Rep 8, 11028 (2018). https://doi.org/10.1038/s41598-018-29231-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29231-x
This article is cited by
-
Green synthesis of silver nanoparticles using Indian Belladonna extract and their potential antioxidant, anti-inflammatory, anticancer and larvicidal activities
Plant Cell Reports (2020)
-
Micropropagation of Atropa acuminata Royle ex Lindl. (a critically endangered medicinal herb) through root callus and evaluation of genetic fidelity, enzymatic and non-enzymatic antioxidant activity of regenerants
Acta Physiologiae Plantarum (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.