Abstract
Recent studies have revealed the prognostic role of the gamma gap, the total serum proteins concentration minus the albumin concentration, for predicting all-cause mortality among adults. This study aims to investigate the relationship between the gamma gap and all-cause mortality among nonagenarians and centenarians via a secondary data analysis of a prospective observational study. The analysis included 801 participants (260 men and 541 women, mean age: 93.7 ± 3.5 years), 46 of which were lost at the 4-year follow-up. The mean gamma gap was 2.7 ± 0.5 g/dl. After adjusting for relevant confounders, the gamma gap was significantly associated with 4-year all-cause mortality (hazard ratio [HR] per 1-SD = 1.22, 95% confidential interval [CI]: 1.12–1.78). Using different cut-off points, the elevated gamma gap could be defined as ≥2.9, 3.0, 3.1, or 3.2 g/dl. The relevant HRs and 95% CIs of the elevated gamma gap for predicting mortality were 1.27 (1.12–1.90), 1.29 (1.03–1.78), 1.21 (1.23–1.66), and 1.26 (1.09–1.69), respectively. In conclusion, the gamma gap is an independent prognostic factor for long-term mortality in nonagenarians and centenarians. A value greater than or equal to 3.1 g/dl may define an elevated gamma gap, but further studies are required.
Similar content being viewed by others
Introduction
The gamma gap, the total serum proteins concentration minus the albumin concentration, has been widely used in clinical practice for several decades1. An elevated gamma gap is usually considered to be an indicator of infection, systemic inflammation, or malignancy2; however, its implications for patient prognosis are not well understood. Previous studies have indicated the potential association between an elevated gamma gap and deleterious health outcomes. For example, a previous study demonstrated that an elevated gamma gap was related to an increased risk of heart failure3, which was possibly the result of systemic inflammation.
A recent cohort study by Juraschek et al. reported that an elevated gamma gap (≥3.1 g/dl) was an independent risk factor for all-cause mortality among a large population of U.S. adults4. A subsequent study confirmed the effect of the gamma gap on all-cause mortality using the same study population with a longer follow-up period5. These findings need to be validated in different populations.
A chronic low-grade inflammation plays a key role in the process of aging6. Older adults, especially the oldest old, are therefore prone to chronic low-grade systemic inflammation and an elevated gamma gap7. Whether the gamma gap is related to an increased risk of death in nonagenarian and centenarian populations is not known. The gamma gap might serve as a valuable and cheap prognostic factor as total serum proteins and albumin (instead of other inflammatory factors, such as C-reactive protein or interleukin 6) are routinely measuredly as prat of a regular examination. Thus, we conducted this study to investigate the possible relationship between the gamma gap and all-cause mortality among a population of nonagenarians and centenarians in western China.
Methods
Study population
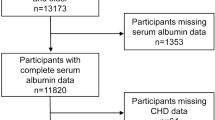
This study was a secondary data analysis of the “Project of Longevity and Aging in Dujiangyan” (PLAD), which was previous published8,9. In brief, all individuals (aged ≥90 years) who lived in Dujiangyan (a small city in western China) were invited to join the baseline investigation in April 2005. A total of 1115 older adults were invited, of which 870 agreed to participate. Well trained staff visited all the participants in their homes or their community centers and performed face-to-face interviews and anthropometric measurements. Biological specimens included fasting venous blood samples.
Ethics
The Research Ethics Committee of Sichuan University (registration number: 2004081233) approved the study protocol. All the participants or their legal proxies signed the informed consents forms. All the methods in this study were in accordance with relevant guideline and regulations.
All-cause mortality
The study population was followed up four years later, in 2009. Mortality data were collected from the local death registration database. Both positive and negative matches were then confirmed with the family members. Individuals who had no record of mortality in the database and whose family members could not be contacted were defined as “loss to follow-up” (45 individuals, 5.5%). The time to death was calculated as the time between the baseline interview and the date of death.
Gamma gap
The measurement of total serum proteins and albumin were performed using a Synchron CX4 Pro (Beckman Coulter, Fullerton, CA). The gamma gap was calculated as “the difference between total protein and albumin (total protein – albumin)”4. Traditionally, an elevated gamma gap was defined as ≥4.0 g/dl; however, previous studies2,4,10 applied a cut-off point of ≥3.1 g/dl to define an elevated gamma gap. In this study, we defined the elevated gamma gap with multiple cut-off points between 1.7 g/dl (10th percentile) and 4.4 g/dl (99th percentile). We also treated the gamma gap as a continuous variable.
