Abstract
Chronic spontaneous urticaria (CSU) is considered in a subset of patients to be an autoimmune disorder. Interleukin(IL)-17, IL-31, and IL-33 are involved in some immune response. The aim of this study was to quantify plasma IL-17, IL-31, and IL-33 levels in CSU patients and to examine their relationships with disease severity. Plasma IL-17, IL-31, and IL-33 concentration were measured in 51 CSU patients and 20 healthy subjects (HCs). Plasma IL-17 (P < 0.001), IL-31 (P < 0.001), and IL-33 (P < 0.001) concentrations were significantly higher in CSU patients when compared with those of HCs. Concerning UAS7, severe group of CSU patients had significantly higher IL-17 levels than the moderate and mild groups (P = 0.028 and 0.007, respectively), and significantly higher IL-33 concentrations than the mild group (P = 0.026). Regarding only pruritus, severe group of patients had significantly higher IL-31 levels than the mild group (P = 0.003). The IL-33 levels in the total IgE positive group were significantly higher than that of negative group (P = 0.010). Our results showed higher plasma levels of IL-17, IL-31, and IL-33 among CSU patients which may highlight a functional role of these cytokines in the pathogenesis of CSU.
Similar content being viewed by others
Introduction
Chronic spontaneous urticaria (CSU) is a common and disabling disease characterized by recurrent itchy wheals and/or angioedema for more than 6 weeks due to known or unknown causes1. These symptoms are the consequence of skin mast cells degranulation with release of histamine and other vasoactive mediators. Autoantibodies (anti-IgE, anti-FcξRI) may be involved in only one-third of the cases2, suggesting that other circulating mediators, including cytokines, may be involved in the pathogenesis of CSU. The CSU shows both autoimmune and allergic disease characteristics3, which is associated with an imbalance between cytokines and T lymphocyte subgroups. Several data support the participation of interleukins in the pathophysiology of chronic urticaria4,5.
IL-17, IL-31, and IL-33 are multifunctional cytokines playing key roles in inflammation and immunity. IL-17 produced by CD4+T-helper subset that named T helper (Th) type 176, is binding to an IL-17 receptor expressed on epithelial, endothelial, and fibroblastic stromal cells. IL-17 is associated with many autoimmune disorders, including rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis and asthma7,8. IL-31 is mainly produced by Th2 cells9 and mast cells10. IL-31 acts on a broad range of immune- and non-immune cells and therefore possesses potential pleiotropic physiological functions, including regulating hematopoiesis and immune response, causing inflammatory bowel disease, airway hypersensitivity and dermatitis9. Moreover, IL-31 has also been described to play a key role in the pathogenesis of atopic dermatitis11 and contribute to itching via activation of the IL-31 receptor on sensory nerve cells12, therefore is considered to be an IL that can lead to skin inflammation. IL-33 is released in the extracellular space following cell injury. Its receptor ST2 is an IL-1R-related protein expressed on Th2 cells, mast cells, basophils and eosinophils13,14. Consequently, IL-33 has been shown to be involved in Th2-mediated immune responses, such as asthma, parasitic infections15, and atopic dermatitis16.
In these regard, IL-17, IL-31, and IL-33 might be involved in the pathogenesis of CSU, and their levels could be biomarker of disease severity or treatment response in CSU. So far, there are few available data regarding behavior of IL-17, 1L-31, and IL-33 in patients with CSU. Therefore, the aim of this study was to measure the values of plasma IL-17, 1L-31, and IL-33 levels in patients with CSU and analyze their relations to disease severity.
Results
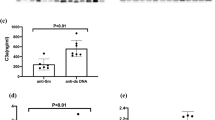
Plasma levels of IL-17 (256.71 ± 25.07 ng/l vs. 181.79 ± 16.62 ng/l, P < 0.001; Fig. 1A), IL-31 (27.79 ± 3.02 ng/l vs. 18.78 ± 1.71 ng/l, P < 0.001; Fig. 1B), and IL-33(45.53 ± 4.32 ng/l vs. 30.09 ± 2.69 ng/l, P < 0.001; Fig. 1C) were significantly higher in CSU patients compared with those of healthy controls.
Comparison of IL-17, IL-31, and IL-33 levels in plasma between CSU patients and HCs. The levels of plasma IL-17 (P < 0.001) (A), IL-31 (P < 0.001) (B), and IL-33 (P < 0.001) (C) were significantly higher in CSU patients than those in HCs. Horizontal lines represent the mean values for IL-17, IL-31, and IL-33. CSU, chronic spontaneous urticaria; HC, healthy control.
