Abstract
This study aimed to assess the prevalence of Attention Deficit Hyperactivity Disorder (ADHD) and its characteristics and risk factors in children with epilepsy at a tertiary medical center in New Delhi. Children with active epilepsy, aged 6 to 12 years, were assessed for ADHD using DSM-IV-TR criteria. Epilepsy and psychiatric characteristics, sociodemographic indicators, and use of antiepileptic drugs were analyzed for differences between the ADHD and non-ADHD groups. Among the 73 children with epilepsy, 23% (n = 17) had comorbid ADHD, of whom 59% (n = 10) had predominantly inattentive type, 35% (n = 6) combined type, and 6% (n = 1) predominantly hyperactive-impulsive type. Lower IQ scores, epileptiform EEG activity, not attending school, and male sex were significantly associated with comorbid ADHD in children with epilepsy. Groups were similar in terms of age, socioeconomic indicators, family history of psychiatric disorders, seizure frequency in the last six months, seizure etiology, and seizure type. Epilepsy is a common pediatric neurological condition with frequent psychiatric comorbidities, including ADHD. Specialists should collaborate to optimize treatment for children with epilepsy and ADHD, especially for families in developing countries where the burden of disease can be great.
Similar content being viewed by others
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a common comorbidity experienced by children with epilepsy, and is associated with problems in social functioning, learning, quality of life, and academic attainment1,2,3,4. Several factors may contribute to this comorbidity5,6, including underlying brain pathology, chronic effects of seizures, epileptiform brain activity as seen on electroencephalogram (EEG), and effects of antiepileptic drugs7 (AEDs). This comorbidity also presents challenges to clinicians in determining a medication regimen and treatment plan8.
The reported prevalence of ADHD in children with epilepsy from high-income countries ranges from 23 to 70%, depending on sample studied and criteria used for diagnosis1,9,10,11, which is much higher than the reported worldwide pooled prevalence of ADHD of 5.3%12. Prevalence estimates of behavioral comorbidities in epileptic children and adolescents in India ranges from 40 to 54%4,13,14. In India, several studies have investigated psychopathology in children with epilepsy4,13,14,15,16,17,18; however, research focused on characterizing the ADHD comorbidity in epilepsy has been limited19.
This study aimed to quantify the prevalence of ADHD and its subtypes among children with epilepsy at an urban tertiary medical center. Measures of epilepsy severity, academic and sociodemographic factors, and use of AEDs were compared to describe the clinical profile of this pediatric group.
Methods
Participants
In this cross-sectional, observational study, children diagnosed with active epilepsy attending the outpatient services of the Child Neurology Division, Department of Pediatrics, All India Institute of Medical Sciences (AIIMS), New Delhi, were screened for eligibility. Active epilepsy was defined as two or more unprovoked seizures occurring 24 hours apart after four weeks of age, with at least one epileptic seizure in the previous five years, regardless of AED treatment, based on International League Against Epilepsy definitions20. The inclusion criteria were (1) children aged 6 to 12 years and (2) duration of epilepsy ≥ 6 months. Patients were excluded if they had an intellectual disability or comorbid chronic systemic disease. In total, 512 patients were screened for eligibility, 172 fulfilled the inclusion criteria, 62 had IQ ≤ 70, and 10 had comorbid systemic illness. Thus 100 patients were enrolled in a general epilepsy and psychopathology cohort (ages 6 to 16) whose findings have been previously reported13, and those aged 6 to 12 (n = 73) were included in this ADHD study.
Patients’ health records were retrospectively reviewed during the clinical course of their epilepsy. Epilepsy-related factors included age at seizure onset, duration of epilepsy, etiology, predominant seizure type, EEG pattern, seizure frequency in last six months, and AED therapy. Socioeconomic status categorization was based on monthly income per Agarwal’s six-stage classification21. Data collection took place between October 2008 and August 2009.
