Abstract
Galectin-1 (Gal-1) is a pleiotropic lectin involved in the modulation of immune responses. Using a model of rat experimental autoimmune orchitis (EAO), we investigated the role of Gal-1 in testicular inflammation. EAO is characterized by leukocytic infiltrates in the interstitium, damage of spermatogenesis and production of inflammatory mediators like TNFα and MCP1 causing infertility. In normal rat testis Gal-1 was mainly expressed in Sertoli cells and germ cells. In the inflamed testis, Gal-1 expression was significantly downregulated most likely due to germ cell loss. Analyses of lectin binding and expression of glucosaminyl- and sialyltransferases indicated that the glycan composition on the cell surface of Sertoli and peritubular cells becomes less favourable for Gal-1 binding under inflammatory conditions. In primary Sertoli cells Gal-1 expression was found to be upregulated after TNFα challenge. Pretreatment with Gal-1 synergistically and specifically enhanced TNFα-induced expression of MCP1, IL-1α, IL-6 and TNFα in Sertoli cells. Combined stimulation of Sertoli cells with Gal-1 and TNFα enhanced the phosphorylation of MAP kinases as compared to TNFα or Gal-1 alone. Taken together, our data show that Gal-1 modulates inflammatory responses in Sertoli cells by enhancing the pro-inflammatory activity of TNFα via stimulation of MAPK signalling.
Similar content being viewed by others
Introduction
Infertility and subfertility affect 10–15% of couples and approximately 50% of cases are caused either by factors associated with the male alone or in combination with the female1. Infection and inflammation of the male genital tract are considered as one of the most important identifiable etiologies for male infertility2,3. Orchitis is characterized by the presence of inflammatory infiltrates in the testicular interstitium and associated disruption of seminiferous tubules, that can lead to partial or total impairment of spermatogenesis4,5. Acute epididymitis, orchitis or combined epidididymo-orchitis caused by infection show apparent clinical symptoms that can often be successfully treated with antibiotics and antiphlogistics2. Post- or non-infectious chronic orchitis is more hazardous because it is not associated with discomfort or pain, is difficult to diagnose and compromises testicular function6,7,8,9.
Experimental autoimmune orchitis (EAO) is a rodent model for studying organ-specific autoimmunity and chronic testicular inflammation that reproduces pathological changes also seen in some cases of human immunological infertility10,11,12. The initial phase of EAO involves the production of auto-antibodies against testicular antigens, increased migration and infiltration of leukocytes like macrophages, T lymphocytes and dendritic cells and elevated production of pro-inflammatory cytokines like TNFα and IL-6 or chemokines like MCP-113,14,15. The chronic phase of the disease consists of granuloma formation, progressive apoptosis of germ cells, shrinkage of seminiferous tubules and decreased testicular weight16,17,18.
Galectins are a family of lectins characterized by a common structural fold and at least one conserved carbohydrate recognition domain (CRD) that recognizes β-galactose-containing glycoconjugates19,20. Gal-1 has a single CRD, requires reducing conditions to maintain its activities and is widely expressed in tissues of many vertebrates21. Through binding to specific glycan structures, Gal-1 is involved in a variety of physiologic and pathologic processes including pathogen recognition, selective induction of Th1 and Th17 apoptosis22, inhibition of T cell trafficking23, expansion of tolerogenic dendritic cells and regulatory T cells24,25, maintenance of maternal-fetal tolerance26, induction of pro-angiogenesis in anti-VEGF refractory tumors27 and suppression of an autoimmune pathology28. Gal-1 plays a role as the master regulator of clinically relevant inflammatory-response genes in osteoarthritic chondrocytes by stimulating NFκB-mediated inflammation19. Notably, the formation of galectin-glycan lattices decorating the cellular surface is a result of synchronized activities of glycan-modifying enzymes, glycosyltransferases and glycosidases21. Interestingly, Gal-1 expression in the testis exhibits a stage-specific pattern during the spermatogenic cycle, and immunostaining of Gal-1 in Sertoli cells is found mainly at stages X–II29. Moreover, Gal-1 is also expressed in human Sertoli cells30,31, but whether Gal-1 affects its immunoregulatory functions has not been elucidated yet.
In the present study, we investigated the expression of Gal-1 in rat EAO testis and the ability of Gal-1 to induce an inflammatory response in Sertoli cells. Moreover, the glycan profiles in EAO testes and TNFα challenged Sertoli as well as peritubular cells were investigated by using lectin binding assays.
Results
Due to germ cell loss expression of Gal-1 in EAO testis is decreased
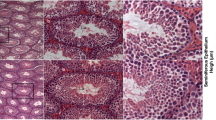
As described earlier11,13 histopathological changes in EAO testis include strong infiltration of the interstitium by leukocytes and loss of the germinal epithelium (Fig. 1c) that is accompanied by a reduced testicular weight11. Testes from untreated and adjuvant controls showed a completely normal morphology (Fig. 1a,b).
In normal rat testes Gal-1 is expressed mainly in Sertoli cells and germ cells but not in macrophages. Hematoxylin-eosin (HE) staining in cryostat sections from normal (a), adjuvant control (b) and EAO (c) rat testes. Localization of Gal-1 (Alexa 546, orange) in normal (d,g,j), adjuvant control (e,h,k) and EAO (f,i,l,m,n) testis. Vimentin (Alexa 488, green) was used as a marker of Sertoli cells (d,e,f). Insets show Gal-1 (Alexa 546, orange) stained in germ cells (thin arrow) and Sertoli cells (thick arrow) (d,f). Staining of Gal-1 and CD68 (Alexa 488, green) or CD163 (Alexa 488, green) in the region of granulomas (m,n). Testicular macrophages were stained with CD68 and CD163 antibodies. Gal-1 was expressed in some CD68 macrophages (m) found around granulomas (thick arrow), but not in CD163 macrophages (n) (thin arrow).