Assessment of covariates
We included the following covariates: age, gender, smoking status (current smoker or not), alcohol consumption (current drinker or not), physical activity (yes/no), and body mass index (BMI, kg/m2).
The following chronic diseases were also included as covariates: hypertension (yes/no), cardiovascular disease (yes/no), diabetes (yes/no), chronic respiratory disease (yes/no), chronic liver disease (yes/no), stroke (yes/no), chronic kidney disease (yes/no), osteoarthritis (yes/no), and cancer (yes/no). The diagnosis of these chronic diseases was based on the formal medical records of each individual. The definitions of these diseases are listed in Supplementary Table 1.
Based on previous studies4,5, we also included the following covariates: total cholesterol (TC, mmol/L), triglyceride (TG, mmol/L), low-density lipoprotein cholesterol (LDL-C, mmol/L) high-density lipoprotein cholesterol (HDL-C, mmol/L), alanine aminotransferase (ALT, U/L), total bilirubin (TB, μmol/L), white blood cell count (WBC, SI), and hemoglobin (HGB, g/L). Furthermore, we included the following geriatric syndromes as covariates: self-reported falls in the previous year (yes/no), incontinence (yes/no), cognitive impairment (yes/no, assessed using Mini Mental State Examination11), polypharmacy (yes/no, defined as the concomitant use of five or more medications12), and depression (yes/no, assessed using Geriatric Depression Scale-Chinese Edition13).
Statistical analyses
Baseline characteristics were presented as a proportion (%) and mean ± standard deviation (SD) of the whole study population and between elevated and normal gamma gap groups. To compare the differences between groups, the Pearson chi-squared test (or Fisher’s exact test where an expected cell count was <5) and one-way ANOVA were applied for categorical data and continuous data, respectively. Weighted Cox proportional hazard models were applied to compare the relationship between the gamma gap (as a dichotomous variable using multiple cut-off points between 1.7 and 4.4 g/dl) with all-cause mortality. Model 1 was adjusted for age, gender, smoking status, alcohol consumption, and BMI. Model 2 was further adjusted for TC, TG, HDL-C, LDL-C, ALT, TB, WBC, and HGB. We also treated the gamma gap as a continuous variable (per 1 g/dl, or per 1-SD) and reanalyzed the association between the gamma gap and all-cause mortality using the same Cox proportional hazard models listed above. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. The Kaplan-Meier curves and the log-rank tests were performed using MedCalc Statistical Software version 15.2 (MedCalc Software bvba, Ostend, Belgium). All other statistical analyses were performed using SPSS 20.0 (IBM, SPSS Statistics, Armonk, NY, USA). P <0.05 was considered statistically significant.
Results
Baseline characteristics of the study population
Participants with missing data on the gamma gap (69 individuals) were excluded in the subsequent analyses. As a result, 801 participants (260 men and 541 women) were included in the baseline analyses. All the participants were Han Chinese. The mean age of the study population was 93.7 ± 3.5 years. Among the whole study population, 180 participants (22.5%) had an elevated gamma gap (defined as ≥3.1 g/dl).
Table 1 shows the characteristics of the whole population the gamma gap values. Compared with those with a normal gamma gap, the participants with an elevated gamma gap (≥3.1 g/dl) were more likely to have higher levels of TB and WBC, but a lower level of HDL-C. The participants with an elevated gamma gap were less likely to exercise. Additionally, the participants with an elevated gamma gap appeared to be older than those with a normal gamma gap, but the difference was not statistically significant.
Association between the gamma gap and 4-year all-cause mortality
In the included population, 36 participants were lost during the 4-year follow-up, and therefore, 765 participants (249 men and 516 women) were included in the survival analyses. During the 4-year follow-up, 139 men (55.8%) and 269 women (52.1%) died (p = 0.338). The mortality of the participants with an elevated gamma gap (defined as ≥3.1 g/dl) was significantly higher than those with a normal gamma gap (61.9% vs. 50.8%, p = 0.009).
When the gamma gap was treated as a continuous variable, a higher gamma gap was independently associated with a higher risk of 4-year all-cause mortality after adjusting for the relevant confounders (HR per 1 g/dl = 1.09, 95% CI: 1.01–1.69; HR per 1-SD = 1.22, 95% CI: 1.12–1.78) (Table 2).