Cognitive assessment
IQ was evaluated with Malin’s Intelligence Scale for Indian Children22 (MISIC), an Indian adaptation of the Wechsler Intelligence Scale for Children. MISIC assesses verbal IQ, performance IQ, and full-scale IQ for children between 6 and 16 years of age. For the present study, verbal IQ was assessed, encompassing six domains: information, general comprehension, arithmetic, analogies and similarities, vocabulary, and digit span. Intellectual disability was defined as a verbal IQ of 70 or lower.
Psychiatric evaluation
Preliminary assessment of behavior problems used parent/caregiver ratings on the Child Behavior Checklist23 (CBCL/4–16). For participants scoring above the CBCL total score threshold for their age and sex24, an experienced psychiatrist and pediatric neurologist used the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision25 (DSM-IV-TR) criteria for evaluation and diagnosis of 15 clinical disorders, including ADHD. Seventeen participants met the diagnostic criteria for ADHD.
EEG assessment
Interictal digital EEG recording (Medelec profile; Oxford Instruments, Oxfordshire, UK) using an international 10–20 system of electrode placement was done at AIIMS for participants who did not have a good-quality EEG recording from the previous three months. Both awake and sleep states were recorded. Hyperventilation, photic stimulation, and sleep were used as activation procedures. EEG records were reported by two pediatric neurologists as either normal or showing epileptiform discharges.
Statistics
IBM SPSS version 24 (Chicago, USA) was used for univariate analyses and descriptive statistics. Independent samples t-tests were used for normally distributed continuous variables. Chi-square (χ2) tests for association were used for categorical variables. The Mann-Whitney U Test was used for epilepsy duration and IQ, which had nonparametric distributions as assessed by Shapiro-Wilk’s test (p < 0.05). Post-hoc pairwise comparisons using Fisher’s exact tests (2 × 2) were used for seizure frequency.
Ethics
This study was approved by the Institutional Ethics Committee at AIIMS, and the study was carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from the parents/caregivers of all participants.
Data availability
Data analyzed during this study are included in the Supplementary Dataset.
Results
ADHD and sociodemographic characteristics
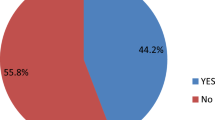
Of the 73 participants, 17 (23.3%) had both epilepsy and ADHD: within this group, 10 (58.8%) had ADHD predominantly inattentive type, 6 (35.3%) had ADHD combined type, and one (5.9%) had ADHD predominantly hyperactive-impulsive type.
Sociodemographic and family background information is presented in Table 1. IQ was significantly lower by almost 1.5 standard deviations in the ADHD group compared to participants without ADHD (mean score 84 vs 92, p < 0.0001). The ADHD group had significantly more males. Almost a quarter of the ADHD group did not attend school compared to 1.8% of non-ADHD participants (χ2(1) = 9.664, p = 0.0019), with epilepsy given in all cases as a reason for not attending. The group difference in school attendance was not driven by age. Seven participants with ADHD (41.2%) met the diagnostic criteria for an additional behavioral disorder, while 14 participants (25%) in the non-ADHD group did (see Supplementary Dataset). Age, socioeconomic indicators, and family history of psychiatric disorders were similar between groups.
Epilepsy characteristics
Comparison of clinical epilepsy characteristics is presented in Table 2. Age at onset of epilepsy ranged from one month to 11 years of age. Epilepsy duration ranged from 6 months to 10 years 5 months. Epileptiform discharges on EEG were positively associated with ADHD diagnosis (χ2(1) = 4.718, p = 0.030), with 52.9% of children with both epilepsy and ADHD showing epileptiform discharges, compared with 25% of those without ADHD. The ADHD and non-ADHD groups were comparable in terms of seizure etiology and seizure type. Post-hoc analysis of seizure frequency in the last six months did not indicate group differences in any of the frequency categories.
