Abstract
Cyclophilin 1 (TvCyP1), a cyclophilin type peptidyl-prolyl isomerase present in the human parasite Trichomonas vaginalis, interacts with Myb1 and assists in its nuclear translocation. Myb1 regulates the expression of ap65-1 gene that encodes for a disease causing cytoadherence enzyme. Here, we determined the crystal structures of TvCyP1 and its complex with the minimum TvCyP1-binding sequence of Myb1 (Myb1104–111), where TvCyP1 formed a homodimer, unlike other single domain cyclophilins. In the complex structure, one Myb1104–111 peptide was bound to each TvCyP1 protomer, with G106-P107 and Y105 fitting well into the active site and auxiliary S2 pocket, respectively. NMR data further showed that TvCyP1 can catalyze the cis/trans isomerization of P107 in Myb1104–111. Interestingly, in the well-folded Myb1 protein (Myb135–141), the minimum binding sequence adopted a different conformation from that of unstructured Myb1104–111 peptide, that could make P107 binding to the active site of TvCyP1 difficult. However, NMR studies showed that similar to Myb1104–111 peptide, Myb135–141 also interacted with the active site of TvCyP1 and the dynamics of the Myb135–141 residues near P107 was reduced upon interaction. Together, the structure of TvCyP1 and detailed structural insights on TvCyP1-Myb1 interaction provided here could pave the way for newer drugs to treat drug-resistant strains.
Similar content being viewed by others
Introduction
Cyclophilins are a family of ubiquitous and versatile enzymes exhibiting peptidyl-prolyl isomerase (PPIase) activity1. They perform a wide variety of biological functions either by the catalysis of cis/trans isomerization of prolyl peptide bonds or as binding partners of biological substrates. The various biological functions of cyclophilins include, but not limited to protein folding and trafficking, immune response, signal transduction, viral infection and transcription regulation2,3. Human cyclophilin A (HcypA) is the well-studied cyclophilin that is found to interact with several proteins leading to various diseases including HIV infection and cancer3. In spite of identifying a large number of interacting proteins of HcypA, the underlying mechanism of HcypA action and the physiological outcome of interactions are not known in most cases.
Catalytically active cyclophilins with a single cyclophilin domain reported till now are monomeric in nature. The PPIase activity of cyclophilins can be inhibited by cyclosporin A (CsA), sanglifehrin A (SfA) or their non-immunosuppressive analogues by binding to the active site of cyclophilins4,5. Besides the active site where substrate proline binds and catalysis occurs, cyclophilins also possess an auxiliary S2 pocket guarded by “gatekeeper residues”, where the preceding residues of the isomerized proline interact6. The “gatekeeper region” of the S2 pocket shows maximum chemical diversity among various isoforms of cyclophilins. Hence, the region surrounding the S2 pocket is the most ideal site for designing drugs with enhanced specificity and binding affinity for particular isoforms of the cyclophilin family.
Parasitic cyclophilins influence different stages of a parasite’s development, and CsA or its analogues have been found with anti-parasitic activity7,8. Most of the parasitic cyclophilins reported to date possess high sequence similarity and identity to HcypA9,10. In most cases, the biological substrates of these cyclophilins are unknown and extensive structural studies on cyclophilin- substrate interactions have not been reported.
TvCyP1 is a cyclophilin present in the parasite Trichomonas vaginalis (T. vaginalis) showing high sequence identity to HcypA. T. vaginalis is the most common, sexually transmitted, non-viral pathogen infecting humans, causing Trichomoniasis. The infection causes several adverse reproductive outcomes and even cervical and prostate cancers11,12,13,14. T. vaginalis also maintains a symbiotic relationship with various other parasitic organisms, which can eventually lead to an inflammatory response or resistance to metronidazole (MTZ)15. The availability of only a single class of drugs to treat the disease is a major concern for public health, with the emergence of drug resistant strains, especially due to the higher transmission of HIV and HPV in infected individuals16,17,18,19.
Cytoadherence is one of the most important contact-dependent mechanisms by which T. vaginalis begins and continues chronic infection. Among the several hydrogenosomal enzymes that also function as adhesins, AP65, encoded by ap65-1 gene, plays an important role in cytoadherence of the parasite to human vaginal epithelial cells20,21. AP65 is a 65 kDa hydrogenosomal malic enzyme primarily involved in carbohydrate metabolism, but also act as adhesin at the surface of the parasite. The temporal and iron-inducible expression of AP65 is regulated by the coordinated actions of three Myb-like transcription factors, Myb1, Myb2 and Myb3, by their differential and competitive selection of the entry sites in the promoter of ap65-1 gene22,23,24. Nuclear import of these Myb proteins is a crucial checkpoint that regulates transcription, which in Myb2 and Myb3 occurs via their own DNA binding domains (DBDs)25,26. However, for Myb1, the nuclear translocation is regulated by TvCyP1. The interaction of TvCyP1 with Myb1 leads to the cytoplasmic release of Myb1 from membrane-bound vesicles, an important step preceding translocation to nucleus. We also identified a minimum TvCyP1 binding sequence in Myb1 (Myb1104–111), in which 105YGP107 was found critical in TvCyP1 interaction. Mutations of G106 and P107 in Myb1 changed the cytoplasmic retention and nuclear translocation of overexpressed Myb1 protein27.
In this study, we determined the crystal structures of TvCyP1 in the absence and presence of Myb1104–111. Unlike other dimeric or multimeric single domain cyclophilins28,29, TvCyP1 formed a homodimer without hindering the substrate binding site and existed as a dimer even in solution. In the complex structure, P107 of one Myb1104–111 molecule was bound to the active site of each TvCyP1 protomer. Presence of tyrosine preceding the Gly-Pro bond in Myb1104–111 allowed optimal interaction with the S2 pocket. Using NMR, we have also confirmed the catalytic behavior of TvCyP1 and inhibition of catalysis by CsA. Although our attempts to crystallize the TvCyP1–Myb135–141 complex were not successful, with NMR and site-directed mutagenesis, we showed that P107 of Myb135–141 also interacts with the active site of TvCyP1 similar to Myb1104–111 peptide fragment. NMR Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion experiments suggested that many Myb135–141 residues exhibit millisecond time-scale dynamics and these conformational exchanges were notably reduced for the residues surrounding P107 upon addition of TvCyP1. By and large, our study reports for the first time the structure of a catalytically active dimeric cyclophilin possessing only a single cyclophilin domain and provides structural details of its interaction with an intriguing biological substrate. The data shown here could provide further insights into the design of newer drugs to treat Trichomoniasis.
Results
TvCyP1 is a divergent, single domain cyclophilin and forms a stable dimer in solution
TvCyP1 is a single domain cyclophilin that shows high sequence identity to human and parasitic cyclophilins, all of which are monomers (Supplementary Fig. S1). Like Caenorhabditis elegans Cyp3 (Ccyp3; PDB: 1DYW) and Brugia malayi CypB (BcypB; PDB: 4JCP), TvCyP1 is a divergent loop cyclophilin and possesses an additional loop in the residues 49–55 (KSGMPLS)30. Although TvCyP1 contains the conserved E84, which helps to lock and hitch the loop into a certain conformation, the divergent loop shows variation from the consensus loop sequence (**GK*LH), observed in all other divergent cyclophilins. Because of the presence of serine instead of a highly conserved H54 seen in Ccyp3, TvCyP1 lacks the metal ion coordination site formed by the imidazole sidechain of H54 and sulfhydryl groups of C168 and C40 (numbering based on 1DYW)30.
