Abstract
Recently, studies on the relationship between gut dysbiosis and Parkinson’s disease (PD) have increased, but whether a specific gut bacterium may cause PD remains unexplored. Here, we report, for the first time, that a specific gut bacterium directly induces PD symptoms and dopaminergic neuronal damage in the mouse brain. We found that the number of Enterobacteriaceae, particularly Proteus mirabilis, markedly and commonly increased in PD mouse models. Administration of P. mirabilis isolated from PD mice significantly induced motor deficits, selectively caused dopaminergic neuronal damage and inflammation in substantia nigra and striatum, and stimulated α-synuclein aggregation in the brain as well as in the colon. We found that lipopolysaccharides, a virulence factor of P. mirabilis, may be associated in these pathological changes via gut leakage and inflammatory actions. Our results suggest a role of P. mirabilis on PD pathogenesis in the brain.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a progressive neurological disease, accompanied by motor symptoms, such as rigidity, bradykinesia, tremor, and postural abnormalities, due to degeneration of dopaminergic and γ-aminobutyric acid neurons in the basal ganglia, including substantia nigra (SN)1,2,3. PD has key pathological features, including nigrostriatal Lewy bodies, composed primarily of accumulated α-synuclein and neuroinflammatory responses characterized by reactive gliosis4. Up to 60% of dopaminergic neurons have already been lost by the time PD is diagnosed based on motor impairment, but the current therapeutics, consisting mainly of dopamine replacement drugs such as levodopa and several dopamine agonists, are restricted to simply relieving the clinical symptoms. Indeed, these drugs don’t cure the disease and even cause severe adverse events like dyskinesia and dopamine dysregulation syndrome5,6,7. Thus, there is a continuing need for a novel approach in the development of diagnostics and therapeutics for PD4,8.
Before the onset of motor symptoms, PD patients suffer from many non-motor symptoms, including olfactory, gastrointestinal, cardiovascular, and urological problems, indicating that PD may start from non-neurocentric tissues outside the brain9,10. Previous studies on pathological changes in the intestine of PD patients and PD animal models suggest the possibility that gut alteration is associated with PD pathogenesis. Both accumulated α-synuclein and 3-nitrotyrosine, which induce apoptotic cell death in dopaminergic neurons11, are increased significantly in colonic submucosal nerve fibers of early PD subjects versus healthy controls12,13. Accumulated α-synuclein was observed in the intestine and brain of Thy1-α-syn or A53T mutant α-syn transgenic mice before the onset of motor signs14,15. Moreover, it was shown experimentally that monomeric, oligomeric, and fibrillar α-synuclein forms can migrate from the gut to the brain via the vagal nerve16. From a different perspective, studies on permeability and inflammation in the intestines of PD patients and PD animal models have been reported. Both intestinal penetrability and lipopolysaccharide (LPS)-binding protein in plasma were increased significantly in PD patients17. Colonic inflammation was upregulated by pro-inflammatory cytokine production and glial cell expression in PD patients18. Increased intestinal permeability and α-synuclein accumulation were observed in the large intestine of PD mice administered LPS systemically19. Levels of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), were also increased in colonic tissues from 6-hydroxydopamine (6-OHDA)-lesioned PD rats20. Taken together, these previous studies suggest that intestinal pathological changes may influence the pathogenesis of PD.
Gut microbial imbalance is an important factor in intestinal pathology21. In fact, gut microbial changes in PD patients have been published. In 16 S ribosomal RNA (rRNA) gene pyrosequencing analysis in the fecal microbiota of PD patients, a higher abundance of the Enterobacteriaceae family was seen in non-tremor dominant PD patients, who were observed to have faster progression, worse outcomes, and more severe colonic α-synuclein pathology12,22. Several experimental studies revealed that exposure to curli, a bacteria-producing functional amyloid protein, enhanced α-synuclein aggregation both in rats and in nematodes, and gut microbiota regulate α-synuclein dependent neuroinflammation and motor dysfunction in α-synuclein overexpressing PD mice23,24. Although these previous studies raise the possibility that intestinal pathology by alteration of gut microbiota may be involved in the onset or aggravation of PD, it still remains unexplored what gut bacterial strains can cause PD.
We therefore first explored the claim that treatment with a specific gut bacterium may induce motor deficits and nigrostriatal dopaminergic neuronal damage. To find a specific gut bacterium, we measured the number of bacterial colonies at the family level in the feces of PD animal models and identified this bacterium from the increased bacterial family. Firstly, we examined whether this bacterium exacerbates PD symptoms and dopaminergic neuron death at the premotor phase. Then, we observed motor behaviors and brain tissues, including SN, striatum (ST), hippocampus (HP), and cortex after the oral administration of this bacterium to normal mice. We also examined how this bacterium injured dopaminergic neurons by assessing the changes of inflammatory factors and α-synuclein in the brain as well as in the colon.
Results
A specific gut bacterium, Proteus mirabilis, is identified from the genera Proteus which is isolated from the commonly increased Enterobacteriaceae family in the feces of PD mice models
Enterobacteriaceae is a representative pathogenic bacterial family that changes in disease states25. It has been reported that the Enterobacteriaceae family triggers colonic inflammatory conditions and is distinctly increased in the colon of PD patients22,26,27,28. As shown in Fig. 1A, we explored whether these bacterial colonies changed in PD mice models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), MPTP/probenecid (MPTP/p), and 6-OHDA toxicity. The number of Enterobacteriaceae increased significantly compared with those of each control group for three PD mice models (Fig. 1B–D). We found that, in the Enterobacteriaceae, the number of Proteus sp. was increased significantly in MPTP/p mice versus the normal group, whereas Escherichia coli (E. coli) and Klebsiella sp. hardly differed from each normal group (Fig. 1E). We confirmed that Proteus sp. was increased significantly in MPTP-induced PD mice (Fig. 1F). Then, we identified the increased Proteus sp. as Proteus mirabilis by 16 S rRNA gene sequencing (Table S1). Next, we assessed whether treatment with P. mirabilis influenced the mouse brain as well as colon.
