Abstract
Adenosine A2A receptor antagonists are an alternative treatment strategy for Parkinson’s disease. Several randomized placebo controlled studies have tested the effect of A2A receptor antagonist istradefylline, and more robust evidence has been acquired. This meta-analysis aimed to provide evidence for its efficacy and safety on patients with Parkinson’s disease. After a systematic literature search, we calculated the pooled standardized mean difference and risk ratio for continuous and dichotomous variables, respectively. Further, sensitivity analyses were performed to confirm the effect estimated by meta-analyses. Publication bias was assessed by funnel plot and deviation of intercept. Six studies satisfied our inclusion criteria. Istradefylline (40 mg/day) decreased off time and improved motor symptoms of Parkinson’s disease in homogeneous studies. Istradefylline at 20 mg/day decreased off time and improved motor symptoms, but heterogeneity was found in the analysis of the former among studies. There was a significant effect of istradefylline on dyskinesia in homogeneous studies. Publication bias, however, was observed in the comparison of dyskinesia. Other adverse events showed no significant difference. The present meta-analysis suggests that istradefylline at 40 mg/day could alleviate off time and motor symptoms derived from Parkinson’s disease. Dyskinesia might be worsened, but publication bias prevents this from being clear.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is characterized by degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc), which induces motor symptoms including tremor, rigidity, akinesia, bradykinesia, and postural instability. A reduced concentration of dopamine in the striatum induces hyperactivation of the globus pallidus internus via inhibition of the direct pathway and excitation of the indirect pathway. The motor output from the striatum is considered to consist of direct and indirect pathways1, which mainly express dopamine D1 and D2 receptors, respectively. Recent transgenic mouse models have allowed for confirmation of the existence of two distinct pathways2,3.
Patients with PD are usually treated with dopamine-related drugs including levodopa, monoamine oxidase B inhibitors and dopamine agonists, which in turn increase the risk of motor and non-motor complications4,5,6,7. Non-dopaminergic agents are thus needed for improving PD therapy and limiting side effects. Caffeine, a non-specific adenosine A2A receptor antagonist, could reduce the risk of the onset of PD and subsequent dyskinesia caused by long-term dopaminergic drug therapy8,9,10. In this context, the A2A receptor antagonist istradefylline was originally developed to address motor and non-motor complications related to advanced use of dopaminergic drugs.
The effect of istradefylline was tested in several randomized placebo-controlled studies11,12,13,14,15,16,17, and was validated by other meta-analyses18,19. However, previous meta-analyses calculated a summary effect using the mean difference without standardization, although different estimators and subjects were involved in each study. In addition, an assessment of tolerability and publication bias and sensitivity analyses, were not performed. Furthermore, the first published meta-analysis estimated a summary effect using only three studies for each dosage, and excluded the work of Stacy et al.17 in the analysis of the effect of istradefylline (20 mg/day) on off time18. The second published meta-analysis combined all studies regardless of dosage, and did not assess adverse events19. To more robustly analyze the evidence for use of istradefylline, a detailed and systematic meta-analysis was performed.
Methods
The general methodology is comparable to our previously published meta-analyses20,21.
Study Selection
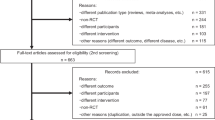
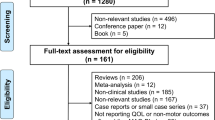
Inclusion criteria in the present meta-analysis comprised the following: (1) 20 mg/day or 40 mg/day istradefylline use for PD; (2) placebo-controlled randomized trial with more than 10 subjects in each group; (3) assessment of off time or unified Parkinson’s disease rating scale (UPDRS) III during the on period; (4) written in English. A systematic literature search of PubMed, Web of Science and Cochrane Library was performed in May 2016 using the following syntax: (“Parkinson’s disease” or “PD”) and (“Istradefylline”) and (“randomized,” “random,” or “randomly”). As indicated in Fig. 1, six studies were finally included in the present meta-analysis. We contacted the corresponding author if incomplete data were detected. Three researchers independently performed the above-mentioned search and study selection. Finally, we resolved any discrepancies after discussion. Risk of bias was evaluated by the Cochrane Collaboration’s tool for risk of bias.