To evaluate whether TvCyP1 exists as a monomer or dimer in solution, we performed size-exclusion chromatography with multi-angle light scattering (SEC-MALS) and analytical ultracentrifugation–sedimentation velocity (AUC-SV). The results from both the experiments confirmed that the protein exists as a dimer in solution (Fig. 1a,b). This is in contrast to the behavior of all other single domain cyclophilins in solution. In addition, the sensitivity of 3D triple resonance experiments was not sufficient to assign any of the side chain carbons and protons, possibly because of shorter transverse relaxation rate (T2), typical of a larger-sized protein. Presence of only a single set of peaks in [1H, 15N]-transverse relaxation optimized spectroscopy–heteronuclear single quantum coherence (TROSY-HSQC) spectrum further confirmed the symmetric structure of TvCyP1 in solution (Supplementary Fig. S2).
Dimeric nature of TvCyP1 in solution. (a) SEC-MALS analysis of 3 mg/mL TvCyP1 showing the presence of a dimer in solution. Molecular weight of TvCyP1 monomer is 19.25 kDa. (b) AUC-SV profile of TvCyP1 (30 μM concentration) showing continuous sedimentation coefficient distribution. The experimental data analysis by using Sedfit yielded an estimated molecular weight (MW) of 37.5 kDa, very close to the dimeric MW (38.5 kDa).
Crystal structure of TvCyP1
To elucidate the structure of TvCyP1, we crystallized it and obtained hexagonal crystals that gave optimal quality X-ray diffraction and belonged to the space group P6122 with the following unit cell dimensions: a = b = 38.51 Å, c = 365.70 Å, and α = β = 90°, γ = 120°. The structure of TvCyP1 was solved by molecular replacement by using the structure of Ccyp3 (PDB: 1DYW)30 as a search model, which shares 70% and 82% sequence identity and similarity, respectively, with TvCyP1. The final crystal structure was refined to 2.27 Å resolution with Rwork and Rfree values of 21.20% and 27.80%, respectively (Table 1).
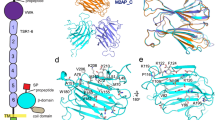
The crystal structure of TvCyP1 displays the canonical cyclophilin fold with a β-barrel structure composed of eight anti-parallel β-strands capped by two α-helices at the top and bottom (Fig. 2a). With one of the crystallographic symmetry mates, TvCyP1 seems to form an anti-parallel side-to-side dimer, which was earlier confirmed in solution. An interesting structural feature of TvCyP1 is the presence of an extra small beta sheet between α1 and β4. The prototypical cyclophilin, HcypA, shows high sequence identity (62%) to TvCyP1. Superposition of HcypA (PDB: 1OCA)31 with TvCyP1 revealed high structural identity (root mean square deviation [RMSD] = 0.910 Å) between HcypA and the protomer of TvCyP1 (Fig. 2b). The active site pocket is far from the dimer interface so that substrate binding to the active site of each protomer is not hindered (Fig. 2c). The dimer interface forms a pocket and is stabilized by hydrogen bonds, salt bridges and hydrophobic interactions between non-conserved residues residing in the loops between β1 and β2, α1 and β3 and α3 and β8. Hydrogen bond interactions between conserved G51 of the divergent loop and R161, R14 and conserved K57 and a salt bridge interaction between D16 and K57 are seen at the dimer interface (Fig. 2d). Hydrophobic interactions at the interface are formed by the residues M60, F78, M163 and M165 (Fig. 2e). Like Ccyp3, TvCyP1 does not form a disulfide bridge between conserved residues C169 and C41, and these cysteines could play a role in a signaling mechanism during oxidative stress conditions30.
Dimeric structure of TvCyP1 by x-ray crystallography. (a) Structure of TvCyP1 dimer showing secondary structural elements (PDB: 5YB9). The divergent loop of TvCyP1 is highlighted in red. (b) Structural alignment of TvCyP1 protomer (blue) with HcypA (orange). Both structures show the typical cyclophilin fold and exhibit high similarity. (c) Surface representation of TvCyP1 highlighting the active site pocket and S2 pocket. The active site residues and “gatekeeper” residues that guard the S2 pocket are shown in sticks. The structure shows the antiparallel nature of TvCyP1 homodimer. (d and e) Close-up views of polar/salt bridge (d) and hydrophobic interactions (e) at TvCyP1 dimer interface. The residues that form a dimer interface from either of the protomers are shown in different colored sticks. Dotted lines indicate hydrogen bond/salt bridge interactions. Oxygen, nitrogen and sulfur atoms are shown in red, blue and yellow, respectively.
Structure of TvCyP1–Myb1104–111 complex
We reported earlier that Myb1 has a minimum TvCyP1 binding sequence, Myb1104–111, with 105YGP107 critical for TvCyP1 interaction27. To further confirm this observation, we designed four different fluorescein isothiocyante (FITC)-labeled peptides starting from Myb1104–108 (104EYGPK108) to Myb1104–111 (104EYGPKWNK111) for fluorescence polarization (FP) experiments. Binding isotherms from FP experiments confirmed the eight-residue Myb1 peptide, Myb1104–111, having the strongest binding to TvCyP1, with Kd value of 16.81 ± 0.58 μM (Fig. 3a).
The minimum binding sequence in Myb1 and the complex structure of TvCyP1 with the same. (a) Binding curves from fluorescence polarization experiments of Myb1 fragments with TvCyP1. Error bars are standard deviation (SD). Binding affinities were derived from a one-site binding model by using GraphPad Prism 6. 104EYGPKWNK111 (Myb1104–111) showed the highest binding affinity to TvCyP1 (16.81 ± 0.58 μM). (b) Electrostatic surface of TvCyP1 dimer in complex with the minimum binding sequence of Myb1, Myb1104–111 (shown as green and magenta sticks; PDB: 5YBA). Each protomer binds to one molecule of Myb1 peptide. Although the peptide used was Myb1104–111, electron densities of only 105YGPKWN110 (protomer I) and 104EYGPK108 (protomer II) were obtained in the complex structure. Intramolecular salt bridge interaction between K108 and E104 is shown as a green dashed line (in 104EYGPK108 bound to protomer II). (c and d) Close-up views of interactions between TvCyP1 and Myb1 peptide. Residues of TvCyP1 interacting with Myb1 peptide are shown as orange sticks. Fragments of Myb1 peptide, 105YGPKWN110 and 104EYGPK108, bound to either protomer, are represented as green and magenta sticks in (c) and (d), respectively. The hydrogen bond between the C=O of P107 and the side chain NH2 of the catalytic residue R63 is shown as a black dashed line. See Supplementary Fig. S2 for a close-up view of the conformation of the Myb1 peptide bound to either protomer and the conformation of the same sequence in Myb135–141. See Supplementary Fig. S3 also for a 2D diagram by Ligplot+ showing detailed interactions between Myb1 peptide and TvCyP1.
To obtain a greater clarity on the interaction between TvCyP1 and Myb1104–111, we co-crystallized them. The orthorhombic crystals gave optimal-quality X-ray diffraction and belonged to the space group P212121, with the following unit cell dimensions: a = 37.79 Å, b = 78.07 Å, c = 117.71 Å, and α = β = γ = 90°. The structure was solved and refined to 2.06 Å resolution with Rwork and Rfree values of 16.79% and 21.29%, respectively (Table 1). The asymmetric unit contained two TvCyP1 molecules forming an anti-parallel side-to-side dimer with non-crystallographic symmetry.
