Abstract
Both viral and bacterial infections can be associated with wheezing episodes in children; however, information regarding combined infections with both viral and bacterial pathogens in full term neonates is limited. We sought to investigate the effects of viral–bacterial codetection on pneumonia severity and recurrent wheezing. A retrospective cohort study was conducted on neonates admitted to our hospital with pneumonia from 2009 to 2015. Of 606 total cases, 341 were diagnosed with RSV only, and 265 were diagnosed with both RSV and a potential bacterial pathogen. The leading four species of bacteria codetected with RSV were Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus and Enterobacter cloacae. Neonates with RSV and a potential bacterial pathogen were significantly more likely to have worse symptoms, higher C-reactive protein values and more abnormal chest x-ray manifestations with Bonferroni correction for multiple comparisons (P < 0.01). On Cox regression analysis, an increased risk of recurrent wheezing was found for neonates positive for RSV–Staphylococcus aureus and RSV–Klebsiella pneumoniae. Our findings indicate that the combination of bacteria and RSV in the neonatal airway is associated with more serious clinical characteristics. The presence of RSV and Staphylococcus aureus or Klebsiella pneumoniae may provide predictive markers for wheeze.
Similar content being viewed by others
Introduction
Pneumonia is the primary infectious cause of death worldwide in children under five years of age. The greatest risk of death from pneumonia occurs in the neonatal period1,2. It is estimated that 0.136 million neonatal deaths were caused by pneumonia in 20133.
Some studies have reported increasing detection rates for mixed viral–bacterial infections in children with community-acquired pneumonia4,5 and have also found an interplay between viruses and bacteria. Viral respiratory infections can elevate nasopharyngeal bacterial colonization density and promote bacterial infections in children6,7,8. In addition, respiratory viruses play an important role in wheezing episodes which may mark the beginning of asthma9,10,11. Recently, bacterial colonization has also been found to be associated with wheezing and asthma6,12. Other research has focused primarily on children with low birth weights13, whereas the interaction of pulmonary viruses and bacteria are relatively unclear in full term neonates. Nevertheless, it has been hypothesized that adverse exposures in early postnatal life might influence lung growth and development, and lead to persistently smaller airways and impaired lung function. Therefore, in this study, we investigated the effect of viral–bacterial codetection on pneumonia severity in full term neonates, and sought to determine if codetection increases the risk of recurrent wheezing, rhinitis and eczema.
Patients and Methods
Patients
This was a retrospective cohort study and conducted at Children’s Hospital of Chongqing Medical University, a tertiary university hospital that has a 214-bed neonatal unit with an annual admission rate of over 6000 neonates. Inclusion criteria included the following: 1) hospitalized neonates (≤28 days of age) with an admitting diagnosis of clinical pneumonia between Jan 2009 and Dec 2015, and 2) respiratory samples collected within 24 hours of admission were positive for a viral respiratory pathogen. Exclusion criteria included the following: (1) premature infants (gestational age <37 weeks at birth); and (2) neonates with congenital malformation, immunodeficiency, severe haemolytic disease, or severe malnutrition. Clinical pneumonia was defined by the presence of a clinical sign (such as cough, wheeze, phlegm production, tachypnoea, cyanosis, or fever) and rales or rhonchi on chest auscultation14,15. Chest x-ray showing lung consolidation or infiltrate was used to diagnose pneumonia. We followed up the included neonates until December 31, 2016. Follow-up times ranged from one to seven years. Patients with incomplete data were excluded from data analysis.
This study was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University (file number: 62/2017). Informed consent was obtained from the parents when patients were admitted to the hospital. All methods were performed in accordance with the relevant guidelines and regulations.
Methods
Sample collection
Nasopharyngeal aspirates were taken for viral testing16, and sputum samples were obtained for bacterial culture6. Within 2 hours of collection, the samples were transported to the microbiology laboratory, and the sputum samples were cultured on chocolate and blood agar plates for bacteria. Viral antigens for respiratory syncytial virus (RSV); adenovirus (ADV); influenza virus A (IVA); influenza virus B (IVB); and parainfluenza virus (PIV) I, PIV II, and PIV III were tested for using direct immunofluorescence16. Codetection was defined as a positive detection of >1 clinically–relevant microbe.