In order to investigate testicular expression and localization of Gal-1 in the EAO model, rat testes from untreated, adjuvant control and EAO mice were processed for immunofluorescence staining, Western blot and qRT-PCR analyses. Immunofluorescence staining revealed that in normal testis Gal-1 was localized in seminiferous tubules, mainly in the cytoplasm of Sertoli cells as co-localization with vimentin showed, as well as in germ cells (Fig. 1d–f). In normal testis Gal-1 was detected in the basal and apical cytoplasm of Sertoli cells (Fig. 1d), but not in CD68/CD163 macrophages (Fig. 1g–l). Interestingly, in inflamed testis Gal-1 was also detected in a few CD68+ macrophages located in the vicinity of granulomas (Fig. 1m). Of note, the expression of Gal-1 was not observed in CD163+ macrophages both in normal and EAO testes (Fig. 1j–l,n). Gal-1 protein levels in EAO testes were downregulated as compared to normal and adjuvant control testes (Fig. 2a,b, Supplementary Fig. 1). Similarly, relative expression of Gal-1 mRNA was also reduced in inflamed testes (Fig. 2c). Because the ratio of testicular cell types is changed in EAO testis due to the loss of germ cells and infiltration of immune cells, the relative expression of Gal-1 mRNA was normalized to the Sertoli cell specific transcript Sox9 (Fig. 2d). These data indicate that the mRNA expression of endogenous Gal-1 in Sertoli cells was not changed in EAO testis as compared to control testis at the investigated time point.
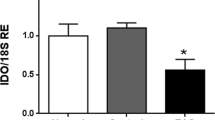
Changes in the expression of Gal-1 in EAO testes are due to germ cell loss. Western blot (a) and densitometric (b) analysis of Gal-1 expression in testes from untreated, adjuvant control and EAO animals. Gal-1 mRNA relative expression (RE) was normalized to three house keeping genes (β-actin, Hprt and 18 s rRNA) (c) or Sertoli cell marker Sox9 (d). The blots were cropped and the full-length blots are presented in the supplementary data (Supplementary Fig. 1); (n = 5, *P < 0.05, **P < 0.01, ***P < 0.001).
Increase of St6gal1 mRNA expression and terminal α-2-6-sialylation in EAO testis
Gal-1 binds to N-acetyllactosamine (LacNAc) present on branches of N- and O-glycans on the cell surface which are synthesized by the synchronized activity of glycosyltransferases21. There are three important post-translational mechanisms to form Gal-1 binding sites including: (a) activity of core 2 glucosaminyl (N-acetyl) transferase 1 (Gcnt1) for synthesis of core 2 O-glycans, which are the backbone of Gal-1 ligands, (b) suppression of ST6 beta-galactoside α-2-6-sialyltransferase 1 (St6gal1) activity, that abrogates Gal-1 binding to some terminal N-acetylglucosamines by adding α-2-6-sialic acid and (c) branching of N-glycans by mannosyl (α-1,3-)-glycoprotein ß-1,2-N-acetylglucosaminyltransferases (Mgat) like Mgat5 (Fig. 3a)21. Our results show that the level of St6gal1 mRNA in EAO testes was upregulated (Fig. 3b). At the same time, binding of SNA, that recognizes terminal α-2-6 sialic acid residues (red triangles in Fig. 3a), was increased as compared to untreated and adjuvant control testis (Fig. 3e). In contrast, Mgat5 mRNA expression was downregulated and, consequently, binding of Phaseolus vulgaris agglutinin (L-PHA), that recognizes ß-5-ß-4-N-acetyl-glucosamine (blue squares in Fig. 3a), was reduced in EAO testis (Fig. 3c and Supplementary Fig. 2). These data indicate that α-2-6-sialylation of O- and N-glycans is increased in inflamed testis. Notably, expression of Gcnt1 mRNA was unchanged (Fig. 3d), whereas binding of peanut agglutinin (PNA), that recognizes asialo-galactose ß-1-3-N-acetylgalactosamine (core-1) in O-glycans, was decreased in EAO testis (Supplementary Fig. 2). However, we did not observe any significant change in the binding of Maackia amurensis agglutinin (MAA), that is recognizing NeuNAcα (2-3) Galβ (1-4) GlcNAc/Glc, in EAO testis (Supplementary Fig. 2).
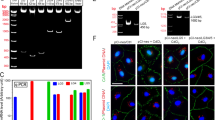
Expression analysis of transferases involved in glycan biosynthesis and lectin-FITC binding in EAO testes. (a) Schematic representation of N- and O-glycan biosynthesis. St6gal1 (b), Mgat5 (c) and Gcnt1 (d) relative mRNA expression was normalized to β-actin, Hprt and 18s rRNA (n = 5). (e) SNA-FITC binds stronger to testicular sections from EAO rats; (**P < 0.01, ***P < 0.001).