We evaluated different cut-off points to define an elevated gamma gap. After adjusting for the relevant confounders, an elevated gamma gap was significantly associated with 4-year all-cause mortality when defined as ≥2.9 g/dl (HR = 1.27, 95% CI: 1.12–1.90), ≥3.0 g/dl (HR = 1.29, 95% CI: 1.03–1.78), ≥3.1 g/dl (HR = 1.21, 95% CI: 1.13–1.66), or ≥3.2 g/dl (HR = 1.26, 95% CI 1.09–1.69). The traditional value used to define an elevated gamma gap ( ≥4.0 g/dl) was not significantly associated with 4-year all-cause mortality after full adjustment (HR = 1.98, 95% CI: 0.91–3.71) (Table 2).
Figure 1 shows the Kaplan-Meier survival curves of the participants with or without an elevated gamma gap defined by different cut-off points. The log-rank test indicated that the survival curve of the participants with an elevated gamma gap was significantly different compared to those with a normal gamma gap, irrespective of which cut-off point was applied.
Discussion
The gamma gap is a useful marker of inflammation, malignancies, and other diseases2; however, its role as a prognostic factor for mortality is not clear. Our study demonstrated that the gamma gap is an independent predictor of all-cause mortality in a study population of nonagenarians and centenarians after a 4-year follow-up. This finding is in accordance with two previous studies with a large sample size4,5. These studies used the data from the National Health Nutrition Examination Survey (NHANES), and the mean ages of the study populations were 41.8 and 46.0 years. Our study expanded the potential prognostic role of the gamma gap for predicting all-cause mortality from nonagenarians and centenarians.
Aging itself is closely associated with chronic low-grade inflammation6,14. This chronically activated inflammation with advancing age has been termed as “inflamm-aging”15. Inflamm-aging has been identified as a risk factor for many age-related diseases (e.g., Alzheimer’s disease, cardiovascular disease, frailty, and sarcopenia) and mortality among older adults6. Therefore, in theory, the gamma gap (as a nonspecific inflammatory marker) should be increased in our study population. In contrast, using the same cut-off point, participants with an elevated gamma gap among our study population (22.5%) was lower than younger adults in a previous study (31.7%)5. One possible reason for this finding might be selective survival bias.
Loprinzi et al.5 recently reported that physical activity might play a beneficial role for reducing all-cause mortality among people with an elevated gamma gap. In addition, many previous studies have revealed the relationship between physical activity and reducing the risk of mortality in different populations16,17. For example, in a prospective cohort study with 204,542 adults aged 45–75 years, an inverse dose-response relationship was identified between physical activity and mortality18. We did not measure physical activity among our study participants; however, our study demonstrated that the participants with an elevated gamma gap were less likely to exercise. One of the proposed mechanisms responsible for this finding is that exercise can reduce systemic inflammation14,19.
One strength of our study is the exploration of optimal cut-off points to define the elevated gamma gap in nonagenarians and centenarians. We found that by using the cut-off points of 2.9, 3.0, 3.1 or 3.2 g/dl (instead of the traditional value of 4.0 g/dl), the elevated gamma gap had similar HRs for predicting long-term mortality in our study population. Previous studies also demonstrated that the cut-off point of 3.1 g/dl was suitable to define an elevated gamma gap in adults2,4,5,10,20. Putting these findings together, we suggest that an elevated gamma gap could also be defined as ≥3.1 g/dl in the oldest adults.
Our study has some limitations. First, because the gamma gap is considered a marker of inflammation, we need to adjust for other inflammatory factors as potential confounders such as C-reactive protein (CRP) or interleukin (IL)-6. We did not include these factors in the Cox proportional hazard models due to a lack of relevant data. Second, as with all cohort studies, selective survival prior to entry in the cohort needs to be considered a potential bias.
Conclusion
The gamma gap is an independent prognostic factor for 4-year all-cause mortality among a study population of nonagenarians and centenarians. The elevated gamma gap can be defined as ≥3.1 g/dl in the oldest adults. Further prospective studies are required to validate these findings in different ethnic populations with various diseases, especially in older adults. The relationship between the gamma gap and disease-specific mortality also needs to be explored.
References
Salt, H. B. Serum globulin fractions in chronic rheumatic diseases; an electrophoretic study. Clin Chem 2, 35–44 (1956).
Edwards, M. K. & Loprinzi, P. D. Atherogenic index of plasma and the gamma gap: Considerations by physical activity. Int J Cardiol 222, 946–948, https://doi.org/10.1016/j.ijcard.2016.08.086 (2016).