Data collection
Data on patient demographics, microbiology results, laboratory parameters and chest x-ray findings were obtained from the hospital electronic medical records system. We used questionnaires to obtain information on the subsequent development of recurrent wheezing, rhinitis and eczema during the first 3 years of life. The questionnaires were based on the Japanese version of the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three Questionnaire17. During the follow-up period, we called parents and asked them questions according to the questionnaire. Wheeze was defined as wheezing or whistling sounds, persistent troublesome cough or breathlessness6. A wheezing episode was defined as a respiratory episode with wheezing for more than 1 day. The interval between two episodes was defined as a period of at least 7 days without respiratory symptoms. Recurrent wheezing was defined as having three or more episodes of wheezing18. Survival time of wheeze for each child was defined as time from neonatal pneumonia to the first occurrence of wheeze.
Statistical analysis
Continuous variables were analysed using the Mann–Whitney U test because data were not normally distributed. Categorical variables were analysed using the chi-square test, and multiple comparisons were adjusted using the Bonferroni correction (P < 0.01). The association between codetection of virus with bacteria, covariates, and subsequent development of recurrent wheezing, rhinitis and eczema were assessed by logistic regression analysis to estimate odds ratios (ORs). Considering the inconsistent follow-up time, survival statistics were used to analyse the risk of recurrent wheezing. The cumulative risks of recurrent wheezing during the first 3 years stratified according to codetection by RSV and bacteria were estimated by the Kaplan–Meier estimator. Changes in risk were quantified by Cox regression.
Continuous variables were reported as medians (25th–75th percentiles), and categorical variables as frequencies (percentages). Statistical analyses were performed using the IBM Statistical Package for Social Sciences (SPSS) version 21. P < 0.05 was considered statistically significant.
Data availability statement
Requests for materials should be addressed to Q.L (email: qilu_qi@163.com)
Results
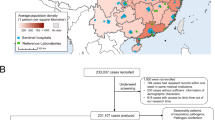
During the study period, a total of 27,169 neonates were admitted to the hospital for clinical pneumonia. Of these, 8128 (29.9%) had nasopharyngeal aspirates collected to check for respiratory viruses within 24 hours of admission.
Detections and Codetections
Among the 860 cases with positive virus detection, RSV (810; 94.2%) was most frequently detected. In RSV-positive patients, Escherichia coli (98/12.1%), Klebsiella pneumoniae (81/10.0%), Staphylococcus aureus (53/6.5%) and Enterobacter cloacae (33/4.1%) were the most commonly detected bacteria (Fig. 1).
Enrollment and outcomes. We studied 606 neonates, 52 (8.6%) neonates were lost to follow up, 554 neonates had follow-up results. n0 = number of neonates who were followed up, n1 = number of neonates who had recurrent wheezing. n2 = number of neonates who had allergic rhinitis, n3 = number of neonates who had eczema.
Other viruses were identified in 50 cases, including 33 samples with parainfluenza virus, 13 with influenza virus and 4 with adenovirus. Escherichia coli was codetected with these viruses more often than other bacteria, with a total of 11 cases.
606 neonates were followed up, and 554 (91.4%) of them had follow-up results during the first 3 years of life.
Clinical Characteristics
In consideration of sample size, RSV-positive cases were further analysed. We assessed whether the main four species of codetected bacteria in RSV-positive neonates influenced disease severity. There were no significant differences in the baseline characteristics of age, sex, weight, gestational age at birth, birth weight, and caesarean section rate between RSV-positive neonates with and without bacterial pathogen codetection with Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, or Enterobacter cloacae. Furthermore, the rates of nasal obstruction, cough, cyanosis, moist rales and diarrhoea did not differ between the groups.
Neonates with codetection of RSV and a potential bacterial pathogen were significantly more likely to present with shortness of breath (P < 0.001), wheezing (P = 0.008), chest retraction (P = 0.009) and a higher oxygen requirement (P < 0.001), higher C-reactive protein values (P < 0.001) and more abnormal chest x-rays (P < 0.001) than those with detection of RSV only.
Neonates with codetection of RSV and Staphylococcus aureus had higher white blood cell counts, higher blood platelet counts, and a higher incidence of fever. However, clinical symptoms and laboratory tests were not significantly different between the RSV only and the RSV–Enterobacter cloacae group.
After adjustment for multiple comparisons by Bonferroni correction (P < 0.01), neonates with RSV and a potential bacterial pathogen were significantly more likely to have worse symptoms, higher C-reactive protein values and more abnormal chest x-ray manifestations (Table 1).
Codetection in relation to recurrent wheezing, rhinitis and eczema
After controlling for confounding variables with logistic regression analysis, neonates with codetection of RSV–Staphylococcus aureus and RSV–Klebsiella pneumoniae had increased risks of recurrent wheezing (OR 7.16, 95% CI, 2.70–18.94, P < 0.001 and OR 3.66; 95% CI, 1.49–9.94, P = 0.005; respectively) compared with those with RSV only. There was no statistically significant difference among the groups in the risks of rhinitis and eczema (Fig. 2).