In the initial refinement cycle, continuous electron densities (at 1σ cutoff) located in the active site of TvCyP1 were observed and identified as parts of the Myb1104–111 peptide: 105YGPKWN110 in one protein molecule and 104EYGPK108 in the other (Fig. 3b). The 106GP107 atoms were inserted deeply into the hydrophobic active site pocket of TvCyP1, whereas Y105 nestled in the S2 pocket. The complex is formed by several hydrophobic and hydrogen bond interactions of Myb1104–111 peptide with the active site and “gatekeeper residues” (Fig. 3c,d and Supplementary Fig. S3). In the active sites of both protomers, TvCyP1 anchors the oxygen of P107 to the hydrophobic pocket by means of hydrogen bond with the guanidinium of its catalytic residue, R63. The overall structures of TvCyP1 in the absence and presence of Myb1104–111 were similar, with RMSD 0.225 Å for the main chain. Both structures presented good overall stereochemistry (Table 1) with small RMSD, which suggests that the presence of Myb1104–111 did not greatly alter the protein conformation and thus the crystal packing contacts. Presence of Y105 preceding G106 in Myb1104–111 enabled optimal interaction with the S2 pocket in TvCyP1.
Myb1104–111 peptide bound to both protomers of TvCyP1 showed a difference in the conformation because of different side-chain orientations of K108. In protomer I, 105YGPKWN110 was in an open conformation, while in protomer II, 104EYGPK108 adopted a closed conformation due to an intramolecular salt bridge interaction between E104 and K108 (Fig. 3b). However, the two Myb1104–111 peptides bound to each protomer adopted a similar conformation of the critical interacting residues 105YGP107.
Peptidyl-prolyl isomerase activity of TvCyP1
Since TvCyP1 is the only known dimeric cyclophilin with the active sites exposed, we tried to obtain direct evidence for catalysis activity by using NMR rotating-frame Overhauser effect spectroscopy (ROESY). In a ROESY spectrum, the sign of the cross-peaks from chemical exchange is the same as the diagonal peaks, while the cross-peaks between neighboring hydrogens display an opposite sign. In order to monitor the catalysis of cis/trans isomerization of P107 in Myb1104–111 by TvCyP1, we completed the 1H resonance assignments of Myb1104–111 peptide. Separate resonance signals were observed for the cis and trans conformations of residues from Y103 to K111 (Supplementary Fig. S4), indicating that each conformation lies in a distinct chemical environment and the cis/trans isomerization is slow on NMR time scale (exchange rate <0.1 s−1). Because of the slow exchange rate of the un-catalyzed isomerization, only the cross-peaks between neighboring hydrogens (with opposite sign relative to the diagonal peaks) are observed in the ROESY spectrum (Fig. 4a). Presence of catalytic amounts of TvCyP1 enhanced the cis/trans isomerization of P107, as revealed by the appearance of exchange cross-peaks connecting cis and trans Hα peaks of G106, P107 and K108 (Fig. 4b). The addition of 1.0 molar equivalent of CsA, a strong binding HcypA inhibitor, hindered catalysis, as observed by the disappearance of exchange cross-peaks (Fig. 4c). This finding suggests that CsA or CsA analogues can bind to the active site of TvCyP1 with greater affinity than Myb1104–111 and hence can be used to inhibit the TvCyP1–Myb1 interaction. All these data provide a direct evidence for the catalysis of cis/trans isomerization of P107 in Myb1104–111 peptide by TvCyP1.
Catalysis of the cis/trans isomerization of Myb1104–111 peptide by TvCyP1. (a) Selected region of the 300-ms mixing-time ROESY spectrum of Myb1104–111 peptide (4.4 mM). The positive peaks are colored in blue and negative peaks in red. The 1H resonances of G106, P107 and K108 are marked, and the resonances from cis and trans conformers are labeled as C and T respectively. The negative cross-peaks between neighboring hydrogens are shown in red and connected by red dashed lines. (b) Exchange between cis and trans conformations was accelerated in the presence of 22 μM TvCyP1, as evidenced by the appearance of positive exchange cross-peaks between cis and trans resonances shown in blue and connected by black dashed lines. (c) Inhibition of TvCyP1 isomerase activity by CsA (22 μM) resulted in a loss of the exchange cross-peaks between cis and trans resonances.
Identification of TvCyP1-binding site on Myb135–141 protein
Unlike the typical substrates for cyclophilins that exhibit flexible structures to fit into the active site pocket, Myb1 is a well-folded protein with TvCyP1-binding key residues, 105YGP107, lying in a short loop structurally restrained by two stable helices. Hence, the conformation of the minimum binding sequence, 104EYGPKWNK111, in Myb1 protein is very different from those observed in the complex structure (Fig. 5a). Our previous NMR structural study on Myb135–141 indicated that all residues from E104 to K111 adopt a trans conformation and there was no NMR signal from the cis conformer32. As a result, it was not possible to study the catalysis of cis/trans isomerization of 106GP107 bond in Myb135–141 protein by TvCyP1. Also, we tried to co-crystallize the TvCyP1–Myb135–141 protein complex but could not obtain any crystal.
TvCyP1 interacts with the P107-containing loop in Myb135–141 protein. (a) Conformations of the minimum binding sequence in protomer I (left), protomer II (middle) and Myb135–141 protein (right), showing that the structure of this sequence in 3 states is different from each other. (b) The intensity ratio of the amide proton resonances, with and without irradiation of the aliphatic protons, plotted against the Myb135–141 residue number. The dashed line signifies one SD from the average intensity ratio (~0.8). (c) Mapping the cross-saturation transfer NMR results on Myb135–141 protein structure. Proline residues are shown as black spheres. The residues exhibiting intensity ratios lower that 0.8 are shown as red spheres and others as cyan spheres. Since TvCyP1 is a PPIase, significant reduction in peak intensities of residues near P107 indicates that TvCyP1 binds to the loop containing P107.
To identify the TvCyP1-binding site on Myb135–141 protein, we used NMR. The titration of unlabeled TvCyP1 to 15N-labeled Myb135–141, which includes the R2-R3 DNA binding domains of Myb1, resulted in significant line-width broadening of all residues but very little chemical shifts perturbation (Supplementary Fig. S5). To identify the TvCyP1 binding site on Myb135–141 protein, we used cross-saturation transfer NMR that helps to identify the residues involved in direct intermolecular contacts33. We prepared uniformly 15N, 2H (>95%)-labeled Myb135–141 to avoid excitation of its aliphatic protons by the radio-frequency pulses. The labeled Myb135–141 protein mixed with unlabeled TvCyP1 was dissolved in 50% 2H2O to reduce saturation transfer from spatially crowded amide protons by 1H2O. The plot of intensity ratios of amide peaks with and without saturation versus residue number showed a significant decrease in amide peak intensity for the residues near P107, with the minimum at G106 and K108 (Fig. 5b). Although there are 5 proline residues in Myb135–141, P70, P83, P89, P92 and P107, cross-saturation transfer NMR clearly showed that TvCyP1 interacts with the P107-containing loop in Myb135–141 protein (Fig. 5c).
Mapping the Myb135–141 protein interaction region on TvCyP1
Further, we tried to map the Myb135–141 protein binding site on TvCyP1 by NMR. To achieve this goal, we firstly completed the 1H, 13C and 15N backbone chemical shift assignment of TvCyP1 using standard triple-resonance NMR spectra acquired from a 2H, 13C, 15N (>95%)-labeled protein sample. The 1H, 15N TROSY-HSQC of TvCyP1 features an extremely well dispersed set of resonances (Supplementary Fig. S2), agreeing with the well-folded structure of this protein. To map the Myb135–141 protein interaction site on TvCyP1, we titrated 2 folds of unlabeled Myb135–141 protein to 15N-labeled TvCyP1, resulting in severe line-width broadening of the peaks of the active site and “gatekeeper” residues of TvCyP1 (Fig. 6a,b and Supplementary Fig. S6). Apart from the active site and “gatekeeper” residues, certain residues in faraway loops were also perturbed or line-width–broadened during titration owing to conformational exchange during substrate turnover34. To confirm the specificity of interaction, we produced a Myb135–141 mutant protein with P107 mutated to alanine (P107A-Myb135–141) and titrated unlabeled P107A-Myb135–141 to 15N-labeled TvCyP1 (Supplementary Fig. S7). The nearly identical NMR spectra from TvCyP1 with and without P107A- Myb135–141 suggested that TvCyP1 recognizes P107A-Myb135–141 weakly. Of note, despite 5 proline residues in Myb135–141, only P107 can bind to TvCyP1 active site pocket specifically.