The binding of SNA to Sertoli and peritubular cells is increased after TNFα stimulation, whereas binding of L-PHA is decreased
Since inflammatory conditions influence the glycophenotype of cells, we investigated the binding of different lectins that selectively recognize specific oligosaccharide structures to TNFα stimulated primary Sertoli cells and peritubular cells by flow cytometry (Fig. 4a). The binding of SNA to Sertoli (Fig. 4b) and peritubular cells (Fig. 4c) challenged by TNFα was significantly increased compared to untreated cells. In contrast, the binding of L-PHA to TNFα stimulated Sertoli cells (Fig. 4b) and peritubular cells (Fig. 4c) was significantly reduced as compared to control cells. Increased binding of MAA was only found in TNFα stimulated Sertoli cells, whereas the binding of PNA to stimulated Sertoli and peritubular cells was unchanged (Fig. 4).
Influence of TNFα stimulation on the glycan profile of Sertoli cells and peritubular cells. (a) Flow cytometric analysis of cell-surface glycans in Sertoli cells and peritubular cells after stimulation with 25 ng/ml TNFα was detected by staining cells with FITC-labeled lectins (SNA, MAA, L-PHA, or PNA) (black filled histograms) or without (open histograms). Numbers in the upper-right corner represent the median of fluorescence intensity (black filled histograms). The binding of FITC-labelled lectins to Sertoli cells (b) or peritubular cells (c) was quantified as relative median fluorescence intensity (rMFI); (rMFI = (MFI with lectin – MFI without lectin)/MFI without lectin) (n = 3–5, *P < 0.05, **P < 0.01, ***P < 0.001).
Gal-1 is upregulated in Sertoli cells after TNFα stimulation
The inflammatory cytokine TNFα is highly upregulated in EAO testis and is involved in testicular damage11,32. To examine the influence of an inflammatory environment on Gal-1 expression, TNFα was used to stimulate primary Sertoli cells. After stimulation a dose-dependent increase in Gal-1 expression was observed in Sertoli cells as compared to untreated cells (Fig. 5 and Supplementary Fig. 3).
Analysis of Gal-1 expression in primary Sertoli cells. Western blot (a) and densitometric (b) analysis of Gal-1 expression in primary Sertoli cells after TNFα stimulation. The blots were cropped and entire/uncut blots are presented in the supplementary data (Supplementary Fig. 3) (n = 5, *P < 0.05).
Gal-1 and TNFα synergistically induce an inflammatory response in Sertoli cells
To determine whether binding of Gal-1 to Sertoli cells can modulate the inflammatory response, we analyzed expression of inflammatory cytokines in TNFα-stimulated (25 ng/ml) Sertoli cells and stimulated cells that were pretreated with Gal-1 (5 µg/ml). In Sertoli cells treated with TNFα only, mRNA expression of IL-1α (Fig. 6a), MCP1 (Fig. 6c) and IL-6 (Fig. 6e) was increased. In contrast, mRNA expression of TGFβ2 was not affected after TNFα stimulation as compared to untreated cells although it was increased when compared to stimulation with Gal-1 alone (Fig. 6d). Pretreatment of Sertoli cells with recombinant Gal-1 prior to the addition of TNFα synergistically induced expression of IL-1α, TNFα, MCP1, and IL-6 mRNA (Fig. 6a–c,e). These effects were abrogated by the addition of lactose to the Gal-1 solution 5 min prior to stimulation of Sertoli cells. Of note, Sertoli cells did not respond with an inflammatory response after treatment with Gal-1 alone (Fig. 6).
Gal-1 and TNFα act synergistically on the expression of pro-inflammatory mediators in Sertoli cells. Primary Sertoli cells were pretreated with Gal-1 (5 µg/ml) for 2 h, and then stimulated with TNFα (25 ng/ml) for 6 h. Lactose (Lac, 50 mM) was used as an inhibitor of Gal-1 binding. Relative mRNA expression of IL-1α (a), TNFα (b), MCP1 (c), TGFβ2 (d), and IL-6 (e) was normalized to Hprt; (n = 3–5, *P < 0.05, **P < 0.01, ***P < 0.001).
Gal-1 and TNFα synergistically activate phosphorylation of MAPK p38 and JNK
To better understand the mechanisms underlying the synergistic effects of Gal-1 and TNFα on the expression of pro-inflammatory cytokines IL-1α and MCP1, we evaluated the activation kinetics of mitogen-activated protein kinases (MAPK) in Sertoli cells following TNFα and Gal-1 treatment. Sertoli cells stimulated with TNFα showed increased phosphorylation of p38 and JNK from 15–30 min after stimulation (Fig. 7a, lanes 2 and 3). Interestingly, pretreatment of Sertoli cells with Gal-1 prior to TNFα stimulation synergistically enhanced phosphorylation of p38 and JNK 15–30 min after stimulation (Fig. 7a, compare lanes 2 and 3 with lanes 7 and 8; Supplementary Fig. 4; Fig. 7c,d). In contrast, no activation of MAPK was detected when Sertoli cells were treated with Gal-1 alone (Fig. 8a, compare lanes 1 and 2, and 6 and 7, Fig. 8b–g and Supplementary Figs 5 and 6). Gal-1 induced phosphorylation of p38 and JNK in the presence of TNFα was specific, because the effect could be abrogated by adding 50 mM lactose (Fig. 8a, lanes 5 and 10, Fig. 8d,e,g; Supplementary Fig. 6).