Data Synthesis and Statistics
Detailed analysis methods are described in our previously published meta-analysis20. Briefly, we used the standardized mean difference (SMD) between the istradefylline and placebo groups, considering off time, UPDRS III score during the on phase, and UPDRS II score, to assess the effect of istradefylline 12 weeks after treatment. We estimated standard deviation (SD) for change from baseline based on a 95% confidence interval (CIs). In contrast to continuous data, a pooled risk ratio (RR) along with 95% CIs was calculated for dichotomous data. We investigated heterogeneity of the included studies with I-squared (I2). Adverse events described in more than 3 papers were defined as the targeted event. Sensitivity analyses were performed to establish robust evidence if there was a significant difference. All analyses were performed using Review Manager (RevMan 5.2) for Windows (http://ims.cochrane.org/revman) and R software (http://www.r-project.org/).
Publication bias
In case of significant differences, publication bias was assessed by visual inspection and Egger’s test as described previously22.
Results
Study Characteristics and Risk of bias
Six placebo-controlled randomized studies met our inclusion criteria (n = 1175 istradefylline subjects, and n = 643 placebo subjects). The summary of the included studies is shown in supporting Table 1.
In terms of risk of bias, we cannot exclude the possibility of selection bias in several studies owing to a lack of description (please see supporting Fig. 1).
Off time and UPDRS III
For off time, the patients on 20 mg/day istradefylline showed significant improvement across heterogeneous studies (SMD = −0.23, 95% confidence interval (CI) = −0.40 to −0.06, P = 0.009, I2 = 55%, Fig. 2A). This heterogeneity disappeared when “Pourcher 2012” was excluded (I2 = 0%, Fig. 2B). In contrast, at 40 mg/day, istradefylline reduced off time across homogeneous studies (SMD = −0.28, 95% CI = −0.44 to −0.12, P = 0.0005, I2 = 34%, Fig. 2A). The significant effect of 20 mg/day istradefylline was lost without “Mizuno 2010” or “Mizuno 2013” (P = 0.06, supporting Table 2), but that of 40 mg/day was observed regardless of any exclusions (“LeWitt 2008,” P = 0.009; “Mizuno 2010,” P = 0.01; “Mizuno 2013,” P = 0.01; “Pourcher 2012,” P < 0.00001; supporting Table 2).
Forest plots of the standardized mean difference in off time. (A) Istradefylline 40 mg/day showed a significant reduction of off time in homogeneous studies. In contrast, istradefylline 20 mg/day showed a significant reduction of off time in heterogeneous studies. (B) Heterogeneity disappeared in istradefylline 20 mg/day use without “Pourcher 2012.”
Istradefylline improved UPDRS III at on phase regardless of daily dose (20 mg, SMD = −0.15, 95% CI = −0.27 to −0.02, P = 0.02; 40 mg, SMD = −0.24, 95% CI = −0.37 to −0.11, P = 0.0002; Fig. 3A). These studies were homogeneous (I2 = 0%, Fig. 3A). Furthermore, sensitivity analysis revealed a robust effect of 40 mg/day istradefylline (“LeWitt 2008,” P < 0.0001; “Mizuno 2010,” P = 0.008; “Mizuno 2013,” P = 0.01; “Pourcher 2012,” P = 0.01; supporting Table 2). In contrast, there was no significant effect of istradefylline 20 mg/day without “Hauser 2008,” “Mizuno 2010,” or “Mizuno 2013” (“Hauser 2008,” P = 0.09; “Mizuno 2010,” P = 0.17; “Mizuno 2013,” P = 0.11; supporting Table 2).
Forest plots of the standardized mean difference in unified Parkinson’s disease rating scale III (UPDRS III) and the pooled risk ratio of dropout. (A) Istradefylline improved UPDRS III regardless of any dosage in homogeneous studies. (B) There was no significant difference of the incidence of dropout between placebo and istradefylline. The included studies were homogeneous.
No subgroup difference in off time or UPDRS III was seen between 20 mg/day and 40 mg/day istradefylline, which suggested that there was no dose effect (off time, I2 = 0%, Fig. 2A; UPDRS III, I2 = 2.0%; Fig. 3A).
Tolerability
No significant difference was found between istradefylline and placebo groups in regard to dropouts (20 mg/day, P = 0.98; 40 mg/day, P = 0.99; Fig. 3B).
UPDRS II
Istradefylline did not improve UPDRS II scores at any daily doses (20 mg, P = 0.64; 40 mg, P = 0.44; please see supporting Fig. 2).