Interaction of TvCyP1 with Myb135–141 protein is similar to that of its binding to Myb1104–111peptide. (a) Plot of the ratio of intensities of TvCyP1 peaks in the presence and absence of Myb135–141 (1:2) against the residue number. Gaps indicate unassigned or proline residues. Red bars indicate the intensity ratio less than the lower control line (Average − SD). Residues that disappeared completely are shown as negative red bars. See Supplementary Fig. S6 for titration HSQC overlay. See also Supplementary Fig. S7 for the interaction between TvCyP1 and Myb135–141 mutant P107A. (b) Cartoon structure of TvCyP1 protomer showing line-width–broadened or disappeared residues on titration of Myb135–141 highlighted in red. (c) The CSP values for backbone amide resonances of TvCyP1 on titration with Myb1104–111 (1:2). Red bars indicate CSP values more than the upper control line (Average + SD) and yellow bars indicate backbone amide resonances that disappeared on titration. See Supplementary Fig. S6 for titration HSQC overlay. (d) Cartoon structure of TvCyP1 protomer showing chemical shift-perturbed and disappeared residues on titration of Myb1104–111 highlighted in red and yellow, respectively.
Although we tried to measure the binding affinity of TvCyP1–Myb135–141 interaction using several biophysical techniques, we were not successful. Hence, to compare TvCyP1–Myb1104–111 and TvCyP1–Myb135–141 interactions, we also titrated Myb1104–111 to 15N-labeled TvCyP1. The titration resulted in a notable chemical shift perturbation (CSP) of several TvCyP1 residues (Fig. 6c and Supplementary Fig. S6). Structural mapping of these residues showed that most of the TvCyP1 residues that underwent significant chemical shift perturbation on Myb1104–111 peptide titration also exhibited severe line-width broadening on Myb135–141 protein titration (Fig. 6d). Although, in the well-folded Myb135–141 protein, the minimum binding sequence adopts a very different conformation from that observed in the complex structure, our NMR studies suggest that the interaction between TvCyP1 and Myb135–141 protein is similar to the highly specific recognition between TvCyP1 and Myb1104–111 peptide.
Interaction with TvCyP1 reduces the slow dynamics in Myb135–141 around P107
We carried out NMR Carr-Purcell-Meiboom-Gill (CPMG) relaxation dispersion experiments at 1H frequency of 600 and 850 MHz on Myb135–141 in the free form and in complex with TvCyP1 to probe the millisecond time scale conformational exchange of Myb135–141 in 2 different states. The 15N relaxation dispersion profiles of individual residues of Myb135–141 were fitted to a two-site exchange process using CR7235 or TSMFK0136 model by the software relax37 (Fig. 7a) and the extracted dynamic parameters are listed in Supplementary Tables S1 and S2 for the CR72 and TSMFK01 models, respectively. Plenty of residues in free Myb135–141 displayed conformational exchange (Fig. 7b), suggesting that Myb1 is a highly dynamic protein. However, the exchange rates for the residues around P107 are too different to be fitted globally to a single model. The residues with very slow exchange rates were fitted to the TSMFK01 model and those with faster exchange rates were fitted to the CR72 model, shown as yellow and red spheres respectively in Fig. 7b,c. In free Myb135–141, E104 and several residues in α5 exhibit faster dynamic motions (Fig. 7b). And most of these motions are restricted to become very slow dynamics by TvCyP1 binding (Fig. 7c). Together, NMR CPMG relaxation dispersion data show that the highly dynamic motions of the Myb1 residues around P107 are significantly restricted on interaction with TvCyP1.
Myb1 dynamics is reduced by the binding of TvCyP1. (a) Relaxation dispersion data for E104 of Myb135–141 in the free form (black circles) and in complex with TvCyP1 (red circles) measured at 850 MHz. Lines show individual fits to a two-site exchange process, demonstrating very slow exchange fit with the TSMFK model or faster exchange fit with the CR72 model, corresponding to yellow and red spheres, respectively, in the structural map in (b) and (c). Black spheres denote proline residues not observed by 1H,15N HSQC NMR and white spheres indicate overlapped residues that could not be analyzed or the residues that do not show slow dynamics. (b) Mapping the models of dispersion data of free Myb135–141, showing that a lot of residues in Myb1 have faster conformational exchange fit with the CR72 model. (c) In the presence of TvCyP1, the dynamics of E104 and several residues in α5 are restricted to become very slow dynamics and can be fitted with the TSMFK model.
Discussion
Dimerization via the interaction of hydrophobic residue pairs in a mirror-image–like fashion as seen in TvCyP1 has been observed in various dimeric bacterial FKBP-type PPIases38,39, but not in any other cyclophilins. Most of the cyclophilins known to date are monomeric in nature, with certain exceptions such as Aspergillus fumigatus cyclophilin (Asp f11; PDB: 2C3B), cyclophilin from Mimivirus (Mimicyp; PDB: 2OSE) and cyclophilin from Hirschia baltica (AquaCyp300; PDB: 5EX1). However, AquaCyp300 is not a single domain cyclophilin because N- and C-terminal extensions together with the large insertion in the cyclophilin domain form a contiguous structural entity termed the NIC domain40. The dimer interface in AquaCyp300 is formed by residues of both the cyclophilin domain and NIC domain. Although Asp f11 and Mimicyp are single-domain cyclophilins with swapped dimeric and trimeric structures, respectively, they were found to be monomeric in solution28,29. Moreover, multimerization in these two proteins resulted in obstruction of the active site, thereby hindering substrate interaction. In contrast, TvCyP1 exhibits a natural tendency to form a dimer even in solution, as observed from a single dimeric peak in SEC-MALS and AUC-SV analyses, and has the active site of both protomers exposed for interaction with the substrate. Hence, TvCyP1 is the only single-domain dimeric cyclophilin reported so far that can still bind to its substrates.
The complex structures of cyclophilin with its biologically relevant substrate are available for only HcypA. The complex structures of HcypA with HIV capsid protein and CrkII protein show high structural similarity because both contain a Gly-Pro motif binding to the active site with proline fitting deep inside the hydrophobic pocket of the active site41,42. However, neither of these biological substrates show strong interaction with the S2 pocket because of the absence of residues that exhibit surface complementarity to the S2 pocket of HcypA.
Myb1 is an ideal cyclophilin substrate due to the presence of Tyr-Gly sequence preceding the interacting proline that enables a strong interaction with the active site and S2 pocket of TvCyP16,43. As a result, in the TvCyP1–Myb1104–111 complex structure, we observed that Y105 was inserted well inside the S2 pocket. However, Myb135–141 is a well-folded protein with interacting proline, lying in a short loop surrounded by two stable helices. Hence, in the well-folded Myb1 protein, the interaction of Y105 with the S2 pocket is structurally hindered and therefore could occur only if some conformational changes happen at helix 4, where it is located. This was found to be true from CPMG relaxation dispersion experiments which showed that Myb135–141 underwent slow conformational exchange. The slow dynamics near the region containing P107 was greatly reduced upon interaction with TvCyP1 further suggesting that the interaction is facilitated by dynamics.