Effects of Gal-1 and TNFα on MAPK phosphorylation in Sertoli cells. (a) Isolated Sertoli cells were pretreated with Gal-1 (5 μg/ml; 2 h) and then stimulated with TNFα (25 ng/ml) for 0–120 min. Subsequently, phosphorylation of MAP kinases ERK1/2, p38 and JNK was investigated by Western blotting. Blots are representative of at least three independent experiments. The blots were cropped and the full-length blots are presented in Supplementary Fig. 4. Densitometric analyses of Fig. 7a are shown in Fig. 7b,c; (n = 3, *P < 0.05).
Lactose abrogates effects of Gal-1 on MAPK phosphorylation in TNFα treated Sertoli cells. (a) Gal-1 was pre-incubated with lactose (50 mM) for 5 min prior to addition to Sertoli cells. After 2 h Sertoli cells were stimulated with TNFα (25 ng/ml) for the indicated times. Subsequently phosphorylation of MAP kinases ERK1/2, p38 and JNK was investigated by Western blotting. Blots are representative of at least three independent experiments. The blots were cropped and the entire/uncut blots are presented in the Supplementary Fig. 6. Densitometric analyses of Fig. 8a are shown in Fig. 8b–g. Stimulation with TNFα was for 15 min (b,d,f) or 30 min (c,e,g); (n = 3–4, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Treatment of Sertoli cells with p38 and JNK inhibitors abrogates Gal-1 and TNFα induced expression of IL-1 α, TNFα, IL-6 and MCP1 mRNA
In order to determine whether the effect of Gal-1 and TNFα on the inflammatory cytokine response is induced specifically through MAPK signaling, we used a p38 inhibitor (SB 203580, 5 mM) and a JNK inhibitor (SP600125, 20 mM) during stimulation with Gal-1 and TNFα. In the presence of both inhibitors Gal-1 and TNFα stimulated mRNA expression of IL-1α (Fig. 9a), TNFα (Fig. 9b), MCP1 (Fig. 9c) and IL-6 (Fig. 9e) was completely abrogated.
Inhibitors of p38 and JNK MAP kinases reverse the synergistic effect of Gal-1 and TNFα on expression of inflammatory mediators in Sertoli cells. Primary Sertoli cells were pretreated with Gal-1 (5 µg/ml) for 1 h prior to addition of p38 inhibitor SB 203580 (5 mM; p38i) and JNK inhibitor SP600125 (20 mM; JNKi) for 1 h. Afterwards Sertoli cells were stimulated with TNFα (25 ng/ml) for 6 h. Relative mRNA expression of IL–1α (a), TNFα (b), MCP1 (c), TGFβ2 (d) and IL-6 (e) was normalized to Hprt; (n = 4–7, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Treatment of Sertoli cells with either p38 inhibitor alone (Supplementary Fig. 7) or JNK inhibitor alone (Supplementary Fig. 8) considerably reversed synergistic expression of IL-1α, TNFα, MCP1 and IL-6 mRNA after induction with Gal-1 and TNFα.
Discussion
An increasing body of evidence indicates that Gal-1 has immunoregulatory functions in autoimmune disease models including systemic lupus erythematosus33, lysolecithin-induced demyelination34 and experimental autoimmune encephalomyelitis (EAE)35 through mechanisms like selective induction of Th1 and Th17 cell apoptosis, inhibition of T cell trafficking, expansion of tolerogenic dendritic cells and regulatory T cells. Taking into account that Gal-1 is also abundantly expressed in immune privileged organs such as the placenta, ovary and testis28, we propose that it could play a role in the maintenance of immune privilege and conversely inflammation. We therefore investigated the expression of Gal-1 and relevant glycans in the inflamed rat testis and asked whether Gal-1 can modulate the pro-inflammatory response of Sertoli cells challenged with TNFα.
In line with previous studies29,30,31,36, we also found that Gal-1 is mainly expressed in Sertoli cells and germ cells of the normal rat testis and shows a stage-specific expression pattern. In the EAO testis, however, some CD68+ macrophages in the vicinity of granulomas were positive for Gal-1 too. Since 60–70% of macrophages in granuloma lesions are CD68 positive and local macrophage proliferation plays a key role in granuloma formation37, it is possible that Gal-1 induces macrophage proliferation and promotes granuloma formation. This is supported by Kanda et al. who showed that Gal-1 can induce proliferation of vascular endothelial cells38. Several studies have also shown that Gal-1 could be involved in the resolution of inflammation by regulating inflammatory signaling as well as accumulation and phagocytosis in macrophages and microglial cells39,40,41. Similar to the inflamed testis, Gal-1 expression was limited to macrophages in the vicinity of spinal cord lesions39. Moreover, Gal-1 was preferentially expressed by peritoneal CD11bhigh macrophages as compared to CD11blow macrophages. The CD11bhigh macrophages had a distinct phenotype characterized by a decreased expression of TNFα and IL-1β, and increased expression of TGFβ. Expression of Gal-1 declined once the cells were converted to the CD11blow phenotype as shown in a mouse peritonitis model40. It can be speculated that Gal-1 facilitates the resolution of macrophage-mediated inflammation during peritonitis.