P. mirabilis is an isolated bacterium from the increased bacterial colonies in PD animal models. (A) Schematic diagram of isolation and identification processes of P. mirabilis. (B–D) The colonies of Enterobacteriaceae were increased in the feces of MPTP/p, MPTP, and 6-OHDA-induced PD mice compared with the normal group, respectively. (E) The colonies of Proteus sp. in MPTP/p mice were only increased compared with the normal group whereas those of E. coli and Klebsiella sp. were no different from each normal group. (F) The colonies of Proteus sp. in MPTP mice were also increased compared with the normal group. Values were expressed as means ± SEM. #p < 0.05 and ##p < 0.01 vs. each normal group (unpaired t-test; n = 4). n.s; not significant, CFU; colony-forming unit.
Administration of P. mirabilis exacerbates striatal dopaminergic neuronal damage and induces motor deficits at the premotor phase of PD
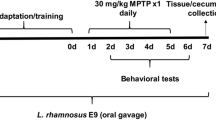
To examine whether P. mirabilis impairs motor function during the premotor stages of PD, we treated MPTP (15 mg/kg/day, i.p., for 5 days)-injected mice with P. mirabilis. The motor function of the mice injected with MPTP at 15 mg/kg alone was not impaired, while the mice co-treated with P. mirabilis showed significant movement impairments, similar to that of PD mice induced by MPTP (30 mg/kg/day, i.p., for 5 days) injection, which showed severe damage in motor function (Fig. 2A). Moreover, P. mirabilis treatment reinforced the MPTP-induced dopaminergic neuronal degeneration in the ST (Fig. 2B). These results show the possibility that P. mirabilis may be concerned with PD pathology.
Aggravation of MPTP-induced motor impairment and dopaminergic neuronal damage by P. mirabilis treatment. (A) P. mirabilis exacerbated MPTP-induced motor deficits (pole test and rotarod test; n = 10). (B) P. mirabilis also reinforced MPTP-induced striatal dopaminergic neuronal damage compared with the premotor stage induced by MPTP (15 mg/kg/d, i.p., for 5 d) injection (MPTP 15; n = 5). Values were expressed as means ± SEM. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. normal group (unpaired t-test); $p < 0.05 and $$p < 0.01 vs. the MPTP 15 group (unpaired t-test). IHC; immunohistochemistry.
P. mirabilis treatment impairs motor function, not affecting memory function
We explored whether treatment with P. mirabilis alone induces movement deficits in normal mice. We found that motor ability in the rotarod test was significantly impaired at the 16th day after the last administration of P. mirabilis (Fig. 3). The locomotor activity in the open field test was also significantly decreased at the 8th and 16th days after last administration of P. mirabilis (Fig. 3). On the other hand, P. mirabilis administration did not affect memory function (Fig. S1). These results show that P. mirabilis may induce motor impairment while it has no impact on the memory function.
P. mirabilis treatment impairs motor function in mice. Two behavioral tests (rotarod test and open field test) were performed at 8th and 16th day after last administration of P. mirabilis. Values were expressed as means ± SEM. #p < 0.05 and ##p < 0.01 vs. each normal group (unpaired t-test; n = 8). n.s; not significant.
P. mirabilis treatment selectively damages dopaminergic neurons in the brain
To examine whether dopaminergic neuronal damage in the brain was provoked by P. mirabilis itself, we analyzed histological changes at the 8th and 16th days after P. mirabilis administration for 5 days. Surprisingly, severe damage in dopaminergic neurons was observed in both the SN (Fig. 4A) and the ST (Fig. 4B) in the P. mirabilis-treated mice versus the normal group, while hippocampal and cortical neurons were not damaged by P. mirabilis treatment (Fig. S2). These results suggest that P. mirabilis causes damages to brain dopaminergic neurons selectively.
Dopaminergic neuronal damage in normal mice with PM administration. (A) Dopaminergic neuronal cell death was shown in the SNpc of mice brain at 8th and 16th day after PM treatment. (B) The loss of dopaminergic terminals was also shown in the ST of mice brain at the same time points. Values were expressed as means ± SEM. ##p < 0.01 and ###p < 0.001 vs. each normal group (ANOVA following Tukey’s post hoc test; n = 8). IHC; immunohistochemistry.
P. mirabilis treatment induces neuroinflammation in the nigrostriatal brain regions
To demonstrate how P. mirabilis treatment selectively induces dopaminergic neuronal damage in the brain, we examined whether P. mirabilis provokes neuroinflammation. Interestingly, the number of activated microglia was significantly increased in the SN (Fig. 5A) and ST (Fig. 5B) of P. mirabilis-treated mice brains at the 16th day after P. mirabilis treatment compared with the normal group, but this phenomenon was not observed in the hippocampal and cortical regions of the brain (Fig. S3). Thus, this result indicates that P. mirabilis-induced brain damage is specific and selective, particularly in dopaminergic neurons-enriched brain areas.
Glial activation in normal mice with P. mirabilis administration. (A) The number of activated microglia (Iba1 (green)-positive cells) was significantly increased in the SNpc of the brain (TH (red)-positive area) after P. mirabilis treatment, respectively. (B) This tendency was also consistent with that found in the ST region. Values were expressed as means ± SEM. ###p < 0.001 vs. normal group (ANOVA following Tukey’s post hoc test; n = 8). IF; immunofluorescence.