In the complex structure, we observed a difference in the conformation of Myb1104–111 peptide bound to both protomers of TvCyP1 because of different side-chain orientations of K108. Structural comparison showed that the conformation of 104EYGPK108 in protomer II is more similar to the conformation of the loop in Myb1 containing P107 (Fig. 5a). Hence, with some conformational changes at Y105, the minimum binding sequence in Myb135–141 upon interaction with TvCyP1 could probably adopt a similar structure as that of 104EYGPK108, bound to protomer II.
Nuclear translocation of Myb1 is an important step regulating the expression of disease causing ap65–1 gene. Hence, preventing the nuclear import by blocking the interaction between Myb1 and TvCyP1 can be a good strategy to treat the disease. From ROESY experiments, we observed that CsA can inhibit Myb1104–111 binding to the active site. Although CsA has long been proposed to be an inhibitor for cyclophilin4, the deleterious effect of its immunosuppressive activity44 has always been a major hindrance in using it as a drug to treat diseases. Moreover, CsA is not specific to any of the isoforms of cyclophilins6. Many attempts to design and synthesize an isoform-specific drug for cyclophilins have not been successful and the S2 region has been proposed as an ideal site for isoform-specific drug development6. However, because HcypA and TvCyP1 possess the same set of “gatekeeper” residues, a drug targeting the S2 region of TvCyP1 may also be able to interact with HcypA in humans. However, the harmful effect of this unwanted interaction would probably not be as detrimental as immunosuppression and hence is worth studying. An alternative and probably better drug target site would be the dimer interface. As shown in Fig. 2c, there is a pocket formed by the dimer interface in TvCyP1. A CsA analogue that can recognize both the active site and the dimer interface pockets may specifically recognize TvCyP1 but not the monomeric HcypA.
Collectively, our study has helped in understanding the unique dimeric structure of TvCyP1 and obtaining detailed information about its interaction with the biological substrate Myb1. TvCyP1 has also been found to interact with Myb327, and future work will elucidate the details of the Myb3–TvCyP1 interaction. It is also possible that TvCyP1 dimerization may help in the simultaneous binding of Myb1 and Myb3 at the active sites of either protomer. Indeed, much needs to be studied at the cellular level to further understand the role of TvCyP1 and the significance of its dimerization on the parasite’s development and pathogenicity. However, our current data shows that TvCyP1 is a good drug target and provides a rationale to design drugs specific for the protein or to disrupt its interaction with Myb1.
Methods
Expression and purification of TvCyP1 and TvCyP1 mutants
TvCyP1 gene was cloned into pET-28a vector (Novagen) and was expressed with an N-terminal His-tag followed by a TEV digestion recognition site in the Escherichia coli strain BL21(DE3). After TEV protease digestion, TvCyP1 protein had an additional Glycine and Serine at its N-terminus. For unlabeled or uniformly labeled samples, E. coli cells were cultured in lysogeny broth medium or M9 minimal medium at 37 °C, respectively. The recombinant TvCyP1 was expressed at 16 °C by the addition of 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) with the culture medium reaching optical density at 600 nm of 0.6. Cells were harvested 16 hr after induction and resuspended in Tris-HCl (pH 8.0) and lysed by using a M-110S microfluidizer (Microfluidics). The insoluble fraction was removed by centrifugation at 12,000 rpm for 30 min. The supernatant was passed through Q ion-exchange resin (Q sepharose fastflow, GE healthcare) and the flow-through containing TvCyP1 was further purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity resin (Qiagen, Hilden, Germany) equilibrated with 20 mM Tris-HCl, 100 mM NaCl, and 10 mM Imidazole (pH 8.0). TvCyP1 was eluted from the Ni-NTA resin in 500 mM imidazole, 100 mM NaCl and 20 mM Tris solution at pH 8.0. The eluate was treated with TEV protease overnight at room temperature and then diluted to a final imidazole concentration of 10 mM and passed through the Ni-NTA resin again. The flow-through containing TvCyP1 was buffer-exchanged in 20 mM NaH2PO4, 50 mM NaCl and 0.5 mM NaN3 (pH 6.0) by centrifugation with 10,000 Da MWC membrane ultrafiltration (Millipore). The 2H/13C/15N-labeled TvCyP1 was prepared in the same way, but the host cells were grown in M9 minimal medium containing D2O supplemented with 15N-NH4Cl (1 g/L) and 13C-glucose (2 g/L). The protein purity was verified by SDS-PAGE, and the concentration of TvCyP1 monomers was assessed by using a molar absorption coefficient of E280 = 8,480 M−1. TvCyP1 mutants were expressed and purified using the same protocol.
Preparation of Myb1, Myb1 mutants and Myb1 peptides
The gene encoding Myb135–141 was cloned into the pET-29b (Novagen) vector and expressed in E. coli BL21(DE3) cultured in lysogeny medium or minimal medium for 1H/15N-labeled samples. The expressed Myb135–141 has two extra residues in the C-terminus, leucine and glutamic acid, followed by a His-tag. Myb135–141 was expressed and purified as described32. 2H,13C,15N-labeled Myb1 was prepared in the same way, but the host cells were grown in M9 minimal medium containing D2O supplemented with 15NH4Cl (1 g/L) and 13C, 2H glucose (2 g/L). The purity of Myb135–141 was confirmed by SDS-PAGE and the concentration was calculated by using a molar absorption coefficient of E280 = 33,460 M−1. The final protein sample used for experiments was in a buffer containing 20 mM NaH2PO4, 50 mM NaCl and 0.5 mM NaN3 (pH 6.0). Myb1 mutants were expressed and purified using the same protocol. Myb1 peptides were purchased from Yao-Hong Biotechnology Inc. (Taiwan) and FITC-labeled Myb1 peptides were synthesized by the peptide synthesis facility at the Institute of Biological Chemistry, Academia Sinica.
Size-exclusion chromatography coupled to multi-angle static light scattering (SEC-MALS)
A total of 300 μg of TvCyP1 was injected into a size-exclusion chromatography column (ENrich™ SEC. 70 10 × 300 Column, Bio-Rad Laboratories, Inc.) and analyzed by static and dynamic light scattering45 at a flow rate of 0.5 mL min−1 in 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0) at 25 °C. The column is in line with four detectors: a static light-scattering detector (miniDAWN TREOS, Wyatt Technology), a quasi-elastic light-scattering detector (QELS, Wyatt Technology), a refractive index detector (Optilab T-rEX, Wyatt Technology) and an ultraviolet-visible (UV) detector (Agilent, USA). Bovine serum albumin (Sigma, A1900) was used for system calibration. Molecular weight was calculated by using ASTRA 6 (Wyatt Technology) with the dn/dc value set to 0.185 mL g−1.
Analytical ultracentrifugation
AUC-SV experiments were conducted on a Beckman Coulter ProteomeLab XL-I ultracentrifuge with an AN 50Ti rotor. The sample was collected from a Superdex 75 gel filtration column (GE Healthcare Life Sciences) after equilibrating the column extensively with 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0). We loaded 400 μl of 29 μM TvCyP1 and collected data at 20 °C at 50,000 rpm at 280 nm. The scans were collected every minute, with a total 150 scans obtained per sample. Buffer density, viscosity and partial specific volume were calculated by using SednTerp. The data were edited and processed by using SedFit software (https://sedfitsedphat.nibib.nih.gov).
Fluorescence polarization measurements
Myb1 peptides for fluorescence polarization experiments were labeled with FITC at the N-terminus. The indicated amounts of TvCyP1 were added to wells containing 0.5 μM FITC-labeled Myb1 peptide in 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0) at 298 K. Triplicate measurements of the reactions were acquired by using the SpectraMax Paradigm microplate reader (Molecular Devices, CA, USA) with excitation wavelength 485 nm and emission wavelength 535 nm. Data were analyzed and fitted to a one-site binding model by using GraphPad Prism 6 (San Diego, CA, USA).