Gal-1 expression was downregulated in the inflamed testis, whereas its mRNA expression did not change when qPCR results were normalized to the Sertoli cell specific transcript Sox9. This suggests that downregulation of Gal-1 in the EAO testis is the consequence of germ cell loss. These results are in line with data published in a mouse model of EAO42. However, direct in vitro treatment of isolated Sertoli cells with TNFα led to upregulation of Gal-1 expression and addition of exogenous Gal-1 potentiated the TNFα effect on the expression of IL-1α, IL-6, MCP1 and also TNFα itself. Gal-1 could play different roles whether it acts early or late during the course of acute or chronic inflammation. Therefore, higher Gal-1 levels induced by inflammatory mediators in the inflamed testis could play a pro-inflammatory role in contrast to an anti-inflammatory function sustaining immune privilege in the healthy testis. In the latter state Gal-1 produced by Sertoli cells in the absence of an inflammatory stimulus like TNFα is able to promote differentiation of tolerogenic dendritic cells and regulatory T cells, further supporting a role of endogenous Gal-1 in the maintenance of testicular immune privilege43. Of note, the number of regulatory T cells is increased during the course of EAO44,45 although these cells are not able to prevent tissue damage.
As shown by Wang et al. the glycocalyx signature of cells is changed in autoimmune diseases like multiple sclerosis and its animal model EAE46. Deficiency of the N-glycan branching enzyme Mgat5 in mice promotes T cell activity, endocytosis of CTLA-4 and autoimmunity, including a spontaneous multiple sclerosis (MS)-like disease47. Our data show that in EAO testis terminal sialic acid and expression of the sialyltransferase St6gal1 were upregulated, while Mgat5 mRNA expression and L-PHA binding were decreased. Moreover, SNA binding to primary Sertoli cells and peritubular cells was increased after TNFα stimulation, whereas L-PHA was downregulated. Collectively, these findings suggest that under inflammatory conditions the glycan composition on the Sertoli and peritubular cell surface becomes less favorable for Gal-1 binding, a means to dampen excessive immune reactions elicited otherwise by concerted TNFα and Gal-1 action in sterile testicular inflammation. Similar to our findings, Benjamin et al. reported that α-2-6 sialic acid was significantly increased on the surface of adipocytes after induction of insulin resistance with TNFα48. Likewise, primary human umbilical vein endothelial cells showed considerable expression of L-PHA-reactive Mgat5-modified N-glycans, that decreased significantly following exposure to pro-inflammatory cytokines like IFNγ and IL-17, whereas α-2-6 sialic acid expression was increased following IFNγ/IL-17 stimulation27.
We could also show that Gal-1 and TNFα synergistically increased the expression of inflammatory mediators in Sertoli cells, whereas Gal-1 alone had no effect. This synergistic effect is specific because it was abrogated in the presence of lactose and is mediated through phosphorylation of MAPKs p38 and JNK. A previous study showed that binding of Gal-1 to N-glycan modified CD45 can prolong retention of CD45 on the surface of microglial cells and augment its phosphatase activity41. Thus, lectin-glycan interactions can control cell responses by adjusting thresholds of cellular activation.
Recent studies show that mice lacking Gal-1 developed a reduced incidence and severity of symptoms in experimental models of epileptic seizures49 and orchitis42, which indicates a proinflammatory function of Gal-1. In EAO mice deficient for Gal-1 decreased numbers of apoptotic germ cells were found compared to normal EAO mice42. Considering that Gal-1 was found to be strongly expressed on apical stalks of Sertoli cells during spermiation, it could be involved in the elimination of defective germ cells. This hypothesis is supported by studies showing that Gal-1 induces T cell and Leydig tumor cell apoptosis by binding to Fas50. Moreover, upregulation of Fas expression was found in aberrant meiotic and postmeiotic germ cells51. Because the number of Fas positive apoptotic germ cells was upregulated in EAO testes52, Gal-1 and Fas could also mediate germ cell apoptosis in orchitis.
Considering our findings that TNFα stimulates Gal-1 expression in Sertoli cells and the addition of exogenous Gal-1 enhances pro-inflammatory effects of TNFα, we propose that under normal conditions Gal-1 induces apoptosis of defective germ cells, whereas under inflammatory conditions Gal-1 adopts a pro-inflammatory and pro-apoptotic function involved in the induction of immune responses and germ cell sloughing.
In conclusion, our data show that Gal-1 and changes in the cellular glycocalyx are involved in the regulation of immune responses in the testis. Under inflammatory conditions Gal-1 specifically enhances TNFα-stimulated production of pro-inflammatory mediators in Sertoli cells. Thus, targeting the Gal-1-glycan axis may offer a new therapeutic strategy for the treatment of autoimmune based testicular inflammation.
Materials and Methods
Induction of EAO
Adult male Wistar rats (Charles River Laboratories, Sulzfeld, Germany) aged 60–70 days were fed with standard food pellets and water ad libitum. EAO was induced by immunization with testicular homogenate in complete Freund’s adjuvant as previously described44. Briefly, animals were injected with testicular homogenate in complete Freund’s adjuvant three times every 14 days (Fig. 10). Control animals received 0.9% NaCl instead of testicular homogenate in complete Freund’s adjuvant. Normal untreated rats were also included. Animals were sacrificed 50 days after the first immunization; testes were collected and frozen in liquid nitrogen. All animal experiments were approved by the local animal ethics committee (Regierungspraesidium Giessen GI 20/23 – Nr. 33/2008). All experiments involving animals were carried out in strict accordance with the recommendations in the guide for the Care and Use of Laboratory Animals of the German law of animal welfare. All methods were carried out in accordance with the approved guidelines.