The following AEDs were used by study participants: valproate (27 participants), phenytoin (22), carbamazepine (20), clobazam (9), lamotrigine (6), oxcarbazepine (3), clonazepam (1), levetiracetam (1), and zonisamide (1). Three participants reported not currently taking any AEDs. Use of AED polytherapy was positively associated with ADHD diagnosis, but not significantly (χ2(1) = 2.952, p = 0.086).
Discussion
At a tertiary medical care center in New Delhi, a high prevalence (23.3%) of ADHD was identified in children with epilepsy. In agreement with several previous studies9,12, ADHD inattentive type was more frequent in this clinical group than hyperactive-impulsive or combined types. Most epilepsy characteristics did not differ significantly between the groups with or without ADHD, but abnormal EEG was associated with ADHD diagnosis. Children with both epilepsy and ADHD had lower IQ scores and were significantly less likely to be attending school, with epilepsy being the primary reason. Sociodemographic profiles of the two groups were similar.
This prevalence rate is similar to the 23.4% of children with epilepsy and comorbid ADHD reported by Philip et al.19 in a pediatric neurology outpatient population in Karnataka, India (n = 94). A recent register-based study in Sweden26 of 1,899,654 individuals found that 13.5% of those with epilepsy also had a diagnosis of ADHD, while 4.3% of the full sample had ADHD, meaning a 3.5-fold increased risk in the comorbid group. In Asia, recent reports from Thailand27 and China28 found prevalences of 19% and 42%, respectively, of ADHD comorbidity among children with epilepsy. Studies of ADHD prevalence in India are limited, use different methodologies, and report varying figures. Recently reported prevalence figures of ADHD in children in the general population include 1.3% in a study of children in Bengaluru, Karnataka29 (n = 3120), 1.7% in a study of families in central India30 (n = 4278 families), 11.32% in a study of children in Coimbatore, Tamil Nadu31 (n = 770), and 12.66% in a study of children in the state of Assam32 (n = 300).
The population of children with comorbid epilepsy and ADHD differs in several ways from those with primary ADHD. For instance, the gender distribution is less skewed towards boys than in primary ADHD, and inattention symptoms are more frequent and severe than impulsivity/hyperactivity33,34, in line with the results presented here. In an American study, Dunn et al.35 found that of children aged 9 to 14 with epilepsy, 24% had ADHD inattentive type, compared to 14% with hyperactive-impulsive or combined types. In a Nigerian tertiary care facility, Chidi et al.36 found that of the 14% of epileptic children who also had ADHD, the inattentive type was predominant (69%).
Biological mechanisms
Recent neuroimaging research using magnetic resonance imaging (MRI) has sought to shed light on possible shared neural mechanisms underlying epilepsy and ADHD comorbidity1,10,37,38,39. While some evidence implicates shared neurobiological abnormalities40,41, other studies suggest that independent factors are at play10,40. Regions of decreased cortical thickness have been reported in groups with comorbid epilepsy and ADHD, compared to epilepsy alone, which could indicate altered development of functional networks42. Saute et al.38 reported that pediatric epilepsy with comorbid ADHD was associated with reduced cortical thickness in bilateral areas of the frontal, parietal, and temporal regions, along with smaller caudate, thalamus, hippocampus, and brainstem volumes, compared to the epilepsy non-ADHD group; smaller cerebellum and thalamus were related to epilepsy generally, but brain volume and surface area did not show major group differences. Similarly, Dabbs et al.43 reported that ADHD problems assessed with the CBCL were associated with decreased cortical thickness in left superior frontal and lateral occipital regions and right fusiform gyrus. In a functional MRI study of boys with ADHD, Bechtel et al.44 reported similar patterns of brain activity during block-design working memory tasks in the groups with and without comorbid epilepsy, which differed from healthy controls. Neural mechanisms underlying epilepsy and ADHD, as opposed to ADHD or epilepsy alone, remain to be fully elucidated and will benefit from further neuroimaging research.