Protein crystallization and data collection
For crystallization screening experiments, TvCyP1 sample was further purified on a Superdex 75-gel filtration column (GE Healthcare Life Sciences) after equilibrating the column extensively with 20 mM Bis-Tris, 50 mM NaCl and 0.5 mM NaN3 (pH 6.0). Initial protein crystallization trials were performed at 283 K by the sitting-drop vapour-diffusion method with commercial crystallization screen kits, 96-well Intelli-plates (Art Robbins Instruments) and a Phoenix robot (Art Robbins Instruments). For co-crystallizing with Myb1104–111 peptide, purified TvCyP1 protein was mixed with the synthetic Myb1104–111 peptide (Glu-Tyr-Gly-Pro-Lys-Trp-Asn-Lys) in a molar ratio of 1:4 before crystallization trials. Each crystallization drop was prepared by mixing 0.3 μL TvCyP1 or TvCyP1/peptide at 9 mg/mL with an equal volume of mother liquor, and the mixture was equilibrated against 100 μL reservoir solution. The crystals of TvCyP1 in apo and Myb1 peptide-bound forms were grown at 283 K within 7–14 days with the optimal conditions of 100 mM HEPES, pH 7.0, 30% (v/v) Jeffamine M-600 and 100 mM Tris-HCl pH 8.0, 30% (v/v) polyethylene glycol 400, respectively. For diffraction data collection, the crystal was cryoprotected in mother liquor supplemented with 20% glycerol, and flash-frozen in liquid nitrogen at 100 K. The diffraction images of TvCyP1 in apo and Myb1 peptide-bound forms were recorded at the National Synchrotron Radiation Research Center (Taiwan) on a MX300HS detector in TPS 05A and a Q315r detector in TLS BL13C1 beamlines, respectively. Diffraction data were processed and scaled by using HKL2000 software46.
Structure determination and refinement
The crystal structures of TvCyP1 and TvCyP1 in complex with Myb1104–111 peptide were determined by molecular replacement with the Phaser-MR program47, by using the Ccyp3 structure from C. elegans (PDB: 1DYW)30 as a search model. Crystallographic refinement involved repeated cycles of conjugate-gradient energy minimization and temperature-factor refinement with the program phenix.refine in the PHENIX package48. Amino-acid side chains and water molecules were fitted into 2Fo-Fc and Fo-Fc electron-density maps by using COOT49. The model was evaluated by using PROCHECK50 and MOLPROBITY51. The data collection and structure refinement statistics are in Table 1. Final coordinates and structure factors of TvCyP1 and the TvCyP1–Myb1 peptide complex have been deposited in the Protein Data Bank (PDB: 5YB9 and 5YBA, respectively).
NMR experiments
TvCyP1 and Myb135–141 samples for NMR experiments were prepared in 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0). To improve the spectral quality of TvCyP1, the sample used for backbone assignment was 2H,13C,15N-labeled. The NMR spectra were acquired on a Bruker AVANCE 600-, 800- and 850-MHz spectrometers equipped with a z-gradient TXI cryoprobe (Bruker, Karlsruhe, Germany) at 310 K. Backbone assignment of TvCYP1 was based on TROSY-HNCACB and -HNCA spectra52. Backbone assignment of FM-TvCyP1 was based on the HNCACB spectrum with a 13C, 15N-labeled sample. Backbone assignment of Myb135–141 was reported previously32. Observation of chemical-shift changes as well as reduction in peak intensity in 1H,15N TROSY-HSQC of 15N-enriched TvCyP1 upon titrating unlabeled Myb1 peptide or Myb134–141 was used to confirm interactions and to determine the binding site of TvCyP1. The weighted CSPs for backbone 15N and 1HN resonances were calculated with the equation Δδ = [((ΔδHN)2 + (ΔδN/5)2)/2]0.5.
All experiments with Myb1 were performed at 298 K. The sample for cross-saturation transfer experiments contained a 2H, 13C, 15N-labeled Myb135–141 sample (333 μM) and unlabeled TvCyP1 (1000 μM) in 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0). To avoid spin diffusion, the sample was prepared in 50% D2O. The deuteration percentage of Myb135–141 is more than 95%. The saturation pulses were centered at 516.8 Hz (0.608 ppm) at which we observed large proton peaks from TvCyP1 and no peak from Myb135–141. The measurement time was 5.5 h, with relaxation delay and saturation time 5 s.
1H-TOCSY, 1H-COSY and 1H-NOESY were performed at 300 K with a mixing time of 75 ms (TOCSY) and 300 ms (NOESY) on the Myb1104–111 peptide to complete the assignment of proton resonances. The peptide was dissolved in 20 mM monosodium phosphate, 50 mM NaCl, 0.5 mM NaN3 (pH 6.0) to obtain a concentration of 2 mM. All ROESY experiments were performed at 300 K with 300 ms mixing time. For catalysis experiments, Myb1104–111 peptide (2000 μM) was mixed with TvCyP1/FM-TvCyP1 (13.3 μM) at a ratio of 150:1.
The 15N, 2H-labeled Myb135–141 (0.3 mM) in the free form and in complex with unlabeled TvCyP1 (0.36 mM) or unlabeled FM-TvCyP1 (0.36 mM) were prepared for CPMG measurement. The constant-time 15N single-quantum relaxation dispersion experiments36 were acquired at 298 K on both 600 MHz and 850 MHz NMR spectrometers with νCPMG of 50, 100, 200, 300, 400, 500, 600, 700, 800, 900 and 1000 Hz and a total CPMG delay of 40 ms. The spectra with νCPMG of 100 and 700 Hz were collected twice to estimate experimental errors. The peak heights in each spectrum were extracted by using an automated routine in NMRView. The R2,eff was calculated as R2,eff (νCPMG) = (−1/T) ln[I(νCPMG)/I0], where I(νCPMG) and I0 are the intensities of peaks recorded with and without the CPMG intervals. The 15N relaxation dispersion profiles of individual residues at both 600 and 850 MHz were fitted to a two-site exchange process by the software relax37. Two models, the Carver and Richards equation35 (CR72) that describes 2-site exchange for most time scales and TSMFK0136, which is appropriate for 2-site very slow exchange within range of microsecond to second time scale, were fitted. The Akaike’s model selection53 was performed to judge statistical significance of the models. Successful fit to CR72 model yields the population-average transverse relaxation rate (R20), the exchange rate (kex), the population of the major state (pA) and the chemical shift difference between 2 states (Δω). The fit to TSMFK01 yields R2A0, kex, and Δω. The extracted dynamic parameters are listed in Supplementary Tables S1 and S2 for the CR72 and TSMFK01 models, respectively.
All NMR spectra, except the spectra for cross-saturation transfer and CPMG relaxation dispersion experiments, were processed by using Topspin 3.1 (Bruker) and analyzed by using NMRViewJ54. For cross-saturation transfer experiments, the spectra were processed by using Topspin 3.1 (Bruker) and analyzed by using Sparky (T.D. Goddard and D.G. Kneller, Sparky 3, University of California, San Francisco). For CPMG-based relaxation dispersion experiments, the spectra were processed by using NMRpipe55 and analyzed by using NMRviewJ.
Data availability
The datasets generated during the current study are available in the RCSB PDB repository, [https://www.rcsb.org/pdb/home/home.do]. The backbone chemical shift assignment of TvCyP1 generated during the current study are available in BMRB under accession code 12014.