LPS elevated by P. mirabilis treatment induces colonic barrier disruption and inflammation
We were curious as to how P. mirabilis treatment induced dopaminergic neurodegeneration and inflammation in the brain. Because P. mirabilis produces LPS29, which can induce inflammatory conditions through producing various proinflammatory mediators30, we considered that LPS may induce inflammation in the gut and brain, so we measured LPS levels in feces and serum after P. mirabilis treatment. LPS levels in feces and serum were significantly increased at the 16th day after the treatment with PM, respectively (Fig. 6A). The increased colonic translocation of LPS derived from Enterobacteriaceae shows epithelial barrier disruption and stimulation of intestinal immune response to produce cytokines, which could move into the brain and give rise to brain inflammation and apoptotic neuronal cell death31,32,33,34. We explored whether P. mirabilis treatment induced pathological changes in the colon. We found that P. mirabilis treatment significantly reduced the protein level of occludin, a tight junction protein of the epithelial barrier in the colon, compared with the normal group at the 16th day after P. mirabilis administration (Fig. 6B). We found the increased release of the inflammatory cytokine TNF-α in colon of P. mirabilis-treated mice (Fig. 6B). Mice treated with P. mirabilis also exhibited the significant overexpression of toll-like receptor 4 (TLR4), a LPS binding receptor, in the colon (Fig. 6B). These results show that P. mirabilis-derived LPS causes pathological conditions in the colon, including epithelial barrier disruption and inflammation.
P. mirabilis treatment stimulates the elevation of fecal and serum LPS levels, colonic barrier disruption, and inflammation. (A) The levels of endotoxin LPS were elevated in the feces and serum of mice after P. mirabilis administration, respectively. The blots were processed in parallel using the samples derive from the same experiment. (B) P. mirabilis treatment induces the decrease of the epithelial barrier tight junction protein (occludin) and release of inflammatory cytokines (TNF-α) via activation of TLR4 by LPS in distal colon. Values were expressed as means ± SEM. #p < 0.05 and ###p < 0.001 vs. each normal group (unpaired t-test; n = 4). WB; western blotting.
Intra-rectal injection of LPS derived from P. mirabilis induces inflammation in the nigrostriatal regions of the brain
We investigated whether LPS purified from P. mirabilis (LPSP. mirabilis) is directly involved in the neuroinflammation in mice with intra-rectal injections of LPSP. mirabilis. Microglial activation was significantly increased in the nigrostriatal regions such as the SN (Fig. 7A) and ST (Fig. 7B), while it exhibited no differences in the hippocampal and cortical regions at the 16th day after treatment with LPSP. mirabilis (Fig. S4). This result supports that LPS may be involved in the P. mirabilis-induced selective brain damage in the dopaminergic neurons.
LPSP. mirabilis induces inflammation in the nigrostriatal regions of the brain. (A, B) Microglial activation (Iba1 (green)-positive cells) was increased in the SNpc (TH (red)-positive area) and ST of mice with intra-rectal administration of LPSP. mirabilis, respectively. Values were expressed as means ± SEM. #p < 0.05 and ###p < 0.001 vs. each normal group (unpaired t-test; n = 4). IF; immunofluorescence.
P. mirabilis treatment triggers the aggregation of α-synuclein in the colon as well as in the brain
It has been reported that the upregulation of α-synuclein expression occurs concurrently in enteric neurons as well as in dopaminergic neurons in PD35,36. Moreover, it has been shown that α-synuclein in enteric neurons moves into the SN via the vagus nerves in animal models16,37. Thus, we considered the view that P. mirabilis may induce α-synuclein aggregation in the colon and that such aggregated α-synuclein may move into the brain and cause neuroinflammation and dopaminergic neuronal cell death. To assess whether P. mirabilis treatment stimulates α-synuclein expression in neuronal cells, we assessed mRNA levels of α-synuclein after P. mirabilis treatment in SH-SY5Y cells. We found that the mRNA levels of α-synuclein were increased significantly in the P. mirabilis-treated group in a manner similar to those of the LPS-treated group when compared with untreated cells (Fig. S5). This change indicates that P. mirabilis may modulate the expression of α-synuclein. In this regard, we examined whether P. mirabilis treatment stimulates the overexpression of α-synuclein monomer in the colon and brain, but we concluded that it did not show a significant difference compared with normal group (Fig. S6). We focused on the change of α-synuclein aggregates that are toxicant forms of α-synuclein in the colon and brain. We investigated whether P. mirabilis treatment may stimulate the aggregation of α-synuclein in the colon. We found a significant increase of α-synuclein filaments (an aggregated form) in the distal colon at the 16th day after P. mirabilis administration compared with the normal group (Fig. 8A). The immunoreactivity of α-synuclein filaments was significantly stronger than that of the normal group in the SN (Fig. 8B) and ST (Fig. 8C), respectively. These results suggest that P. mirabilis activates notable aggregation of α-synuclein both in the colon and in the brain, particularly in the brain regions of the nigrostriatal pathway. We also suggested the potential that aggregated α-synuclein may migrate from colon to brain via vagus nerve, showing that P. mirabilis treatment did not induce the aggregation of α-synuclein in SN of vagotomized (VGX) mice while P. mirabilis only treatment increases the levels of aggregated α-synuclein (Fig. S7).