Genetic studies, especially at the population level, have also begun to identify risk factors related to these and other neurodevelopmental disorders45. Relatives of those with epilepsy have an increased risk of ADHD themselves26, suggesting genetic and environmental linkages within families, and evidence of an association between maternal epilepsy and ADHD has also been reported46. However, Brikell et al.26 found only a modest genetic correlation between the disorders. In addition to inherited genetic factors, de novo mutations and other rare variants, such as those involved in synaptic function, neurotransmission, and methylation remodeling, should be investigated as potential risk factors (see review by Lo-Castro and Curatolo47).
Treatment challenges and AEDs
A clearer understanding of medication effects on the symptomatology and progression of combined epilepsy and ADHD is needed to tailor medical management in this population34. It is possible that epilepsy alters the ADHD symptoms in children who have both, based on possible shared etiology and pathophysiology44,48. For example, children with ADHD are predisposed to epileptic seizures40 and have an increased rate of EEG abnormalities without a history of epilepsy49.
Determining an effective treatment regimen for children with epilepsy and ADHD is challenging, as clinicians must consider a patient’s seizure frequency, cognitive function, drug interactions, and medication adherence. Use of AEDs, in particular polytherapy, has in some studies been associated with cognitive deficits in children including attention problems and hyperactivity50,51,52. Sleep disturbances due to AEDs, along with subclinical seizures and learning disabilities, can contribute to inattention51, which was the ADHD subtype diagnosed in 59% of children with ADHD in this study. Several studies suggest that use of methylphenidate appears to be effective in children with comorbid epilepsy and ADHD49,52,53. A 2015 Brazilian study54, which administered low to moderate doses of methylphenidate, observed reduced seizure frequency and severity along with improved quality of life among adolescents with ADHD and difficult-to-treat epilepsy. However, increased risk of seizures with high dosage of osmotic release oral system methylphenidate55 and lowered seizure threshold associated with tricyclic antidepressants and bupropion use has also been reported1,41,52,56. Most studies of medication for ADHD in children with epilepsy have had small numbers of participants and/or short study duration52, and clinical trials in developing countries have been limited57,58. Research on other medications, such as amphetamines, atomoxetine, and alpha-2 adrenergic agonists, has also been limited in this population. Medication effects on attentive behavior and seizure threshold should therefore be closely monitored. EEG monitoring is a useful tool in this population to identify whether staring episodes or repetitive movements are related to seizure activity or ADHD. Children with epilepsy in this study experienced ADHD at a high rate, and further research in larger populations should therefore clarify the risk-benefit ratios associated with various medications.
Moreover, Gonzalez-Heydrich et al.59 report that children with epilepsy and ADHD often have additional comorbidities, such as anxiety or oppositional defiant disorder, making treatment decisions complex33. AED polytherapy may also create financial stress for some families3. Holistic management of children with epilepsy would include screening and management of psychiatric problems, including ADHD. School failure and drop-out was a problem for a number of children in this study and underscores the need to engage the education community in this region to improve academic outcomes60. Further studies are required to evaluate the efficacy of behavior therapy, drug therapy, and other treatment modalities for individuals with epilepsy and ADHD, as well as their effect on improving quality of life61.
Strengths and limitations
Limited research in India has examined ADHD comorbidity in children with epilepsy using diagnostic criteria. In this study, standard criteria from the DSM-IV-TR were used for diagnosing ADHD. Participants were recruited from the outpatient population of a government hospital which serves children from all socioeconomic backgrounds.
The study population was drawn from the tertiary care center outpatient population, and all participants in this study had a history of epilepsy, making it difficult to generalize to primary care settings and to the typically developing pediatric population. Future studies should therefore also include a healthy matched group without epilepsy. Within-group analysis, for example more detailed analysis of seizure frequency related to comorbidity, would be strengthened by a larger number of participants. While Malin’s Indian adaptation of the WISC has been widely used across India for cognitive testing in children, future studies should consider using newer assessments, such as the WISC-IV India. Finally, the revised DSM-V was released after the data collection in this study. Using the updated diagnostic criteria for ADHD may have classified additional children with ADHD, meaning that the prevalence figures reported here are likely conservative. The DSM-V designation of mild, moderate, or severe ADHD would have provided extra detail to the current study.