Gopal, D. M. et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J 160, 279–285, https://doi.org/10.1016/j.ahj.2010.05.022 (2010).
Juraschek, S. P., Moliterno, A. R., Checkley, W. & Miller, E. R. 3rd The Gamma Gap and All-Cause Mortality. PLoS One 10, e0143494, https://doi.org/10.1371/journal.pone.0143494 (2015).
Loprinzi, P. D. & Addoh, O. The gamma gap and all-cause mortality risk: considerations of physical activity. Int J Clin Pract 70, 625–629, https://doi.org/10.1111/ijcp.12817 (2016).
Fougere, B., Boulanger, E., Nourhashemi, F., Guyonnet, S. & Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. J Gerontol A Biol Sci Med Sci, https://doi.org/10.1093/gerona/glw240 (2016).
Goncalves de Carvalho, C. M. & Ribeiro, S. M. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr, https://doi.org/10.1038/ejcn.2016.177 (2016).
Zhou, Y., Huang, C. Q., Lu, Z. C. & Dong, B. R. Association of serum uric acid with Pro12Ala polymorphism in PPAR-gamma2 among Chinese nonagenarians/centenarians. Aging Clin Exp Res 24, 139–144, https://doi.org/10.3275/8018 (2012).
Yang, M. et al. Body mass index and disability in chinese nonagenarians and centenarians. J Am Med Dir Assoc 15, 303 e301–306, https://doi.org/10.1016/j.jamda.2013.10.011 (2014).
Loprinzi, P. D. Adequate muscular strength may help to reduce mortality risk in those with an elevated gamma gap. Int J Cardiol 218, 47–49, https://doi.org/10.1016/j.ijcard.2016.05.051 (2016).
Liu, H. C. et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. J Am Geriatr Soc 43, 144–149 (1995).
Lu, J., Yang, M., Luo, L., Hao, Q. & Dong, B. Polypharmacy among nonagenarians/centenarians in rural China. Intern Med J 44, 1193–1199, https://doi.org/10.1111/imj.12534 (2014).
Chan, A. C. Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. Journal of aging and health 8, 238–253 (1996).
Woods, J. A., Wilund, K. R., Martin, S. A. & Kistler, B. M. Exercise, inflammation and aging. Aging dis 3, 130–140 (2012).
Franceschi, C. et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128, 92–105, https://doi.org/10.1016/j.mad.2006.11.016 (2007).
Samitz, G., Egger, M. & Zwahlen, M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol 40, 1382–1400, https://doi.org/10.1093/ije/dyr112 (2011).
Hupin, D. et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged >/=60 years: a systematic review and meta-analysis. Br J Sports Med 49, 1262–1267, https://doi.org/10.1136/bjsports-2014-094306 (2015).
Gebel, K. et al. Effect of Moderate to Vigorous Physical Activity on All-Cause Mortality in Middle-aged and Older Australians. JAMA Intern Med 175, 970–977, https://doi.org/10.1001/jamainternmed.2015.0541 (2015).
Warnberg, J., Cunningham, K., Romeo, J. & Marcos, A. Physical activity, exercise and low-grade systemic inflammation. The Proceedings of the Nutrition Society 69, 400–406, https://doi.org/10.1017/S0029665110001928 (2010).
Edwards, M. K. & Loprinzi, P. D. The Association of Estimated Cardiorespiratory Fitness on mortality risk among those with an elevated gamma gap. Int J Cardiol 227, 508–510, https://doi.org/10.1016/j.ijcard.2016.10.113 (2017).
Acknowledgements
The PLAD study was supported by the Sichuan Provincial Science and Technology Department [grant number 2006Z09-006-4] and the Construction Fund for Subjects of West China Hospital of Sichuan University [grant number XK05001]. Additional funding for this secondary analysis was provided by the Sichuan Provincial Science and Technology Department [grant number 2016JY0058]. The sponsors had no role in the design, methods, data collection, analysis, or preparation of this work.
Author information
Authors and Affiliations
Contributions
M.Y., L.L.X. and J.J.J. wrote the manuscript; M.Y. and B.R.D. conceived and designed the study; J.J.J., L.L.X., X.L., and Q.K.H. recruited the subjects; M.Y. and Q.K.H. analyzed the data. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, M., Xie, L., Liu, X. et al. The gamma gap predicts 4-year all-cause mortality among nonagenarians and centenarians. Sci Rep 8, 1046 (2018). https://doi.org/10.1038/s41598-018-19534-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19534-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.