Accession Numbers
The atomic coordinates and structure factors for TvCyP1 and TvCyP1-Myb1 peptide complex are deposited under RCSB PDB accession codes PDB: 5YB9 and 5YBA respectively. The backbone NMR chemical shift of TvCyP1 has been deposited in the BMRB under accession code 12014.
References
Wang, P. & Heitman, J. The cyclophilins. Genome Biology 6, https://doi.org/10.1186/gb-2005-6-7-226 (2005).
Ferreira, P. A. & Orry, A. From Drosophila to Humans: Reflections on the Roles of the Prolyl Isomerases and Chaperones, Cyclophilins, in Cell Function and Disease. J Neurogenet 26, 132–143, https://doi.org/10.3109/01677063.2011.647143 (2012).
Nigro, P., Pompilio, G. & Capogrossi, M. C. Cyclophilin A: a key player for human disease. Cell Death & Disease 4, https://doi.org/10.1038/cddis.2013.410 (2013).
Mikol, V., Kallen, J., Pflugl, G. & Walkinshaw, M. D. X-ray structure of a monomeric cyclophilin A-cyclosporin A crystal complex at 2.1 A resolution. J Mol Biol 234, 1119–1130, https://doi.org/10.1006/jmbi.1993.1664 (1993).
Kallen, J., Sedrani, R., Zenke, G. & Wagner, J. Structure of human cyclophilin a in complex with the novel immunosuppressant sanglifehrin a at 1.6 angstrom resolution. Journal of Biological Chemistry 280, 21965–21971, https://doi.org/10.1074/jbc.M501623200 (2005).
Davis, T. L. et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol 8, e1000439, https://doi.org/10.1371/journal.pbio.1000439 (2010).
Bua, J., Ruiz, A. M., Potenza, M. & Fichera, L. E. In vitro anti-parasitic activity of Cyclosporin A analogs on Trypanosoma cruzi. Bioorganic & Medicinal Chemistry Letters 14, 4633–4637, https://doi.org/10.1016/j.bmcl.2004.07.003 (2004).
Yau, W. L. et al. Cyclosporin A Treatment of Leishmania donovani Reveals Stage-Specific Functions of Cyclophilins in Parasite Proliferation and Viability. Plos Neglected Tropical Diseases 4, https://doi.org/10.1371/journal.pntd.0000729 (2010).
Peterson, M. R. et al. The three-dimensional structure of a Plasmodium falciparum cyclophilin in complex with the potent anti-malarial cyclosporin A. J Mol Biol 298, 123–133, https://doi.org/10.1006/jmbi.2000.3633 (2000).
Taylor, P., Page, A. P., Kontopidis, G., Husi, H. & Walkinshaw, M. D. The X-ray structure of a divergent cyclophilin from the nematode parasite Brugia malayi. Febs Lett 425, 361–366, https://doi.org/10.1016/S0014-5793(98)00264-6 (1998).
Mitteregger, D. et al. High detection rate of Trichomonas vaginalis in benign hyperplastic prostatic tissue. Medical Microbiology and Immunology 201, 113–116, https://doi.org/10.1007/s00430-011-0205-2 (2012).
Stark, J. R. et al. Prospective Study of Trichomonas vaginalis Infection and Prostate Cancer Incidence and Mortality: Physicians’ Health Study. Journal of the National Cancer Institute 101, 1406–1411, https://doi.org/10.1093/jnci/djp306 (2009).
Gimenes, F. et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nature Reviews Urology 11, 672–687, https://doi.org/10.1038/nrurol.2014.285 (2014).
Ghosh, I. et al. Association between high risk human papillomavirus infection and co-infection with Candida spp. and Trichomonas vaginalis in women with cervical premalignant and malignant lesions. J Clin Virol 87, 43–48, https://doi.org/10.1016/j.jcv.2016.12.007 (2017).
Menezes, C. B., Frasson, A. P. & Tasca, T. Trichomoniasis - are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microbial Cell 3, 404–418, https://doi.org/10.15698/mic2016.09.526 (2016).
Dunne, R. L., Dunn, L. A., Upcroft, P., O’Donoghue, P. J. & Upcroft, J. A. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res 13, 239–249, https://doi.org/10.1038/sj.cr.7290169 (2003).
Fichorova, R. N. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. Journal of Reproductive Immunology 83, 185–189, https://doi.org/10.1016/j.jri.2009.08.007 (2009).
Kissinger, P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. Bmc Infectious Diseases 15, https://doi.org/10.1186/s12879-015-1055-0 (2015).
McClelland, R. S. et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis 195, 698–702, https://doi.org/10.1086/511278 (2007).
Garcia, A. F. & Alderete, J. F. Characterization of the Trichomonas vaginalis surface-associated AP65 and binding domain interacting with trichomonads and host cells. Bmc Microbiology 7, https://doi.org/10.1186/1471-2180-7-116 (2007).
Mundodi, V., Kucknoor, A. S., Klumpp, D. J., Chang, T. H. & Alderete, J. F. Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Molecular Microbiology 53, 1099–1108, https://doi.org/10.1111/j.1365-2958.2004.04192.x (2004).
Ong, S. J., Hsu, H. M., Liu, H. W., Chu, C. H. & Tai, J. H. Multifarious transcriptional regulation of adhesion protein gene ap65-1 by a novel Myb1 protein in the protozoan parasite Trichomonas vaginalis. Eukaryotic Cell 5, 391–399, https://doi.org/10.1128/Ec.5.2.391-399.2006 (2006).
Ong, S. J., Hsu, H. M., Liu, H. W., Chu, C. H. & Tai, J. H. Activation of multifarious transcription of an adhesion protein ap65-1 gene by a novel Myb2 protein in the protozoan parasite Trichomonas vaginalis. Journal of Biological Chemistry 282, 6716–6725, https://doi.org/10.1074/jbc.M610484200 (2007).
Hsu, H. M., Ong, S. J., Lee, M. C. & Tai, J. H. Transcriptional Regulation of an Iron-Inducible Gene by Differential and Alternate Promoter Entries of Multiple Myb Proteins in the Protozoan Parasite Trichomonas vaginalis. Eukaryotic Cell 8, 362–372, https://doi.org/10.1128/Ec.00317-08 (2009).
Hsu, H. M. et al. Iron-Inducible Nuclear Translocation of a Myb3 Transcription Factor in the Protozoan Parasite Trichomonas vaginalis. Eukaryotic Cell 11, 1441–1450, https://doi.org/10.1128/Ec.00190-12 (2012).
Chu, C. H. et al. A Highly Organized Structure Mediating Nuclear Localization of a Myb2 Transcription Factor in the Protozoan Parasite Trichomonas vaginalis. Eukaryotic Cell 10, 1607–1617, https://doi.org/10.1128/Ec.05177-11 (2011).
Hsu, H. M. et al. Regulation of nuclear translocation of the Myb1 transcription factor by TvCyclophilin 1 in the protozoan parasite Trichomonas vaginalis. J Biol Chem 289, 19120–19136, https://doi.org/10.1074/jbc.M114.549410 (2014).
Limacher, A. et al. The crystal structure of Aspergillus fumigatus cyclophilin reveals 3D domain swapping of a central element. Structure 14, 185–195, https://doi.org/10.1016/j.str.2005.10.015 (2006).
Thai, V. et al. Structural, biochemical, and in vivo characterization of the first virally encoded cyclophilin from the Mimivirus. J Mol Biol 378, 71–86, https://doi.org/10.1016/j.jmb.2007.08.051 (2008).
Dornan, J. et al. Biochemical and structural characterization of a divergent loop cyclophilin from Caenorhabditis elegans. J Biol Chem 274, 34877–34883 (1999).