P. mirabilis treatment triggers aggregation of α-synuclein both in the colon and in the brain. The blots were processed in parallel using the samples derive from the same experiment. (A) The protein levels of α-synuclein filament was overexpressed in the distal colon of P. mirabilis -treated mice (n = 3). (B,C) P. mirabilis treatment stimulated immunoreactivity of α-synuclein filament (red) in the SNpc (TH (green)-positive area) and ST brain regions, respectively. Values were expressed as means ± SEM. #p < 0.05 and ###p < 0.001 vs. each normal group (unpaired t-test; n = 4). DB; dot blotting, IF; immunofluorescence, α-syn; α-synuclein.
Discussion
There is increasing evidence that the intestinal microbiota may regulate behavior and neuronal function, affecting neurological disorders or possibly attenuating them. Goehler et al. reported that Campylobacter jejuni, a common food-poisoning bacterium, induces anxiety-like behavior and c-fos protein expression in the amygdala and nucleus of the solitary tract, which process visceral and autonomic information38. Hsiao et al. showed that 4-ethylphenylsulfate, the most markedly increased metabolite from the gut microbiota in maternal immune activation-induced autism mice, induced abnormal autism-like behaviors, whereas commensal Bacteroides fragilis treatment attenuated this behavior39. In the case of PD, a recent evidence reported that short chain fatty acids produced by gut microbiota promote α-synuclein-mediated gliosis and PD motor symptoms23. As shown in Fig. 9, we demonstrated that a specific gut bacterium, P. mirabilis, was involved in the pathogenesis in a mouse model of PD.
First, we observed that gut microbiota dysbiosis had occurred regardless of toxin type in the colons of neurotoxin-induced PD mice. The bacterial colonies of Enterobacteriaceae family were significantly higher than those of each corresponding normal group. Then, we determined the genus of increased bacterial colonies as Proteus and it was identified as P. mirabilis. Interestingly, this result is consistent with a recent report that Proteus was a remarkably increased genus of the bacteria in α-synuclein overexpressing mice with intestinal dysbiosis induced by fecal transplantation from PD patients23.
Thus, our result is consistent with the previous study that Enterobacteriaceae increased significantly in PD patients22. Although E. coli is the most representative bacterium in the Enterobacteriaceae family and its endotoxin increases intestinal permeability and α-synuclein levels17, it showed no change in PD mice, whereas P. mirabilis showed levels increased over 10-fold compared with the normal group in our study. Before now, there has been no clinical report on the quantification of E. coli and P. mirabilis in the intestine, but recently it was reported that P. mirabilis was significantly higher in the urine of PD patients than in controls40,41. In that study, it was suggested that P. mirabilis was a causative bacterium of ‘purple urine bag syndrome’, increasing urinary indoxyl sulfate, a bacteria-generated metabolite. It was also shown that P. mirabilis acts as an inducible bacterium in pathological colonic changes. P. mirabilis stimulates robust IL-1β production in response to dextran sulfate sodium-induced colitis, mediated by the NOD-like receptor family pyrin domain-containing 3 inflammasome activation42. It could also trigger pathological colonic changes, characterized by colonic barrier disruption and elevated TNF-α levels as a key feature in mice with T-bet−/−RAG2−/− ulcerative colitis26. Additionally, P. mirabilis was demonstrated to be one of the causative bacteria in brain infection, according to some case reports43,44,45. These previous studies suggest that P. mirabilis may induce pathological status in both the colon and the brain. Thus, we hypothesized that P. mirabilis may be pathogenic to dopaminergic neurons, and then we evaluated whether P. mirabilis treatment could exacerbate or induce PD-like pathological characteristics in mice. This bacterium worsened the motor symptoms and dopaminergic neuronal damage in mice at the premotor phase of PD induced by MPTP toxicity. Moreover, the treatment of this bacterium alone impaired motor function, induced severe dopaminergic neuronal damage, and activated glial cells in the SN and ST of normal mice. These results suggest that P. mirabilis may be involved in the pathogenesis of PD.
Next, we sought to examine how this bacterium affects brain damage. Because P. mirabilis is a gram-negative pathogenic bacterium that produces LPS endotoxin29, we considered that LPS derived from P. mirabilis in the colon could move to the periphery, and then induce neuroinflammation. We found that LPS levels in feces were elevated significantly at the 16th day after P. mirabilis treatment, and the levels in serum increased consistently. These results suggested the possibility that P. mirabilis-generated LPS may be transferred from the colon to the brain via blood circulation, showing our results that microglial activation was observed in the SN and ST of mice performed intra-rectal injections of LPSP. mirabilis. This is consistent with the report of Qin et al. that increased inflammatory cytokines by systemic injection of LPS transferred inflammatory reactions from periphery to brain inducing microglial activation and dopaminergic neuronal damage in the SN46.
It has been reported previously that LPS derived from bacteria impairs the colonic barrier by reducing tight junction proteins such as occludin, and contributes to the release of TNF-α, which is stimulated by T helper 1 cell differentiation following TLR4-mediated macrophage activation in the lamina propria of the colon47,48,49. In this study, colonic barrier disruption and elevated TNF-α levels via TLR4 activation were seen after P. mirabilis administration. Clairembault and colleagues reported that expression of colonic epithelial barrier tight junction proteins was significantly lower in biopsy tissues from PD patients compared to that of controls50. Pro-inflammatory cytokines, including TNF-α, were also overexpressed in colonic biopsies from PD patients18. Thus, these pathological changes induced by P. mirabilis may represent the condition in the colon of PD patients.