Immunofluorescence staining
Testicular cryosections (10 µm) were fixed in methanol (Sigma-Aldrich, Steinheim, Germany) at −20 °C for 10 minutes and permeabilized in 0.1% Triton X-100. After blocking with 5% goat serum in 2.5% BSA (Carl Roth, Karlsruhe, Germany), sections were incubated overnight with appropriate primary antibody at 4C (Table 1). Sections were washed in PBS and incubated with secondary antibody at room temperature (RT) for 1 hour. Finally, sections were mounted with ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, Carlsbad, USA). Images were taken with an Axioplan 2 microscope (Carl Zeiss, Jena, Germany).
Lectin binding assay
Testicular cryosections were fixed in 2% paraformaldehyde for 30 min. After blocking with 1% BSA in PBS, sections were incubated with a specific lectin (Table 2) conjugated with FITC (EY Laboratories, San Mateo, USA) in 1% BSA-PBS for 30 min. Finally, sections were mounted with ProLong Gold Antifade Mountant with DAPI. Images were taken with an Axioplan 2 microscope (Carl Zeiss, Jena, Germany).
Expression and purification of recombinant Gal-1
A pET-21a (+) vector for bacterial expression of human wild type Gal-1 was generously provided by Dr. Ken-Ichi Kasai and Dr. Jun Hirabayashi (Research Center for Medical Glycoscience, AIST, Tsukuba, Japan). The plasmid was amplified in E. coli DH5α and subsequently transformed into E. coli BL21 (DE3) pLysS for expression of the protein. The resulting protein was purified by affinity chromatography on an asialofetuin Sepharose column53. Purified Gal-1 was dialyzed three times against 100 mM NaHCO3 (pH 6–9) for 2 hours each and subjected to Triton X-114 (Sigma-Aldrich, Steinheim, Germany) extraction for the removal of contaminating endotoxins54. The protein concentration was measured by using the Pierce BCA Protein Assay Kit (Thermo Fisher, Carlsbad, USA) according to the user’s manual.
Isolation and in vitro treatment of testicular cells
Peritubular and Sertoli cells were isolated from 19-day-old Wistar rats (Charles River Laboratories, Sulzfeld, Germany) using enzymatic digestion as described previously55. Briefly, the decapsulated testes were digested in trypsin-DNase I solution for 4–6 min. After washing, the seminiferous tubules were incubated with a collagenase-hyaluronidase-DNase I solution for 10–12 min. The supernatant containing peritubular cells was harvested, and the remaining seminiferous tubules were digested with hyaluronidase-DNase I solution. After filtration through 70 µm nylon mesh, Sertoli cells were seeded onto 6-well plates using serum free RPMI-1640 medium. Following 2 days of culture, contaminating germ cells were removed by hypotonic shock (25 mM Tris buffer, pH 7.5, 1.5 min). Purity of isolated Sertoli and peritubular cells was confirmed to be >95% by using α-smooth muscle actin (for peritubular cells) and vimentin (for Sertoli cells) immunolabeling. For flow cytometric analysis, Sertoli cells and peritubular cells were treated with 25 ng/ml TNFα (PromoCell GmbH, Heidelberg, Germany) for 48 hours.
Flow cytometry
A total of 2 × 105 Sertoli or peritubular cells were used for the lectin binding assay. After washing, cells were incubated for 1 h at RT with 50 µl of the corresponding plant lectin solution (Table 2). In order to exclude dead cells propidium iodide was added before the measurement and samples were analyzed with a MACSQuant 10 flow cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany). Data were collected from 20,000 events and analyzed with FlowJo soſtware version 10.0.8 (Ashland, Oregon, USA).
Western blotting
Testes or Sertoli cells were homogenized in ice-cold RIPA buffer (50 mM Tris-HCl pH 8.0, 1% Nonidet P 40, 0.5% deoxycholate, 0.1% SDS, 150 mM NaCl) containing protease inhibitor cocktail 1 (Sigma-Aldrich, Steinheim, Germany) and Halt Phosphatase Inhibitor Cocktail (Thermo Fisher, Carlsbad, USA). Equal amounts of proteins (20 µg) were separated by SDS-polyacrylamide gel electrophoresis in 15% polyacrylamide gels and electroblotted onto nitrocellulose membranes (GE Healthcare, Freiburg, Germany). The efficiency of the transfer was monitored by Ponceau S staining. Membranes were blocked with 5% non-fat dry milk in TBS containing 0.1% Tween 20 for 1 h at RT. Blots were probed overnight with primary antibody (Table 1). Afterwards, the membranes were washed and incubated with the appropriate secondary HRP-conjugated antibody (Table 1). Signals were visualized using ECL (Millipore Corporation, Billerica, USA) and analyzed by densitometry (Fusion FX, Witec Ag, Luzern, Switzerland) to select and determine the background subtracted density of the bands in all blots. Equal loading was confirmed using β-actin and total protein signals were confirmed as controls for phosphoprotein signals.
RNA extraction and quantitative RT–PCR
Total RNA was isolated by using RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Real-time RT–PCR was performed on a CFX96 Touch thermal cycler (Bio-Rad, Munich, Germany) using the iTaq Universal SYBR Green Supermix (Bio-Rad, Munich, Germany). The primers for rat galectin-1 (Lgals1), mannoside acetylglucosaminyltransferase 5 (Mgat5), core 2 glucosaminyl (N-acetyl) transferase 1 (Gcnt1), and ST6 beta-galactoside α-2-6-sialyltransferase 1 (St6gal1) were purchased as PrimePCR SYBR Green Assays from Bio-Rad. Primer sequences and amplicon sizes are shown in Table 3. β-actin, hypoxanthine guanine phosphoribosyl transferase (Hprt) and 18 s rRNA (Rn18s) were used as housekeeping genes. Relative gene expression was calculated by using the 2−ΔΔCt method56.