Ke, H. M. S. and Differences between Human Cyclophilin-a and Other Beta-Barrel Structures - Structural Refinement at 1.63 Angstrom Resolution. J Mol Biol 228, 539–550, https://doi.org/10.1016/0022-2836(92)90841-7 (1992).
Lou, Y. C. et al. NMR structural analysis of DNA recognition by a novel Myb1 DNA-binding domain in the protozoan parasite Trichomonas vaginalis. Nucleic Acids Research 37, 2381–2394, https://doi.org/10.1093/nar/gkp097 (2009).
Takahashi, H., Nakanishi, T., Kami, K., Arata, Y. & Shimada, I. A novel NMR method for determining the interfaces of large protein-protein complexes. Nat Struct Biol 7, 220–223 (2000).
McGowan, L. C. & Hamelberg, D. Conformational plasticity of an enzyme during catalysis: intricate coupling between cyclophilin A dynamics and substrate turnover. Biophys J 104, 216–226, https://doi.org/10.1016/j.bpj.2012.11.3815 (2013).
Carver, J. P. & Richards, R. E. General 2-Site Solution for Chemical Exchange Produced Dependence of T2 Upon Carr-Purcell Pulse Separation. J Magn Reson 6, 89, https://doi.org/10.1016/0022-2364(72)90090-X (1972).
Tollinger, M., Skrynnikov, N. R., Mulder, F. A. A., Forman-Kay, J. D. & Kay, L. E. Slow dynamics in folded and unfolded states of an SH3 domain. J Am Chem Soc 123, 11341–11352, https://doi.org/10.1021/ja011300z (2001).
Morin, S. et al. relax: the analysis of biomolecular kinetics and thermodynamics using NMR relaxation dispersion data. Bioinformatics 30, 2219–2220, https://doi.org/10.1093/bioinformatics/btu166 (2014).
Budiman, C. et al. Crystal structure of N-domain of FKBP22 from Shewanella sp SIB1: Dimer dissociation by disruption of Val-Leu knot. Protein Science 20, 1755–1764, https://doi.org/10.1002/pro.714 (2011).
Riboldi-Tunnicliffe, A. et al. Crystal structure of Mip, a prolylisomerase from Legionella pneumophila. Nat Struct Biol 8, 779–783, https://doi.org/10.1038/nsb0901-779 (2001).
Jakob, R. P., Schmidpeter, P. A. M., Koch, J. R., Schmid, F. X. & Maier, T. Structural and Functional Characterization of a Novel Family of Cyclophilins, the AquaCyps. Plos One 11, https://doi.org/10.1371/journal.pone.0157070 (2016).
Gamble, T. R. et al. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87, 1285–1294, https://doi.org/10.1016/S0092-8674(00)81823-1 (1996).
Saleh, T. et al. Cyclophilin A promotes cell migration via the Abl-Crk signaling pathway. Nat Chem Biol 12, 117–123, https://doi.org/10.1038/nchembio.1981 (2016).
Howard, B. R., Vajdos, F. F., Li, S., Sundquist, W. I. & Hill, C. P. Structural insights into the catalytic mechanism of cyclophilin A (vol 10, pg 475, 2003). Nat Struct Biol 10 (2003).
Cardenas, M. E., Zhu, D. & Heitman, J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens 4, 472–477 (1995).
Wyatt, P. J. Light-Scattering and the Absolute Characterization of Macromolecules. Analytica Chimica Acta 272, 1–40, https://doi.org/10.1016/0003-2670(93)80373-S (1993).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A 276, 307–326, https://doi.org/10.1016/S0076-6879(97)76066-X (1997).
Mccoy, A. J. et al. Phaser crystallographic software. Journal of Applied Crystallography 40, 658–674, https://doi.org/10.1107/S0021889807021206 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D-Biological Crystallography 66, 213–221, https://doi.org/10.1107/S0907444909052925 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallographica Section D-Biological Crystallography 66, 486–501, https://doi.org/10.1107/S0907444910007493 (2010).
Laskowski, R. A., Macarthur, M. W., Moss, D. S. & Thornton, J. M. Procheck - a Program to Check the Stereochemical Quality of Protein Structures. Journal of Applied Crystallography 26, 283–291, https://doi.org/10.1107/S0021889892009944 (1993).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica Section D-Biological Crystallography 66, 12–21, https://doi.org/10.1107/S0907444909042073 (2010).
Kay, L. E. Pulsed field gradient multi-dimensional NMR methods for the study of protein structure and dynamics in solution. Prog Biophys Mol Bio 63, 277–299, https://doi.org/10.1016/0079-6107(95)00007-0 (1995).
Akaike, H. In Selected Papers of Hirotugu Akaike (eds Emanuel Parzen, Kunio Tanabe, & Genshiro Kitagawa) 199–213 (Springer New York, 1998).
Johnson, B. A. & Blevins, R. A. Nmr View - a Computer-Program for the Visualization and Analysis of Nmr Data. J Biomol Nmr 4, 603–614, https://doi.org/10.1007/Bf00404272 (1994).
Delaglio, F. et al. Nmrpipe - a Multidimensional Spectral Processing System Based on Unix Pipes. J Biomol Nmr 6, 277–293, https://doi.org/10.1007/Bf00197809 (1995).
Karplus, P. A. & Diederichs, K. Linking Crystallographic Model and Data Quality. Science 336, 1030–1033, https://doi.org/10.1126/science.1218231 (2012).
Acknowledgements
We thank the experimental facility and the technical services provided by the Synchrotron Radiation Protein Crystallography Facility of the National Core Facility Program for Biotechnology, Ministry of Science and Technology and the National Synchrotron Radiation Research Center, a national user facility supported by the Ministry of Science and Technology, Taiwan, ROC. We also acknowledge the acquisition of NMR spectra at the High-field NMR Centre in the Institute of Biomedical Sciences, Academia Sinica, Taiwan. We thank Dr. Meng-Ru Ho of the Biophysical Instrumentation Laboratory at the Institute of Biological Chemistry, Academia Sinica, for assisting in FP experiments and SEC-MALS, Ms. Szuhuan Wang of the Biophysics Core Facility, Department of Academic Affairs and Instrument Service at Academia Sinica for performing sedimentation experiments and Ms. Yu-Ling Hwang of the peptide synthesis facility at the Institute of Biological Chemistry, Academia Sinica, for synthesizing FITC-labeled peptides. We also thank Yu-You Cheng for purifying some of the proteins and Laura Smales (BioMedEditing) for copyediting the manuscript. This work was supported by Academia Sinica (104-0210-01-09-02) and the Ministry of Science and Technology, Taiwan, ROC (MOST 103-2311-B-001-026-MY3, 105-2320-B-001-019-MY3 and MOST 107-0210-01-19-01 to C. C., 105-2113-M-002-009 to C.-H.H.).
Author information
Authors and Affiliations
Contributions
T.M. and C.C. designed all the experiments; S.Y.W. cloned the protein constructs; T.M. expressed and purified the proteins; T.M. and S.S. performed mutagenesis experiments; C.C. Chou, C.C. Cho and M.H.L. crystallized the protein and protein-peptide complex, collected diffraction data; C.H.H. determined the crystal structures; S.Y.W. and T.M. assigned the spectra; T.M. performed NMR experiments and fluorescence polarization experiments and analyzed the data, Y.C.L. performed CPMG relaxation dispersion NMR experiments and analyzed the data; T.M. analyzed AUC and SEC-MALS data; C.C. supervised the research; T.M., Y.C.L., C.H.H., J.H.T. and C.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin, T., Lou, YC., Chou, CC. et al. Structural basis of interaction between dimeric cyclophilin 1 and Myb1 transcription factor in Trichomonas vaginalis. Sci Rep 8, 5410 (2018). https://doi.org/10.1038/s41598-018-23821-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23821-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.