Then, we assessed whether P. mirabilis induced overexpression of α-synuclein, a pathological hallmark protein in PD, in the brain and in the colon. In an in vitro experiment, P. mirabilis treatment increased the mRNA levels of α-synuclein significantly in SH-SY5Y cells. Although we could not clarify whether the effect was induced by P. mirabilis itself or LPS generated from the microbiota, this finding indicates that P. mirabilis may be involved in the production of α-synuclein in dopaminergic neurons. We also demonstrated the increase of α-synuclein aggregates both in the colon and in the SN of mice after P. mirabilis treatment. Pan-Montojo and his colleagues reported that α-synuclein first appeared in enteric neurons, moved into the dorsal motor nucleus of the vagus, and was eventually detected in the SN of rotenone-induced PD-like mice37. According to Braak theory, α-synuclein migrates from the gastrointestinal tract to the brain and overexpression of α-synuclein starts in the intestine51,52. Our results in VGX mice showed the consistent with the previous results by Holmqvistet and his colleagues that aggregated α-synuclein that was injected directly into the intestine migrated from the intestinal wall to the brain via the vagal nerve16. It has been also reported that inflammatory factors such as LPS and pro-inflammatory cytokines may trigger aggregation and accumulation of α-synuclein in enteric nerves19,53. Based on these reports, we expect that the increased α-synuclein aggregates by P. mirabilis treatment in the distal colon may move to the SN where it triggered dopaminergic neuronal damage and neuroinflammation. Additionally, increased pro-inflammatory cytokine TNF-α due to LPS insult in the colon may move directly via the blood to the SN and trigger α-synuclein aggregation and dopaminergic neuronal damage. Several studies suggest that other virulence factors of P. mirabilis except LPS may involve in intestinal inflammation or increased intestinal permeability. For example, flagella of P. mirabilis allows invasion into colonic epithelial barrier, thereby induces the increase of permeability and P. mirabilis-generated mannose-resistant Proteus-like fimbriae, which is closely related to biofilm formation as an adhesion factor, exhibits cytotoxicity on eukaryotic epithelial cells54,55. Meanwhile, it is not possible to exclude the possibility that other gut microbiomes except P. mirabilis could be involved in PD pathology in P. mirabilis-treated mice. For example, Chen et al. indicated the causal relationship between E. coli-producing curli amyloid and α-synuclein pathology24. Further researches on the actions of other bacteria after P. mirabilis administration in mice are needed.
In conclusion, we demonstrated that P. mirabilis, which showed increased bacterial colonies in the feces of three PD mouse models, is pathogenic in these animal models. It induced PD-related pathological changes, including dopaminergic neuronal death, neuroinflammation, and α-synuclein aggregation in the brain. We suggest that these pathological changes may be due to LPS as a P. mirabilis-generated virulence factor in the colon. Here, our findings are meaningful in that they provide the experimental evidence that a specific gut bacterium, which is commonly increased in PD mice, may cause the phenotype and pathogenesis of PD. It is required that further exploration to clarify the relevance of P. mirabilis for PD pathology under the conditions with germ-free or P. mirabilis-specific antibiotics treatment.
Methods
Animals
The mice used in the study were purchased from Daehan Biolink (Eumseong, Korea) and included the following: male C57BL/6 mice; 12-week-old (MPTP/p-induced PD mice) and 7-week-old mice (MPTP-induced PD mice, mice administrated with MPTP + P. mirabilis, mice with intra-rectal injection of LPSP. mirabilis, and mice with administrated P. mirabilis alone), male ICR 6-week-old mice (6-OHDA-induced PD mice). After the adaptation periods for 7 days, mice were housed in separate cages per each group (n = 4 per cage) at an ambient temperature of 23 ± 1 °C and relative humidity 60 ± 10% under a 12 h light/dark cycle and were allowed free access to water and food. All animal studies were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 80–23, revised 1996) and approved by the “Animal Care and Use Guidelines” of Kyung Hee University, Seoul, Korea (the approval number: KHUASP(SE)-16-132 and KHUASP(SE)-17-004). No research involved human participants in this study.
MPTP/p-induced PD model
The MPTP/p-induced PD model was made according to previously described methods56. Detailed methods are described in Supplementary Methods.
MPTP-induced PD model
The MPTP-induced PD model was made according to our previously reported methods57. Briefly, mice were injected with MPTP hydrochloride (30 mg/kg/day in saline, i.p. for 5 days). Vehicles of equal volume (0.25 ml) were given to the normal group. Feces were obtained at the 7th day after the last MPTP injection.
6-OHDA-induced PD model
The 6-OHDA-induced PD model was made according to previously reported methods with some modification58. Briefly, mice were anesthetized, and two stereotaxic injections of 6-OHDA (16 μg/2 μl; 6-OHDA group), or an equivalent volume of 0.9% saline containing 0.2% ascorbic acid, were administered into the right ST (normal group), at a rate of 1 μl/min (AP + 0.7, ML + 2.0, DV −3.4 mm from bregma and dura)59. Feces were obtained at the 21st day after 6-OHDA stereotaxic injection.
Isolation and culture of fecal microbiota
Two or three fresh stools per mouse (approximately 0.08–0.12 g, 0.04 g per individual pellet) was septically collected from MPTP/p, MPTP, 6-OHDA-induced mice, and each normal mice, respectively. Each stool was suspended in a diluted anaerobic broth (Nissui Pharmaceutical Co., Japan), inoculated in deoxycholate hydrogen sulphide lactose (DHL) for isolation of Enterobacteriaceae colonies (Eiken, Tokyo, Japan), and cultured anaerobically at 37 °C for 2 days. The grown colonies in the DHL agar plates were identified as P. mirabilis using 16 S rRNA gene sequencing.
Isolation of LPS extracted from P. mirabilis
LPSP. mirabilis was extracted using the hot phenol-water method as a previously reported method with several modifications60. Detailed methods are described in Supplementary Methods.