Adverse Events
Eight adverse events which satisfied our inclusion criteria were detected as targets. The aggravation of dyskinesia occurred more frequently in the istradefylline group than the placebo group (RR = 1.72, 95% CI = 1.26 to 2.34, P = 0.0007, Fig. 4A). The included studies were homogeneous (I2 = 29%, Fig. 4A). A significant effect of istradefylline was observed regardless of any exclusion (“Hauser 2008,” P = 0.005; “LeWitt 2008,” P < 0.006; “Mizuno 2010,” P = 0.002; “Mizuno 2013,” P = 0.002; “Pourcher 2012,” P < 0.0001; “Stacy 2008,” P = 0.004; supporting Table 2). The other motor symptom, tremor, showed no significant difference between istradefylline and placebo groups (RR = 0.81, 95% CI = 0.23 to 2.87, P = 0.75, Fig. 4B). There was no significant effect of istradefylline on the other adverse events (nausea, P = 0.41; constipation, P = 0.14; hallucination, P = 0.12; insomnia, P = 0.81; somnolence, P = 0.94; accident, P = 0.28, please see supporting Fig. 3).
Publication bias
No clear asymmetry of funnel plots was identified, and there were no significant deviations of intercept in all outcomes except for dyskinesia (off time 20 mg/day, P = 0.12; off time 40 mg/day, P = 0.19; UPDRS III 20 mg/day, P = 0.16; UPDRS III 40 mg/day, P = 0.34). For dyskinesia, the funnel plot appeared asymmetrical (dyskinesia, P = 0.007, please see supporting Fig. 4).
Discussion
Our meta-analysis revealed that 40 mg/day istradefylline could improve off time and motor symptoms, and both findings were supported by a sensitivity analysis. In terms of the effect of 20 mg/day istradefylline on off time, however, heterogeneity of the included studies was detected, but its cause remained unknown. Furthermore, we failed to provide robust evidence for 20 mg/day istradefylline for off time or UPDRS III using a sensitivity analysis. It was noted that “Pourcher 2012” contributed to significant heterogeneity for the effect of 20 mg/day istradefylline on off time. The characteristics of “Pourcher 2012” related to this included increased dosage of daily levodopa intake with a wider range, more men, and a longer duration compared to the other studies, which suggests that they analyzed effects in a more advanced stage of PD. These factors could thus affect the results. Taken together, istradefylline could be recommended for use in the early stages of PD. Given the results of the sensitivity analyses, it was possible that the effect of 20 mg/day istradefylline was smaller than that of 40 mg/day. In such a case, istradefylline at 20 mg/day produced variable results owing to the different background of each study, whereas istradefylline at 40 mg/day stably improved off time and motor symptoms. However, considering significant but weak effect on these symptoms, istradefylline appeared suitable for adjuvant therapy at advanced stage in clinical practice.
Istradefylline was also administered at the advanced stage when dyskinesia was seen as motor complication owing to levodopa treatment. PD patients with levodopa-induced dyskinesia were reported to show elevated A2A receptor binding23. Given this finding, A2A receptor antagonists would be expected not to worsen dyskinesia. However, the exaggeration of dyskinesia was demonstrated by our meta-analysis; at the same time, publication bias could have contributed to this effect. Additional studies need to be performed to confirm the effect of istradefylline on levodopa-induced dyskinesia. There was no difference of the proportion of dropouts between istradefylline and placebo groups, which might indicate adequate tolerability in spite of the aggravation of dyskinesia.
No non-motor symptoms were worsened by istradefylline as in supporting Fig. 3. Administration of istradefylline was reported to improve overactive bladder and daytime sleepiness24,25, however, these were not randomized placebo-controlled trials. Further studies are needed to establish robust evidence of efficacy of istradefylline on non-motor symptoms.
A previous meta-analysis reported that coffee could reduce the risk of PD9. Caffeine is considered a nonspecific A2A receptor antagonist8, and istradefylline is expected to have a similar effect to caffeine on neurons, i.e. a neuroprotective effect. Indeed, several papers demonstrated that A2A receptor antagonists prevented neurodegeneration in dopaminergic neurons in MPTP and 6-OHDA rodent models of PD26,27. Deletion of the A2A receptor inhibited loss of dopamine in the striatum, and of dopaminergic neurons in the SNc8. Furthermore, there was a tendency for caffeine to reduce the risk of developing dyskinesia in an observational study10. In this sense, istradefylline could be recommended for use before onset of dyskinesia or wearing off, to promote disease modification owing to neuroprotection of dopaminergic neurons; this is the case despite all previous randomized controlled trials (RCT) targeting patients with PD in an advanced phase.