Odds ratio for recurrent wheezing, allergic rhinitis and eczema. Logistic regression analysis: According to the results of telephone interview of RSV only and RSV with bacteria. Odds ratios were adjusted for the following possible confounders: sex, caesarean section, allergic history, family history of allergies, educational background of mother, living conditions, antibiotic therapy before admission, feeding option, follow-up time, age, gestational age at birth, weight, birth weight. (●P<0.05).
Survival analyses for risk factors associated with recurrent wheezing during the first 3 years of life
The risk of recurrent wheezing was increased in neonates with codetection of RSV–Staphylococcus aureus and RSV–Klebsiella pneumoniae, but not in those with codetection of RSV–Escherichia coli or RSV–Enterobacter cloacae (Table 2). Those with codetection of RSV–Staphylococcus aureus were 4.22 times more likely to have recurrent wheezing than RSV only cases (HR, 4.22; 95% CI, 2.02–8.83). The hazard ratio for the codetection of RSV–Klebsiella pneumoniae was 3.15 (95% CI, 1.51–6.57) for recurrent wheezing.
In the Kaplan–Meier analysis, the risk of recurrent wheezing associated with codetection of RSV–Staphylococcus and RSV–Klebsiella pneumoniae continually increased during the first 3 years of life (Fig. 3). There was no significant difference between the risks of recurrent wheezing for RSV–Klebsiella pneumoniae and RSV–Staphylococcus aureus.
Discussion
RSV was the predominant viral pathogen seen in our study, consistent with results from other studies19,20. RSV was detected in 10% of neonatal pneumonia samples, however, other studies have shown RSV to be present in about 22%–29% of all acute lower respiratory tract infection cases19,20. This could be dueto the fact that we only included full term infants in our study. Infants are most susceptible to RSV infections at 6–11 months of age, when they lack protection from maternal immunoglobulins19.
Several previous studies have shown an association between viruses and bacteria. Hishiki et al. reported that Haemophilus influenzae, Streptococcus pneumoniae and Moraxella catarrhalis were most frequently isolated from paediatric inpatients having RSV bronchopulmonary infection in Japan21. Menno R et al. observed a positive association between RSV and Haemophilus influenzae in the upper respiratory tract of children <2 years of age in the Netherlands22. Our findings differed from those studies, showing RSV was more likely to be codetected with Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus and Enterobacter cloacae. This may be due to differences in geography and population, as the most common gram-negative organisms in China are Escherichia coli, Klebsiella pneumoniae and Haemophilus influenzae, and the most common gram-positive organism is Staphylococcus aureus14.
In our study, codetection of RSV and potential bacterial pathogens contributed to enhanced symptoms of disease, higher C-reactive protein values and more abnormal chest x-ray manifestations. These findings suggest that during RSV infection, these specific bacterial pathogens were cofactors that contributed to the severity of respiratory symptoms and inflammatory reactions. One possible explanation for this pattern was that virally induced inflammation might alter bacterial gene expression, leading to a more pathogenic phenotype, increased bacterial virulence and decreased clearance of bacteria23,24.
The incidence of wheezing is quite high in the first 3 years of life25, and RSV might be the initiator (post-RSV wheezing disorder). Notably, there is increasing evidence that the combination of specific bacteria and rhinovirus is associated with wheezing and asthma. Previous studies showed that the detection of rhinovirus together with M. catarrhalis and S. pneumoniae increased the risk of asthma exacerbations in children 4–12 years of age26. In our study, cases with codetection of Staphylococcus aureus and Klebsiella pneumoniae with RSV were associated with an increased risk of recurrent wheezing compared with RSV only cases in children from birth to age 3. This may result from the fact that specific bacteria are involved in allergic inflammation and may induce immunomodulatory effects and a Th2-type of eosinophilic inflammation in patients with allergic disease12,27.
To our knowledge, this study is the first to research both viral and bacterial detection in full term neonates, and we found a relationship between RSV and specific bacteria. However, some potential study limitations need to be discussed. First, single-centre retrospective cohort analysis has known inherent limitations, and further prospective multi-centre studies are recommended. Second, we had no information on viral and bacterial loads, and quantitative analysis could not be performed in our study. Third, sicker neonates were more likely to have hospital-associated acquisition of bacteria, and, therefore, the bacteria could simply be a marker of disease severity and sequelae. Furthermore, it is worth noting that detection of bacteria in sputum samples might not indicate causative agents. Bacteria may be contaminants from the nasopharynx, because healthy infants often carry pathogenic bacteria there. Thus, because we must be cautious in using the terms ‘causative agent’ or definite ‘coinfection’, we used ‘codetected’ to state the relationship between the detected bacteria and viruses in our study.