Cell culture and P. mirabilis treatment in vitro
SH-SY5Y neuroblastoma cells were purchased from the Korean Cell Line Bank (Seoul, Korea). SH-SY5Y cells were cultured at 37 °C and 5% CO2 in Dulbeco’s Modified Eagle’s medium (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (PAN biotech GmbH Aidenbach, Germany). Detailed methods are described in Supplementary Methods.
P. mirabilis administration in vivo
P. mirabilis was grown in tropic soy broth (200 ml) and harvested by the centrifuge at 10,000 × g for 10 min. Cell supernatants were then washed twice with phosphate buffered saline (PBS). Detailed methods are described in Supplementary Methods.
Intra-rectal injection of LPSP. mirabilis in mice
We performed intra-rectal administration of LPSP. mirabilis according to the previously reported method61. Detailed methods are described in Supplementary Methods.
Behavior test
We performed three motor behavior tests in this study. Detailed methods are described in Supplementary Methods.
Biological sample preparation
For immunohistochemical studies, at 24 h after behavioral tests, mice were perfused transcardially with 0.05 M PBS, and then fixed with cold 4% paraformaldehyde (PFA) in a 0.1 M phosphate buffer. Brains were removed and post-fixed in a 0.1 M phosphate buffer containing 4% PFA overnight at 4 °C and then immersed in a solution containing 30% sucrose in 0.05 M PBS for cryoprotection. Serial 30 µm-thick coronal sections were cut on a freezing microtome (Leica, Germany) and stored in cryoprotectant (25% ethylene glycol, 25% glycerol, and 0.05 M phosphate buffer) at 4 °C until use. For western blot analysis, the mice were decapitated and the brains or distal colons were isolated and stored at −80 °C until use. We selected the distal region of the colon due to the large amount of intestinal bacteria in this region62.
Determination of LPS in feces and serum
The LPS content of the feces, serum, and brain cortex was determined using diazo-coupled LAL assays. Briefly, the feces (approximately 0.08–0.12 g) collected from the mice were placed in 50 ml of PBS in a pyrogen-free tube, sonicated for 1 h on ice, and sterilized by filtration through a 0.45-μm filter followed by re-filtration through a 0.22-μm filter. The serum (5 μl) was diluted 1:10 in pyrogen-free water and heated at 70 °C for 10 min. The LPS levels in the feces and serum were assayed using the LAL Assay Kit (Cape Cod Inc., USA), according to the manufacturer’s protocol.
Immunohistochemistry
For the immunohistochemical study, the brain sections were selected according to mouse brain atlas (SN; from −2.92 to −3.52 mm, ST; from 1.18 to 0.38 mm, HP and primary sensory cortex (PSC); from −1.94 to −2.30 mm following coordinates from the bregma)63. Detailed methods are described in Supplementary Methods. The images were photographed using an optical bright-field or fluorescence microscope (BX51, Olympus, Japan).The number of tyrosine hydroxylase (TH) and ionized calcium-binding adapter molecule 1 (Iba1) positive cells in the SN, ST, HP, and PSC was quantified according to stereological counting64 and they were analyzed with Image J software.
Dot blotting
Dot blotting was performed according to previously described methods65. For dot blot quantification of α-synuclein filament, 8 μg samples of the distal colon were spotted on an Immobilon-P transfer membrane and subsequently blocked for 30 min with 5% skim milk in TBST. After rinsing with TBST, the membrane was incubated with a primary antibody for 1 h at room temperature and then treated with an HRP secondary antibody for 30 min. Immuno-reactive bands and their quantification were equally measured as the methods of western blotting.
Quantitative real time-polymerase chain reaction
Total RNA was extracted from SH-SY5Y cells, using a total RNA extraction kit (Qiagen, Germany). Synthesis of cDNA for detection of α-synuclein, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed with 2 μg of total RNA, oligo(dT) primers (α-synuclein, forward: 5′-AGGCAGCTGGAAAGACAAAA-3 and the reverse: 5′-CAGCTCCCTCCACTGTCTTC-3′; and GAPDH, forward: 5′-TGCAGTGGCAAAGTGGAGAT-3′ and the reverse: 5′-TTTGCCGTGAGTGGAGTCATA-3′), and a reverse transcriptase in a total volume of 40 μl, as described previously. PCRs were performed in a total volume of 50 μl, comprising 4 μl of cDNA product and 25 μl of Premix EX Taq (TaKaRa Bio Inc., Japan), using a TaKaRa thermal cycler and SYBR premix agents, per the instructions provided by TaKaRa. Thermal cycling conditions were as follows: activation of DNA polymerase at 95 °C for 5 min, followed by 40 cycles of amplification at 95 °C for 10 sec and at 60 °C for 30 sec. Gene expression was normalized with respect to GAPDH.
Statistical Analysis
All statistical parameters were calculated using Graphpad Prism 5.0 software (GraphPad Software, Inc., USA) or SPSS 11 software (SPSS Inc., USA). Values were expressed as the mean ± standard error of the mean (SEM). All results were evaluated by the one-way ANOVA analysis (Tukey’s post hoc test) or unpaired t-test. Differences with a p-value less than 0.05 were considered statistically significant. Data that is out of range of mean ± 2-fold standard deviation was excluded. All experiments were repeated for reproducibility.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dickson, D. W. et al. Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15(Suppl 3), S1–5, https://doi.org/10.1016/S1353-8020(09)70769-2 (2009).
Windels, F. & Kiyatkin, E. A. GABA, not glutamate, controls the activity of substantia nigra reticulata neurons in awake, unrestrained rats. J Neurosci 24, 6751–6754, https://doi.org/10.1523/JNEUROSCI.1528-04.2004 (2004).