Although istradefylline was known to block A2A receptors in the indirect pathway, the network-level mode of action for istradefylline has remained an enigma. Prior reports identified abnormal brain networks in PD28,29, for which the elucidation of the effect of istradefylline could lead to the development of novel adenosinergic therapies.
The previous meta-analysis reported significant effect of 40 mg/day istradefylline on reduction of off time with heterogeneous studies, but failed to demonstrate that 20 mg/day istradefylline could improve motor symptoms18. Additional studies and usage of standardized value allowed us to reveal significant effects of istradefylline with homogeneous studies. The assessment of a funnel plot revealed that summary effect of istradefylline on dyskinesia could be biased by publication. Moreover, the present research added new evidence of favorable tolerability.
This meta-analysis had multiple limitations. First, it was possible that limiting the search to studies written in English, and the modest number of included studies, might contribute to bias. Second, Egger’s test detected publication bias in the analysis of dyskinesia. Third, the included studies examined the short-term effect of istradefylline on patients with PD. One open-label study showed the long-term effect of istradefylline30, but we require an RCT with long-term follow up to reveal the longitudinal outcome including the putative neuroprotective effect.
In summary, the present meta-analysis has provided robust evidence that istradefylline at 20 mg/day improves UPDRS III, and at 40 mg/day improves both off time and UPDRS III. The latter findings were further supported by sensitivity analyses. There was no significant difference in dropout ratio between istradefylline and placebo groups, which suggested this drug was well tolerated. However, dyskinesia could be worsened, but publication bias was detected, leaving the issue murky. Istradefylline at 40 mg/day is the only drug to have meta-analysis-based evidence among non-dopaminergic drugs potentially useful in PD, and is recommended for administration in the early phases of PD, or at least before the onset of motor complications.
References
Alexander, G. E. & Crutcher, M. D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences 13, 266–271 (1990).
Kravitz, A. V. et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466, 622–626, https://doi.org/10.1038/nature09159 (2010).
Gerfen, C. R. & Surmeier, D. J. Modulation of striatal projection systems by dopamine. Annual review of neuroscience 34, 441–466, https://doi.org/10.1146/annurev-neuro-061010-113641 (2011).
Chondrogiorgi, M., Tatsioni, A., Reichmann, H. & Konitsiotis, S. Dopamine agonist monotherapy in Parkinson’s disease and potential risk factors for dyskinesia: a meta-analysis of levodopa-controlled trials. European journal of neurology: the official journal of the European Federation of Neurological Societies 21, 433–440, https://doi.org/10.1111/ene.12318 (2014).
Stocchi, F. et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Annals of neurology 68, 18–27, https://doi.org/10.1002/ana.22060 (2010).
Parkinson Study Group CALM Cohort investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Archives of neurology 66, 563–570, https://doi.org/10.1001/archneur.66.1.nct90001 (2009).
Moore, T. J., Glenmullen, J. & Mattison, D. R. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA internal medicine 174, 1930–1933, https://doi.org/10.1001/jamainternmed.2014.5262 (2014).
Kachroo, A. & Schwarzschild, M. A. Adenosine A2A receptor gene disruption protects in an alpha-synuclein model of Parkinson’s disease. Annals of neurology 71, 278–282, https://doi.org/10.1002/ana.22630 (2012).
Hernan, M. A., Takkouche, B., Caamano-Isorna, F. & Gestal-Otero, J. J. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Annals of neurology 52, 276–284, https://doi.org/10.1002/ana.10277 (2002).
Wills, A. M. et al. Caffeine consumption and risk of dyskinesia in CALM-PD. Movement disorders: official journal of the Movement Disorder Society 28, 380–383, https://doi.org/10.1002/mds.25319 (2013).
Fernandez, H. H. et al. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism & related disorders 16, 16–20, https://doi.org/10.1016/j.parkreldis.2009.06.008 (2010).
Hauser, R. A. et al. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Movement disorders: official journal of the Movement Disorder Society 23, 2177–2185, https://doi.org/10.1002/mds.22095 (2008).
LeWitt, P. A. et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Annals of neurology 63, 295–302, https://doi.org/10.1002/ana.21315 (2008).
Mizuno, Y. & Kondo, T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society 28, 1138–1141, https://doi.org/10.1002/mds.25418 (2013).
Mizuno, Y., Hasegawa, K., Kondo, T., Kuno, S. & Yamamoto, M. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Movement disorders: official journal of the Movement Disorder Society 25, 1437–1443, https://doi.org/10.1002/mds.23107 (2010).