Conclusion
Prevalence of comorbid ADHD was 23% in children with epilepsy attending outpatient services at a tertiary medical center in New Delhi, with inattentive type being predominant. In this study, children with comorbid ADHD had lower IQ scores on average, and four of the five children who did not attend school had both ADHD and epilepsy. Clinicians should be sensitive to identify ADHD in children with epilepsy since this may lead to more effective intervention and improved quality of life62. Further research examining potential shared neurological causes as well as strategies to optimize medication and treatment are needed to better serve this pediatric population.
References
Dunn, D. W. & Kronenberger, W. G. Childhood epilepsy, attention problems, and ADHD: review and practical considerations. Semin Pediatr Neurol 12, 222–228, https://doi.org/10.1016/j.spen.2005.12.004 (2005).
Burton, K. et al. Behavioural comorbidity in Tanzanian children with epilepsy: a community-based case-control study. Dev Med Child Neurol 53, 1135–1142, https://doi.org/10.1111/j.1469-8749.2011.04109.x (2011).
Datta, S. S. et al. Impact of pediatric epilepsy on Indian families: influence of psychopathology and seizure related variables. Epilepsy Behav 9, 145–151, https://doi.org/10.1016/j.yebeh.2006.04.011 (2006).
Malhi, P. & Singhi, P. Correlates of quality of life with epilepsy. Indian J Pediatr 72, 131–135 (2005).
Amudhan, S., Gururaj, G. & Satishchandra, P. Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol 18, 263–277, https://doi.org/10.4103/0972-2327.160093 (2015).
Gulati, S., Yoganathan, S. & Chakrabarty, B. Epilepsy, cognition and behavior. Indian J Pediatr 81, 1056–1062, https://doi.org/10.1007/s12098-014-1530-4 (2014).
Parisi, P., Moavero, R., Verrotti, A. & Curatolo, P. Attention deficit hyperactivity disorder in children with epilepsy. Brain Dev 32, 10–16, https://doi.org/10.1016/j.braindev.2009.03.005 (2010).
Besag, F. et al. Psychiatric and Behavioural Disorders in Children with Epilepsy (ILAE Task Force Report): Epilepsy and ADHD. Epileptic Disord. https://doi.org/10.1684/epd.2016.0811 (2016).
Bennett-Back, O., Keren, A. & Zelnik, N. Attention-deficit hyperactivity disorder in children with benign epilepsy and their siblings. Pediatr Neurol 44, 187–192, https://doi.org/10.1016/j.pediatrneurol.2010.10.003 (2011).
Hermann, B. et al. The frequency, complications and aetiology of ADHD in new onset paediatric epilepsy. Brain 130, 3135–3148, https://doi.org/10.1093/brain/awm227 (2007).
Russ, S. A., Larson, K. & Halfon, N. A national profile of childhood epilepsy and seizure disorder. Pediatrics 129, 256–264, https://doi.org/10.1542/peds.2010-1371 (2012).
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J. & Rohde, L. A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164, 942–948, https://doi.org/10.1176/ajp.2007.164.6.942 (2007).
Choudhary, A., Gulati, S., Sagar, R., Kabra, M. & Sapra, S. Behavioral comorbidity in children and adolescents with epilepsy. J Clin Neurosci 21, 1337–1340, https://doi.org/10.1016/j.jocn.2013.11.023 (2014).
Datta, S. S. et al. Behaviour problems in children and adolescents with seizure disorder: associations and risk factors. Seizure 14, 190–197, https://doi.org/10.1016/j.seizure.2005.01.007 (2005).