Blaszczyk, J. W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front Neurosci 10, 269, https://doi.org/10.3389/fnins.2016.00269 (2016).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Muller, T. Motor complications, levodopa metabolism and progression of Parkinson’s disease. Expert Opin Drug Metab Toxicol 7, 847–855, https://doi.org/10.1517/17425255.2011.575779 (2011).
Pires, A. O. et al. Old and new challenges in Parkinson’s disease therapeutics. Prog Neurobiol, https://doi.org/10.1016/j.pneurobio.2017.04.006 (2017).
Antonini, A., Tolosa, E., Mizuno, Y., Yamamoto, M. & Poewe, W. H. A reassessment of risks and benefits of dopamine agonists in Parkinson’s disease. Lancet Neurol 8, 929–937, https://doi.org/10.1016/S1474-4422(09)70225-X (2009).
Cheng, H. C., Ulane, C. M. & Burke, R. E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67, 715–725, https://doi.org/10.1002/ana.21995 (2010).
Cersosimo, M. G. et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260, 1332–1338, https://doi.org/10.1007/s00415-012-6801-2 (2013).
Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15(Suppl 1), 14–20, https://doi.org/10.1111/j.1468-1331.2008.02056.x (2008).
Blanchard-Fillion, B. et al. Metabolism of 3-nitrotyrosine induces apoptotic death in dopaminergic cells. J Neurosci 26, 6124–6130, https://doi.org/10.1523/JNEUROSCI.1038-06.2006 (2006).
Lebouvier, T. et al. Biopsable neural tissues: toward new biomarkers for Parkinson’s disease? Front Psychiatry 1, 128, https://doi.org/10.3389/fpsyt.2010.00128 (2010).
Shannon, K. M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord 27, 709–715, https://doi.org/10.1002/mds.23838 (2012).
Wang, L. et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil 24, e425–436, https://doi.org/10.1111/j.1365-2982.2012.01974.x (2012).
Bencsik, A., Muselli, L., Leboidre, M., Lakhdar, L. & Baron, T. Early and persistent expression of phosphorylated alpha-synuclein in the enteric nervous system of A53T mutant human alpha-synuclein transgenic mice. J Neuropathol Exp Neurol 73, 1144–1151, https://doi.org/10.1097/NEN.0000000000000137 (2014).
Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128, 805–820, https://doi.org/10.1007/s00401-014-1343-6 (2014).
Forsyth, C. B. et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6, e28032, https://doi.org/10.1371/journal.pone.0028032 (2011).
Devos, D. et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50, 42–48, https://doi.org/10.1016/j.nbd.2012.09.007 (2013).
Kelly, L. P. et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29, 999–1009, https://doi.org/10.1002/mds.25736 (2014).
Pellegrini, C. et al. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflammation 13, 146, https://doi.org/10.1186/s12974-016-0608-5 (2016).
Yang, Y. & Jobin, C. Microbial imbalance and intestinal pathologies: connections and contributions. Dis Model Mech 7, 1131–1142, https://doi.org/10.1242/dmm.016428 (2014).
Scheperjans, F. et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30, 350–358, https://doi.org/10.1002/mds.26069 (2015).
Sampson, T. R. et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167, 1469–1480 e1412, https://doi.org/10.1016/j.cell.2016.11.018 (2016).
Chen, S. G. et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci Rep 6, 34477, https://doi.org/10.1038/srep34477 (2016).
Stiles, M. E. & Ng, L. K. Biochemical characteristics and identification of Enterobacteriaceae isolated from meats. Appl Environ Microbiol 41, 639–645 (1981).
Garrett, W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8, 292–300, https://doi.org/10.1016/j.chom.2010.08.004 (2010).
Bloom, S. M. et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 9, 390–403, https://doi.org/10.1016/j.chom.2011.04.009 (2011).
Unger, M. M. et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32, 66–72, https://doi.org/10.1016/j.parkreldis.2016.08.019 (2016).
Sidorczyk, Z., Zahringer, U. & Rietschel, E. T. Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re-mutant. Eur J Biochem 137, 15–22 (1983).
Raetz, C. R. & Whitfield, C. Lipopolysaccharide endotoxins. Annu Rev Biochem 71, 635–700, https://doi.org/10.1146/annurev.biochem.71.110601.135414 (2002).
Jung, H. C. et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 95, 55–65, https://doi.org/10.1172/JCI117676 (1995).
Cario, E. et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 164, 966–972 (2000).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9, 46–56, https://doi.org/10.1038/nrn2297 (2008).
Kell, D. B. & Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: the central roles of LPS and LPS-induced cell death. Integr Biol (Camb) 7, 1339–1377, https://doi.org/10.1039/c5ib00158g (2015).
Gold, A., Turkalp, Z. T. & Munoz, D. G. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzheimer’s disease. Mov Disord 28, 237–240, https://doi.org/10.1002/mds.25298 (2013).
Natale, G., Pasquali, L., Paparelli, A. & Fornai, F. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol Motil 23, 1056–1065, https://doi.org/10.1111/j.1365-2982.2011.01794.x (2011).
Pan-Montojo, F. et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2, 898, https://doi.org/10.1038/srep00898 (2012).
Goehler, L. E., Park, S. M., Opitz, N., Lyte, M. & Gaykema, R. P. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 22, 354–366, https://doi.org/10.1016/j.bbi.2007.08.009 (2008).
Hsiao, E. Y. et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463, https://doi.org/10.1016/j.cell.2013.11.024 (2013).
Cassani, E. et al. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord 21, 389–393, https://doi.org/10.1016/j.parkreldis.2015.02.004 (2015).