Pourcher, E. et al. Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism & related disorders 18, 178–184, https://doi.org/10.1016/j.parkreldis.2011.09.023 (2012).
Stacy, M. et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70, 2233–2240, https://doi.org/10.1212/01.wnl.0000313834.22171.17 (2008).
Chen, W., Wang, H., Wei, H., Gu, S. & Wei, H. Istradefylline, an adenosine A(2)A receptor antagonist, for patients with Parkinson’s Disease: a meta-analysis. Journal of the neurological sciences 324, 21–28, https://doi.org/10.1016/j.jns.2012.08.030 (2013).
Tao, Y. & Liang, G. Efficacy of adenosine A2A receptor antagonist istradefylline as augmentation for Parkinson’s disease: a meta-analysis of randomized controlled trials. Cell biochemistry and biophysics 71, 57–62, https://doi.org/10.1007/s12013-014-0162-7 (2015).
Sako, W., Miyazaki, Y., Izumi, Y. & Kaji, R. Which target is best for patients with Parkinson’s disease? A meta-analysis of pallidal and subthalamic stimulation. Journal of neurology, neurosurgery, and psychiatry 85, 982–986, https://doi.org/10.1136/jnnp-2013-306090 (2014).
Sako, W., Murakami, N., Izumi, Y. & Kaji, R. Reduced alpha-synuclein in cerebrospinal fluid in synucleinopathies: evidence from a meta-analysis. Movement disorders: official journal of the Movement Disorder Society 29, 1599–1605, https://doi.org/10.1002/mds.26036 (2014).
Sako, W., Murakami, N., Izumi, Y. & Kaji, R. The difference of apparent diffusion coefficient in the middle cerebellar peduncle among parkinsonian syndromes: Evidence from a meta-analysis. Journal of the neurological sciences 363, 90–94, https://doi.org/10.1016/j.jns.2016.02.034 (2016).
Ramlackhansingh, A. F. et al. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 76, 1811–1816, https://doi.org/10.1212/WNL.0b013e31821ccce4 (2011).
Kitta, T. et al. Clinical efficacy of istradefylline on lower urinary tract symptoms in Parkinson’s disease. International journal of urology: official journal of the Japanese Urological Association 23, 893–894, https://doi.org/10.1111/iju.13160 (2016).
Suzuki, K. et al. Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: An open-label, 3-month study. Journal of the neurological sciences 380, 230–233, https://doi.org/10.1016/j.jns.2017.07.045 (2017).
Ikeda, K., Kurokawa, M., Aoyama, S. & Kuwana, Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. Journal of neurochemistry 80, 262–270 (2002).
Kalda, A., Yu, L., Oztas, E. & Chen, J. F. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson’s disease. Journal of the neurological sciences 248, 9–15, https://doi.org/10.1016/j.jns.2006.05.003 (2006).
Vo, A. et al. Parkinson’s disease-related network topographies characterized with resting state functional MRI. Human brain mapping. https://doi.org/10.1002/hbm.23260 (2016).
Mattis, P. J. et al. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology 87, 1925–1933, https://doi.org/10.1212/wnl.0000000000003285 (2016).
Kondo, T. & Mizuno, Y. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clinical neuropharmacology 38, 41–46, https://doi.org/10.1097/wnf.0000000000000073 (2015).
Acknowledgements
We would like to thank Mr. Kohei Namba for help to perform the present meta-analysis.
Author information
Authors and Affiliations
Contributions
W.S. conceived the study, and performed statistical analysis. W.S., N.M. and K.M. performed literature search and study selection. W.S. wrote the original draft of the manuscript, with important contributions from Y.I. and R.K. All authors provided significant input into the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sako, W., Murakami, N., Motohama, K. et al. The effect of istradefylline for Parkinson’s disease: A meta-analysis. Sci Rep 7, 18018 (2017). https://doi.org/10.1038/s41598-017-18339-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18339-1
This article is cited by
-
Molecular docking/dynamics simulations, MEP analysis, bioisosteric replacement and ADME/T prediction for identification of dual targets inhibitors of Parkinson’s disease with novel scaffold
In Silico Pharmacology (2023)
-
Evaluating the impact of adjunctive istradefylline on the cumulative dose of levodopa-containing medications in Parkinson’s disease: study protocol for the ISTRA ADJUST PD randomized, controlled study
BMC Neurology (2022)
-
The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease
Purinergic Signalling (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.