Dharmadhikari, A. S. & Sinha, V. K. Psychiatric Comorbidity in Children with Epilepsy: A Cross-sectional 5 Years Rural Prevalence Study. J Neurosci Rural Pract 8, 179–184, https://doi.org/10.4103/jnrp.jnrp_487_16 (2017).
Jayashree, K., Talukdar, B., Srivastava, P. K. & Sharma, U. Cognitive function and behavior in epileptic children of school going age. Indian Pediatr 36, 1032–1038 (1999).
Pradhan, P. V., Shah, H., Rao, P., Ashturkar, D. & Ghaisas, P. Psychopathology and self-esteem in chronic illness. Indian J Pediatr 70, 135–138 (2003).
Vinayan, K. P., Biji, V. & Thomas, S. V. Educational problems with underlying neuropsychological impairment are common in children with Benign Epilepsy of Childhood with Centrotemporal Spikes (BECTS). Seizure 14, 207–212, https://doi.org/10.1016/j.seizure.2005.01.009 (2005).
Philip, J. P., Kamate, N. M. & Attention-deficit, M. hyperactivity disorder in children with idiopathic epilepsy: A cross-sectional study. Indian J Health Sci Biomed Res 9, 31–34, https://doi.org/10.4103/2349-5006.183682 (2016).
Sander, J. W. & Shorvon, S. D. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry 61, 433–443 (1996).
Agarwal, A. Social classification: the need to update in the present scenario. Indian J Community Med 33, 50–51, https://doi.org/10.4103/0970-0218.39245 (2008).
Malin, A. J. Malin’s Intelligence Scale for Indian Children manual. (Indian Psychological Corporation, 1969).
Achenbach, T. Manual for the child behavior checklist/4-16 and1983 profile. (Department of Psychiatry, University of Vermont, 1983).
Srinath, S. et al. Epidemiological study of child & adolescent psychiatric disorders in urban & rural areas of Bangalore, India. Indian J Med Res 122, 67–79 (2005).
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR). (American Psychiatric Association, 2000).
Brikell, I. et al. Familial Liability to Epilepsy and Attention-Deficit/Hyperactivity Disorder: A Nationwide Cohort Study. Biol Psychiatry, https://doi.org/10.1016/j.biopsych.2017.08.006 (2017).
Subchartanan, J. P. & Chonchaiya, S. W. Prevalence of attention deficit hyperactivity disorder in children with epilepsy in a Thai Hospital. Asian Biomedicine 9, 803–807, https://doi.org/10.5372/1905-7415.0906.454 (2015).
Han, Y. et al. [Co-morbidity of attention deficit hyperactivity disorder in children with epilepsy]. Zhongguo Dang Dai Er Ke Za Zhi 14, 89–92 (2012).
Ramya, H. S., Lakshmi, G. A. & Pandit, V. Prevalence of attention deficit hyperactivity disorder in school going children aged between 5–12 years in Bengaluru. Current Pediatric Research 21, 321–326 (2017).
Khairkar, P. et al. A 5-year hospital prevalence of child and adolescent psychiatric disorders from central India. Indian J Pediatr 80, 826–831, https://doi.org/10.1007/s12098-013-1120-x (2013).
Venkata, J. A. & Panicker, A. S. Prevalence of Attention Deficit Hyperactivity Disorder in primary school children. Indian J Psychiat 55, 338–342, https://doi.org/10.4103/0019-5545.120544 (2013).
Choudhury, H. A., Ghosh, P. & Victor, R. Prevalence Of Attention Deficit Hyperactivity Disorder Among Primary School Children In Cachar, Assam, North-East. Indian J Psychiat 59, S199–S199 (2017).
Barkley, R. A. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd edn, (Guilford Press, 2006).
Sherman, E. M., Slick, D. J., Connolly, M. B. & Eyrl, K. L. ADHD, neurological correlates and health-related quality of life in severe pediatric epilepsy. Epilepsia 48, 1083–1091, https://doi.org/10.1111/j.1528-1167.2007.01028.x (2007).