Concerning the urticaria activity, severe group (271.51 ± 19.76 ng/l) of CSU patients had significantly higher IL-17 levels than the moderate (255.21 ± 28.56 ng/l) and mild (248.44 ± 21.30 ng/l) groups (P = 0.028 and 0.007, respectively; Fig. 2A). There was no significant differences in IL-31 levels between different severity group (Fig. 2B). An increased level of IL-33 was observed in severe group (47.41 ± 2.88 ng/l) of CSU patients compared with the mild group (44.62 ± 4.24 ng/l, P = 0.026; Fig. 2C), but no significant differences in IL-33 levels between mild and moderate (45.19 ± 5.04 ng/l) group or moderate and severe group. However, regarding only pruritus, severe (29.41 ± 2.15 ng/l) group of CSU patients had significantly higher IL-31 levels than the mild group (26.49 ± 2.62 ng/l, P = 0.003; Fig. 3), no significant differences between mild and moderate (27.39 ± 3.38 ng/l) group or moderate and severe group.
Comparison of IL-17, IL-31, and IL-33 levels according to disease severity in CSU patients. Severe group of CSU patients had significantly higher IL-17 levels than the moderate and mild groups (P = 0.028 and 0.007, respectively) (A); no significant differences were found in IL-31 levels between different severity group (B). An increased level of IL-33 was observed in severe group of CSU patients compared with the mild group (P = 0.026), no significant differences in IL-33 levels between mild and moderate group or moderate and severe group (C).
We evaluated the correlation of IL-17, IL-31, and IL-33 in plasma by the Spearman’s rank test. Interestingly, both IL-17 (r = 0.333, P = 0.017; Fig. 4A) and IL-31 (r = 0.361, P = 0.009; Fig. 4B) were significantly correlated with IL-33 levels. Nevertheless, the levels of IL-17 in plasma were not relevant to that of IL-31 (Fig. 4C).
A higher level of IL-33 was observed in the total IgE positive group compared with that of negative group (46.73 ± 4.02 vs 43.96 ± 4.31 ng/l, P = 0.010; Fig. 5), but not for IL-17 and IL-31. There were no significant differences in IL-17, IL-31, or IL-33 levels according to the age, gender and presence of angioedema. Plasma levels of IL-17, IL-31, and IL-33 were not significantly correlated with CRP or blood eosinophil count.
Discussion
A full understanding of the pathogenesis of CSU has yet to be achieved. In the present study, to gain better understanding of the role of cytokines in immunopathogenesis of CSU we aimed to determine whether CSU is associated with alterations in IL-17, IL-31, and IL-33. The results of our study showed that plasma levels of IL-17, IL-31, and IL-33 were significantly elevated in patients with CSU. Severe group of CSU patients had significantly higher IL-17 levels compared with the moderate and mild group, and significantly higher IL-33 concentration than the mild group of CSU patients.
The IL-17 levels were significantly higher in CSU patients compared with the control group, and severe group had significantly higher IL-17 levels compared with the other two groups of CSU patients. In agreement with our results, many studies showed that the IL-17 levels of CU patients was higher than that of control17,18,19, and there were significant positive correlation between serum IL-17, IL-23, TNF-α and disease activity18. In another report, IL-17 were significantly higher in autologous serum skin test (ASST) positivity than in ASST negative CSU patients20. Moreover, patients with CSU and ASST positivity, showed increased circulating levels of TNF-α, IL-1β and IL-6. These pro-inflammatory cytokines, in turn, are known to be induced by IL-17, which may contribute to the inflammatory profile founded in CSU17.
The important role of IL-31 in atopic dermatitis, in particular its impact on intensity of pruritus, is well known. An enhanced expression of the specific IL-31RA was discovered in cells of the human and murine dorsal root ganglia and in murine primary afferent fibers of the spinal cord and dermis that are proposed to be involved in the sensation of itch21,22. Furthermore, IL-31 antibodies have been shown to reduce itch significantly in a mouse model of AD23, confirming in patients with moderate-to-severe AD very recently24. Our results agreed with a previous study, in which the researchers demonstrated that IL-31 levels in CSU patients were significantly higher compared with those of controls25. However, there was no difference in IL-31 plasma levels in ASST positive or negative CU patients25. We could not find a correlation between IL-31 plasma levels and the urticaria activity, confirming a previous report26, but if regarding only pruritus, severe group of CSU patients had significantly higher IL-31 levels than the mild group. This may be attributed to the fact that IL-31 is contribute to itching.
IL-33 is being increasingly recognized as an important inflammatory cytokine. The plasma levels of IL-33 were significantly higher in patients with CSU, and severe group had significantly higher concentration compared to the mild group of CSU patients. In support of our finding, elevation of IL-33 was recently demonstrated in the lesional skin of CSU patients27. Besides, among the patients who had received desloratadine for two weeks, there was a significant reduction in IL33 levels of CSU patients28. IL-33 induces increased release of Th2 cytokines such as IL-5 and IL-13 from Th2 cells in vitro and elevated levels of plasma IgE and blood eosinophils in vivo 29. IL-33 also causes activation, maturation, and Th2 cytokine production in mast cells30 and induces eosinophil-dependent cutaneous fibrosis27. Thus, IL-33 may play a pivotal role in the development of inflammatory reactions in CSU.
Inconsistent with our results, there were studies demonstrated the plasma IL-17 levels in CSU patients were not differ from the healthy control20,31 or even lower32. Besides, two reports showed that there was no significant difference in plasma IL-33 levels between patients with urticaria and control subjects33,34. The probable cause of the different result may be impact of genetic variation in the study population or the study was conducted on a small number of patients32.