Tan, C. K., Wu, Y. P., Wu, H. Y. & Lai, C. C. Purple urine bag syndrome. CMAJ 179, 491, https://doi.org/10.1503/cmaj.071604 (2008).
Seo, S. U. et al. Distinct Commensals Induce Interleukin-1beta via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity 42, 744–755, https://doi.org/10.1016/j.immuni.2015.03.004 (2015).
Kassim, Z., Aziz, A. A., Haque, Q. M. & Cheung, H. A. Isolation of Proteus mirabilis from severe neonatal sepsis and central nervous system infection with extensive pneumocephalus. Eur J Pediatr 162, 644–645, https://doi.org/10.1007/s00431-003-1240-9 (2003).
Juyal, D., Rathaur, V. K. & Sharma, N. Neonatal meningoventriculitis due to proteus mirabilis - a case report. J Clin Diagn Res 7, 369–370, https://doi.org/10.7860/JCDR/2013/5146.2772 (2013).
Kourbeti, I. S. et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg 122, 1113–1119, https://doi.org/10.3171/2014.8.JNS132557 (2015).
Qin, L. et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462, https://doi.org/10.1002/glia.20467 (2007).
Guo, S. et al. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol 195, 4999–5010, https://doi.org/10.4049/jimmunol.1402598 (2015).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9, 799–809, https://doi.org/10.1038/nri2653 (2009).
Abreu, M. T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10, 131–144, https://doi.org/10.1038/nri2707 (2010).
Clairembault, T. et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3, 12, https://doi.org/10.1186/s40478-015-0196-0 (2015).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24, 197–211 (2003).
Shannon, K. M., Keshavarzian, A., Dodiya, H. B., Jakate, S. & Kordower, J. H. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27, 716–719, https://doi.org/10.1002/mds.25020 (2012).
Lema Tome, C. M. et al. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease–is there a link? Mol Neurobiol 47, 561–574, https://doi.org/10.1007/s12035-012-8267-8 (2013).
Scavone, P., Villar, S., Umpierrez, A. & Zunino, P. Role of Proteus mirabilis MR/P fimbriae and flagella in adhesion, cytotoxicity and genotoxicity induction in T24 and Vero cells. Pathog Dis 73, https://doi.org/10.1093/femspd/ftv017 (2015).
Okumura, R. et al. Lypd8 promotes the segregation of flagellated microbiota and colonic epithelia. Nature 532, 117–121, https://doi.org/10.1038/nature17406 (2016).
Carta, A. R., Carboni, E. & Spiga, S. The MPTP/probenecid model of progressive Parkinson’s disease. Methods Mol Biol 964, 295–308, https://doi.org/10.1007/978-1-62703-251-3_17 (2013).
Kim, H. G. et al. Effects of the root bark of Paeonia suffruticosa on mitochondria-mediated neuroprotection in an MPTP-induced model of Parkinson’s disease. Food Chem Toxicol 65, 293–300, https://doi.org/10.1016/j.fct.2013.12.037 (2014).
da Conceicao, F. S., Ngo-Abdalla, S., Houzel, J. C. & Rehen, S. K. Murine model for Parkinson’s disease: from 6-OH dopamine lesion to behavioral test. J Vis Exp, https://doi.org/10.3791/1376 (2010).
Franklin, K. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates. (Academic Press 1997).
Rezania, S. et al. Extraction, Purification and Characterization of Lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J Med Biotechnol 3, 3–9 (2011).
Im, E., Riegler, F. M., Pothoulakis, C. & Rhee, S. H. Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am J Physiol Gastrointest Liver Physiol 303, G490–497, https://doi.org/10.1152/ajpgi.00120.2012 (2012).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14, 20–32, https://doi.org/10.1038/nrmicro3552 (2016).
Paxinos, G. & Franklin, K. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates 4th Edition. 4th edn, (Academic Press, 2012).
Mishra, V. et al. Resveratrol Treatment after Status Epilepticus Restrains Neurodegeneration and Abnormal Neurogenesis with Suppression of Oxidative Stress and Inflammation. Sci Rep 5, 17807, https://doi.org/10.1038/srep17807 (2015).
Nuber, S. et al. A progressive dopaminergic phenotype associated with neurotoxic conversion of alpha-synuclein in BAC-transgenic rats. Brain: a journal of neurology 136, 412–432, https://doi.org/10.1093/brain/aws358 (2013).
Acknowledgements
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (MEST) (NRF-2015R1A2A2A01004341). This work was also supported by Medical Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2017R1A5A2014768).
Author information
Authors and Affiliations
Contributions
J.G.C., D.H.K., and M.S.O. designed and coordinated the study. J.G.C., N.K., I.G.J., H.E., S.M.L. and S.E.J. performed the experiment and acquired the data. J.G.C., D.H.K. and M.S.O. wrote the paper. All the authors participated in discussion of the results and reviewed the final draft.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, J.G., Kim, N., Ju, I.G. et al. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep 8, 1275 (2018). https://doi.org/10.1038/s41598-018-19646-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19646-x
This article is cited by
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
The connection between gut microbiota and its metabolites with neurodegenerative diseases in humans
Metabolic Brain Disease (2024)
-
PD-Like Pathogenesis in Caenorhabditis elegans Intestinally Infected with Nocardia farcinica and the Underlying Molecular Mechanisms
Molecular Neurobiology (2024)
-
Oral pathogens exacerbate Parkinson’s disease by promoting Th1 cell infiltration in mice
Microbiome (2023)
-
Parkinson’s disease and gut microbiota: from clinical to mechanistic and therapeutic studies
Translational Neurodegeneration (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.