Dunn, D. W., Austin, J. K., Harezlak, J. & Ambrosius, W. T. ADHD and epilepsy in childhood. Dev Med Child Neurol 45, 50–54 (2003).
Chidi, I. R., Chidi, N. A., Ebele, A. A. & Chinyelu, O. N. Co-Morbidity of attention deficit Hyperactivity Disorder (ADHD) and epilepsy In children seen In University of Nigeria Teaching Hospital Enugu: Prevalence, Clinical and social correlates. Niger Postgrad Med J 21, 273–278 (2014).
Zelko, F. A., Pardoe, H. R., Blackstone, S. R., Jackson, G. D. & Berg, A. T. Regional brain volumes and cognition in childhood epilepsy: does size really matter? Epilepsy Res 108, 692–700, https://doi.org/10.1016/j.eplepsyres.2014.02.003 (2014).
Saute, R. et al. Brain morphology in children with epilepsy and ADHD. PLoS One 9, e95269, https://doi.org/10.1371/journal.pone.0095269 (2014).
McDonald, B. C., Hummer, T. A. & Dunn, D. W. Functional MRI and structural MRI as tools for understanding comorbid conditions in children with epilepsy. Epilepsy Behav 26, 295–302, https://doi.org/10.1016/j.yebeh.2012.10.013 (2013).
Hesdorffer, D. C. et al. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry 61, 731–736, https://doi.org/10.1001/archpsyc.61.7.731 (2004).
Hamoda, H. M., Guild, D. J., Gumlak, S., Travers, B. H. & Gonzalez-Heydrich, J. Association between attention-deficit/hyperactivity disorder and epilepsy in pediatric populations. Expert Rev Neurother 9, 1747–1754, https://doi.org/10.1586/ern.09.128 (2009).
Kleen, J. K. et al. Early-life seizures produce lasting alterations in the structure and function of the prefrontal cortex. Epilepsy Behav 22, 214–219, https://doi.org/10.1016/j.yebeh.2011.07.022 (2011).
Dabbs, K., Jones, J. E., Jackson, D. C., Seidenberg, M. & Hermann, B. P. Patterns of cortical thickness and the Child Behavior Checklist in childhood epilepsy. Epilepsy Behav 29, 198–204, https://doi.org/10.1016/j.yebeh.2013.07.008 (2013).
Bechtel, N. et al. Attention-deficit/hyperactivity disorder in childhood epilepsy: a neuropsychological and functional imaging study. Epilepsia 53, 325–333, https://doi.org/10.1111/j.1528-1167.2011.03377.x (2012).
Moreno-De-Luca, A. et al. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. Lancet Neurol 12, 406–414, https://doi.org/10.1016/S1474-4422(13)70011-5 (2013).
Halmoy, A., Klungsoyr, K., Skjaerven, R. & Haavik, J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 71, 474–481, https://doi.org/10.1016/j.biopsych.2011.11.013 (2012).
Lo-Castro, A. & Curatolo, P. Epilepsy associated with autism and attention deficit hyperactivity disorder: is there a genetic link? Brain Dev 36, 185–193, https://doi.org/10.1016/j.braindev.2013.04.013 (2014).
Chou, I. C. et al. Correlation between epilepsy and attention deficit hyperactivity disorder: a population-based cohort study. PLoS One 8, e57926, https://doi.org/10.1371/journal.pone.0057926 (2013).
Kaufmann, R., Goldberg-Stern, H. & Shuper, A. Attention-deficit disorders and epilepsy in childhood: incidence, causative relations and treatment possibilities. J Child Neurol 24, 727–733, https://doi.org/10.1177/0883073808330165 (2009).
Kwan, P. & Brodie, M. J. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet 357, 216–222, https://doi.org/10.1016/S0140-6736(00)03600-X (2001).
Schubert, R. Attention deficit disorder and epilepsy. Pediatr Neurol 32, 1–10, https://doi.org/10.1016/j.pediatrneurol.2004.06.007 (2005).