Statistical analysis
Data are shown as mean ± SEM. Comparisons of lectin binding in untreated and TNFα treated cells were performed by a two-tailed t-test. One-way ANOVA followed by Tukey’s multiple comparison post hoc test were applied when more than two groups were compared. P-values < 0.05 were considered as statistically significant. All tests were performed using GraphPad Prism 5 software (GraphPad Software, San Diego, USA).
Data availability statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Inhorn, M. C. & Patrizio, P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 21, 411–26 (2015).
Schuppe H. C.; Bergmann M. Inflammatory conditions of the testis. In: Atlas of the human testis. (Springer, 2013).
Weidner, W. et al. Male urogenital infections: impact of infection and inflammation on ejaculate parameters. World J. Urol. 31, 717–723 (2013).
Schuppe, H.-C. & Meinhardt, A. Immune privilege and inflammation of the testis. Chem. Immunol. Allergy 88, 1–14 (2005).
Haidl, G., Allam, J. P. & Schuppe, H.-C. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia 40, 92–6 (2008).
Schuppe, H.-C. et al. Chronic orchitis: a neglected cause of male infertility? Andrologia 40, 84–91 (2008).
Jungwirth, A. et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Eur. Urol. 62, 324–32 (2012).
Fijak, M. & Meinhardt, A. The testis in immune privilege. Immunol. Rev. 213, 66–81 (2006).
Pilatz, A. et al. Acute epididymitis revisited: impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur. Urol. 68, 428–35 (2015).
Tung, K. S. K. & Teusher, C. Mechanisms of autoimmune disease in the testis and ovary. Hum. Reprod. Update 1, 35–50 (1995).
Aslani, F. et al. Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis. Hum. Reprod. 30, 417–431 (2015).
Lustig, L., Rival, C. & Tung, K. S. K. Autoimmune orchitis and autoimmune oophoritis. In: The autoimmune diseases. (Elsevier/Academic Press, 2013).
Fijak, M. et al. Identification of immunodominant autoantigens in rat autoimmune orchitis. J. Pathol. 207, 127–38 (2005).
Rival, C. et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J. Pathol. 215, 108–117 (2008).
Rival, C. et al. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 324, 311–318 (2006).
Guazzone, V. A., Rival, C., Denduchis, B. & Lustig, L. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in experimental autoimmune orchitis. J. Reprod. Immunol. 60, 143–157 (2003).
Guazzone, V. A., Jacobo, P., Theas, M. S. & Lustig, L. Cytokines and chemokines in testicular inflammation: A brief review. Microscopy Research and Technique 72, 620–628 (2009).
Fijak, M., Bhushan, S. & Meinhardt, A. Immunoprivileged sites: the testis. Methods Mol. Biol. 677, 459–70 (2011).
Cerliani, J. P., Blidner, A. G., Toscano, M. A., Croci, D. O. & Rabinovich, G. A. Translating the ‘Sugar Code’ into immune and vascular signaling programs. Trends Biochem. Sci. 42, 255–273 (2017).
Méndez-Huergo, S. P., Blidner, A. G. & Rabinovich, G. A. Galectins: emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immunol. 45, 8–15 (2017).
Cedeno-Laurent, F. & Dimitroff, C. J. Galectin-1 research in T cell immunity: past, present and future. Clin. Immunol. 142, 107–16 (2012).
Toscano, M. A. et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8, 825–34 (2007).
Norling, L. V., Sampaio, A. L. F., Cooper, D. & Perretti, M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 22, 682–90 (2008).
Ilarregui, J. M. et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 10, 981–91 (2009).
Toscano, M. A. et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J. Immunol. 176, 6323–32 (2006).
Blois, S. M. et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 13, 1450–7 (2007).
Croci, D. O. et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156, 744–758 (2014).
Rabinovich, G. A. & Croci, D. O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 36, 322–35 (2012).
Dettin, L., Rubinstein, N., Aoki, A., Rabinovich, G. A. & Maldonado, C. A. Regulated expression and ultrastructural localization of galectin-1, a proapoptotic beta-galactoside-binding lectin, during spermatogenesis in rat testis. Biol. Reprod. 68, 51–59 (2003).
Chui, K. et al. Characterization and functionality of proliferative human sertoli cells. Cell Transplant. 20, 619–635 (2011).
Wollina, U. et al. Sertoli cell expression of galectin-1 and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol. Histopathol. 14, 779–84 (1999).
Theas, M. S. et al. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum. Reprod. 23, 1865–72 (2008).
Hornung, Á., Monostori, É. & Kovács, L. Systemic lupus erythematosus in the light of the regulatory effects of galectin-1 on T-cell function. Lupus 26, 339–347 (2017).
Rinaldi, M. et al. Galectin-1 circumvents lysolecithin-induced demyelination through the modulation of microglial polarization/phagocytosis and oligodendroglial differentiation. Neurobiol. Dis. 96, 127–143 (2016).