Conclusion and Prospection
The combination of bacteria and RSV in the neonatal airway was found to be associated with an additive effect that resulted in more serious clinical symptoms. The presence of RSV and Staphylococcus aureus or Klebsiella pneumoniae in the airway were associated with an increased risk of recurrent wheezing in early life and may provide a predictive marker for wheezing.
References
Duke, T. Neonatal pneumonia in developing countries. Arch Dis Child Fetal Neonatal Ed. 90, F211–219 (2005).
Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 388, 1459–1544 (2016).
Liu, L. et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 385, 430–440 (2015).
Wei, L. et al. Detection of viral and bacterial pathogens in hospitalized children with acute respiratory illnesses, Chongqing, 2009-2013. Medicine (Baltimore). 94, e742 (2015).
Honkinen, M., Lahti, E., Österback, R., Ruuskanen, O. & Waris, M. Viruses and bacteria in sputum samples of children with community-acquired pneumonia. Clin Microbiol Infect. 18, 300–307 (2012).
Bisgaard, H. et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 357, 1487–1495 (2007).
McGillivary, G., Mason, K. M., Jurcisek, J. A., Peeples, M. E. & Bakaletz, L. O. Respiratory syncytial virus-induced dysregulation of expression of a mucosal beta-defensin augments colonization of the upper airway by non-typeable Haemophilus influenzae. Cell Microbiol. 11, 1399–1408 (2009).
Wolter, N. et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. 210, 1649–1657 (2014).
Hall, C. B. et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 360, 588–598 (2009).
Busse, W. W., Lemanske, R. F. & Gern, J. E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 376, 826–834 (2010).
Hansbro, N. G., Horvat, J. C., Wark, P. A. & Hansbro, P. M. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 117, 313–353 (2008).
Davis, M. F., Peng, R. D., McCormack, M. C. & Matsui, E. C. Staphylococcus aureus colonization is associated with wheeze and asthma among US children and young adults. J Allergy Clin Immunol. 135, 811–813.e5 (2015).
der Voort AM, S. et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 133, 1317–1329 (2014).
Wang, H. et al. Neonatal community-acquired pneumonia: pathogens and treatment. J Paediatr Child Health. 46, 668–672 (2010).
Vissing, N. H., Chawes, B. L. & Bisgaard, H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med. 188, 1246–1252 (2013).
Lahti, E. et al. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax. 64, 252–257 (2009).
Asher, M. I. et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 368, 733–743 (2006).
Blanken, M. O. et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 368, 1791–1799 (2013).
Nair, H. et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 375, 1545–1555 (2010).
Rudan, I. et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 3, 010401 (2013).
Hishiki, H. et al. Incidence of bacterial coinfection with respiratory syncytial virus bronchopulmonary infection in pediatric inpatients. J Infect Chemother. 17, 87–90 (2011).
van den Bergh, M. R. et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One. 7, e47711 (2012).
Smith, C. M. et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am J Respir Crit Care Med. 190, 196–207 (2014).
Loh, E. et al. Temperature triggers immune evasion by Neisseria meningitidis. Nature. 502, 237–240 (2013).
Matricardi, P. M. et al. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J. 32, 585–592 (2008).
Kloepfer, K. M. et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 133, 1307.e1–3 (2014). 1301–1307.
Tomassen, P. et al. Staphylococcus aureus enterotoxin-specific IgE is associated with asthma in the general population: a GA(2)LEN study. Allergy. 68, 1289–1297 (2013).
Acknowledgements
This work was supported by the Natural Science Foundation of Chongqing, China [grant number cstc2014jctjA10052], the Affiliated Children’s Hospital of Chongqing Medical University Clinical Research Projects [grant number lcyj2014-8] and the Health and Family Planning Commission of Chongqing Medical Research Project [grant number 2017HRBC008].
Author information
Authors and Affiliations
Contributions
Qi Lu conceived and designed the study. Qin Zhong, Hui Feng, Xu Liu, Qi Zhao and Yue Du extracted the data. Hui Feng and Jia-Rong Wang analyzed the data. Qi Lu and Qin Zhong wrote the manuscript. Hui Feng and Xian-Hong Zhang prepared the figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, Q., Feng, H., Lu, Q. et al. Recurrent wheezing in neonatal pneumonia is associated with combined infection with Respiratory Syncytial Virus and Staphylococcus aureus or Klebsiella pneumoniae. Sci Rep 8, 995 (2018). https://doi.org/10.1038/s41598-018-19386-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19386-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.