Williams, A. E., Giust, J. M., Kronenberger, W. G. & Dunn, D. W. Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges. Neuropsychiatr Dis Treat 12, 287–296, https://doi.org/10.2147/NDT.S81549 (2016).
Torres, A. R., Whitney, J. & Gonzalez-Heydrich, J. Attention-deficit/hyperactivity disorder in pediatric patients with epilepsy: review of pharmacological treatment. Epilepsy Behav 12, 217–233, https://doi.org/10.1016/j.yebeh.2007.08.001 (2008).
Radziuk, A. L. et al. Methylphenidate improves the quality of life of children and adolescents with ADHD and difficult-to-treat epilepsies. Epilepsy Behav 46, 215–220, https://doi.org/10.1016/j.yebeh.2015.02.019 (2015).
Gonzalez-Heydrich, J. et al. Adaptive phase I study of OROS methylphenidate treatment of attention deficit hyperactivity disorder with epilepsy. Epilepsy Behav 18, 229–237, https://doi.org/10.1016/j.yebeh.2010.02.022 (2010).
Alldredge, B. K. Seizure risk associated with psychotropic drugs: clinical and pharmacokinetic considerations. Neurology 53, S68–75 (1999).
Pal, D. K., Das, T., Chaudhury, G., Johnson, A. L. & Neville, B. G. Randomised controlled trial to assess acceptability of phenobarbital for childhood epilepsy in rural India. Lancet 351, 19–23, https://doi.org/10.1016/S0140-6736(97)06250-8 (1998).
Santos, K. et al. The impact of methylphenidate on seizure frequency and severity in children with attention-deficit-hyperactivity disorder and difficult-to-treat epilepsies. Dev Med Child Neurol 55, 654–660, https://doi.org/10.1111/dmcn.12121 (2013).
Gonzalez-Heydrich, J. et al. Psychiatric disorders and behavioral characteristics of pediatric patients with both epilepsy and attention-deficit hyperactivity disorder. Epilepsy Behav 10, 384–388, https://doi.org/10.1016/j.yebeh.2007.01.010 (2007).
Homi Bhesania, N., Rehman, A., Saleh Savul, I. & Zehra, N. Knowledge, attitude and practices of school teachers towards epileptic school children in Karachi, Pakistan. Pak J Med Sci 30, 220–224, https://doi.org/10.12669/pjms.301.4307 (2014).
Salpekar, J. A. & Mishra, G. Key issues in addressing the comorbidity of attention deficit hyperactivity disorder and pediatric epilepsy. Epilepsy Behav 37, 310–315, https://doi.org/10.1016/j.yebeh.2014.04.021 (2014).
Amudhan, S., Gururaj, G. & Satishchandra, P. Epilepsy in India II: Impact, burden, and need for a multisectoral public health response. Ann Indian Acad Neurol 18, 369–381, https://doi.org/10.4103/0972-2327.165483 (2015).
Acknowledgements
We thank the children and their families for participating in the study. One author (K.S.) received support from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (project number 46056907) and the Norwegian Research School in Medical Imaging.
Author information
Authors and Affiliations
Contributions
A.C. and S.G. led the planning, design, and data collection for the study. A.C., N.S., and K.S. analyzed the data, and R.S. helped in data analysis related to psychiatric evaluation. A.C., S.G., and K.S. wrote the manuscript, and S.G. is the guarantor.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choudhary, A., Gulati, S., Sagar, R. et al. Childhood epilepsy and ADHD comorbidity in an Indian tertiary medical center outpatient population. Sci Rep 8, 2670 (2018). https://doi.org/10.1038/s41598-018-20676-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20676-8
This article is cited by
-
Comorbidity of epilepsy and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis
Journal of Neurology (2023)
-
Co-morbidity of attention deficit hyperactivity disorder among children with seizure disorders at University of Gondar referral hospital Ethiopia (2016)
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.