Mari, E. R. et al. Galectin-1 is essential for the induction of MOG35-55 -based intravenous tolerance in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 46, 1783–96 (2016).
Timmons, P. M., Rigby, P. W. J. & Poirier, F. The murine seminiferous epithelial cycle is pre-figured in the Sertoli cells of the embryonic testis. Development 129, 635–47 (2002).
Lan, H. Y., Nikolic-Paterson, D. J., Mu, W. & Atkins, R. C. Local macrophage proliferation in multinucleated giant cell and granuloma formation in experimental Goodpasture’s syndrome. Am. J. Pathol. 147, 1214–20 (1995).
Kanda, A., Noda, K., Saito, W. & Ishida, S. Aflibercept traps galectin-1, an angiogenic factor associated with diabetic retinopathy. Sci. Rep. 5, 17946 (2015).
Gaudet, A. D., Sweet, D. R., Polinski, N. K., Guan, Z. & Popovich, P. G. Galectin-1 in injured rat spinal cord: Implications for macrophage phagocytosis and neural repair. Mol. Cell. Neurosci. 64, 84–94 (2015).
Rostoker, R. et al. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 107, 85–94 (2013).
Starossom, S. C. et al. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity 37, 249–263 (2012).
Pérez, C. V. et al. Dual roles of endogenous and exogenous galectin-1 in the control of testicular immunopathology. Sci. Rep. 5, 12259 (2015).
Gao, J. et al. Murine Sertoli cells promote the development of tolerogenic dendritic cells: a pivotal role of galectin-1. Immunology 148, 253–265 (2016).
Fijak, M. et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol. 186, 5162–72 (2011).
Jacobo, P., Guazzone, V. A., Jarazo-Dietrich, S., Theas, M. S. & Lustig, L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J. Reprod. Immunol. 81, 44–54 (2009).
Wang, D. et al. Uncovering cryptic glycan markers in multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). Drug Dev. Res. 75, 172–188 (2014).
Lee, S.-U. et al. N-glycan processing deficiency promotes spontaneous inflammatory demyelination and neurodegeneration. J. Biol. Chem. 282, 33725–34 (2007).
Parker, B. L. et al. Terminal galactosylation and sialylation switching on membrane glycoproteins upon TNF-alpha-induced insulin resistance in adipocytes. Mol. Cell. Proteomics 15, 141–153 (2016).
Bischoff, V., Deogracias, R., Poirier, F. & Barde, Y.-A. Seizure-induced neuronal death is suppressed in the absence of the endogenous lectin Galectin-1. J. Neurosci. 32, 15590–600 (2012).
Biron, V. A. et al. Galectin-1: biphasic growth regulation of Leydig tumor cells. Glycobiology 16, 810–21 (2006).
Francavilla, S. et al. Fas expression correlates with human germ cell degeneration in meiotic and post-meiotic arrest of spermatogenesis. Mol. Hum. Reprod. 8, 213–20 (2002).
Theas, S., Rival, C. & Lustig, L. Germ cell apoptosis in autoimmune orchitis: involvement of the Fas-FasL system. Am. J. Reprod. Immunol. 50, 166–76 (2003).
Hirabayashi, J., Ayaki, H., Soma, G.-I. & Kasai, K.-I. Production and purification of a recombinant human 14 kDa β-galactoside-binding lectin. FEBS Lett. 250, 161–165 (1989).
Salek-Ardakani, S. et al. High level expression and purification of the Epstein-Barr virus encoded cytokine viral interleukin 10: efficient removal of endotoxin. Cytokine 17, 1–13 (2002).
Bhushan, S. et al. Isolation of Sertoli cells and peritubular cells from rat testes. J. Vis. Exp. e53389, https://doi.org/10.3791/53389 (2016).
Fijak, M. et al. Influence of testosterone on inflammatory response in testicular cells and expression of transcription factor Foxp3 in T cells. Am. J. Reprod. Immunol. 74, 12–25 (2015).
Acknowledgements
The authors would like to thank Prof. Gabriel A. Rabinovich for providing galectin-1 antibody. The financial support of the China Scholarship Council to Tao Lei and of the Medical Faculty of Justus-Liebig University is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
T.L. performed the experiments, analysed and discussed the data and drafted the manuscript. S.M., J.K., F.A., E.W., S.B. and S.F. were involved in performance of experiments. J.K. contributed to discussion of data, writing and editing of the article. A.M. contributed to conception and study design, interpretation and discussion of data and editing of the article. M.F. contributed to conception and study design, performance of experiments, interpretation and discussion of data, drafting and editing of the article. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lei, T., Moos, S., Klug, J. et al. Galectin-1 enhances TNFα-induced inflammatory responses in Sertoli cells through activation of MAPK signalling. Sci Rep 8, 3741 (2018). https://doi.org/10.1038/s41598-018-22135-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22135-w
This article is cited by
-
“Outcome of non-surgical periodontal treatment on Gal-1 and Gal-3 GCF levels in periodontitis patients: a case-control study”
Clinical Oral Investigations (2024)
-
Ensemble learning model for identifying the hallmark genes of NFκB/TNF signaling pathway in cancers
Journal of Translational Medicine (2023)
-
Diabetes as a potential compounding factor in COVID-19-mediated male subfertility
Cell & Bioscience (2022)
-
Galectin-1 as the new player in staging and prognosis of COVID-19
Scientific Reports (2022)
-
Regulation of wound healing and fibrosis by galectins
Journal of Molecular Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.