Intriguingly, IL-33 levels were both correlated with IL-17 and IL-31 in CSU patients. Many studies provided indirect evidence for a functional link between these cytokines in many human diseases. Vocca et al. reported IL-33/ST2 axis was involved in Th2/IL-31 and Th17 immune response during the progression of allergic airway disease35. Nygaard et al. found a moderate, positive correlation between IL-33 and IL-31 in atopic dermatitis36. These authors speculate that the activation of the IL-33-ST2 axis, as a biomarker of Th2/IL-31 immune response, may be a critical crossroad between the immune system and epidermal homoeostasis36. On the other hand, Meephansan et al. found IL-17A induced IL-33 in epidermis through EGFR, EPK, p38 and JAK/STAT1 pathways, which were necessary for induction of IL-3337. There may be a functional link between these cytokines, but the exact mechanism is not yet clear and needs further study.
A correlation between IL-33 and IgE has been reported. A study demonstrated that mast cells produce IL-33 after IgE-mediated activation and that the IL-33/ST2 pathway was critical for the progression of IgE-dependent inflammation38. Futhermore, IL-33 enhances IgE-mediated degranulation and migration as well as IgE- and IL-3-mediated cytokine and chemokine production in human and mouse basophils39. On the other hand, long-term exposure of human and mouse muscle cell to IL-33 resulted in attenuation of IgE/Ag-FcεRI-mediated degranulation due to down-regulation of PLCγ1 and Hck expression, although short term exposure to IL-33 did not influence that degranulation directly40. In our study, we found the IL-33 levels in the total IgE positive group were significantly higher than that of negative group, providing evidence for this functional link between IL-33 and IgE in CSU.
Our study has two limitations. One is the relatively small number of study subjects. The other is the absence of a positive control group, such as atopic dermatitis or psoriasis as an inflammatory dermatosis. Therefore, further studies with a larger sample size and a positive control group are required to confirm our results.
In summary, our results showed high plasma levels of IL-17, IL-31, and IL-33 among CSU patients which may highlight a functional role of these cytokines in the pathogenesis of this common skin disease, and may provide the rationale for new treatment strategies in chronic urticaria. However, more studies are needed on more patients to study different Th1, Th2 and Th17 cytokines in plasma and skin of CSU patients.
Methods
Study subjects
We studied 51 CSU patients and 20 sex-matched and age-matched healthy controls as control. CUS was diagnosed according to the EAACI/GA2−LEN/EDF/WAO guidelines. We excluded patients with clinical evidence of urticaria vasculitis and physical urticaria, such as dermographism, cholinergic urticaria, and cold urticaria. Anti-histamines were discontinued 1 week before the study and none of the patients was taking any other drugs for more than 8 weeks preceding the study. All the controls did not take any medication for at least 2 weeks before the study. Disease activity in all CSU patients was determined by use of UAS7 during 7 days. Weekly UAS were graded as follows: 0–14(mild), 15–28(moderate) and 29–42(severe). Weekly pruritus intensity was graded as: 0–7(mild), 8–14(moderate) and 15–21(severe). The characteristics of patients (n = 51) are shown in Table 1.
Total IgE were measured using immunoblot assay (MEDIWISS Analytic GmbH, Moers, Germany), when the value higher than 100 kU/l meant positive.
The study protocol was approved by the Institutional Review Board for Human Studies of Affiliated Hospital, School of Medicine, Ningbo University (Ningbo, China). All subjects provided written informed consent before participation and methods in this study were performed in accordance with the relevant guidelines and regulations.
Assay of IL-17, IL-31, and IL-33
Plasma was collected and stored at −80 °C. Concentration of IL-17, IL-31, and IL-33 in plasma was measured by the enzyme-linked immunosorbent assay (ELISA) using a commercial kit (IL-17: Shanghai Future Industry Co., Ltd., Shanghai, China; IL-31: Shanghai Future Industry Co., Ltd., Shanghai, China; IL-33: Shanghai Future Industry Co., Ltd., Shanghai, China;). The assays were conducted according to the manufacturer’s guidelines. The samples were analyzed in batches to minimize interassay variability.
Statistical analysis
Data were delivered as medians and ranges. Kruskal-Wallis variance analysis was used for screening differences between the groups. Mann–Whitney U test was used to compare data between the patient groups and the healthy controls. Spearman’s rank test was used for correlations. The probability value of P < 0.05 was assumed significant. The data were analyzed with SPSS statistics 18.0.
References
Zuberbier, T. et al. The EAACI/GA2LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 69, 868–887 (2014).
Altrichter, S. et al. IgE mediated autoallergy against thyroid peroxidase–a novel pathomechanism of chronic spontaneous urticaria? PLoS. One. 6, e14794 (2011).
O’Shea, J. J., Ma, A. & Lipsky, P. Cytokines and autoimmunity. Nat Rev Immunol. 2, 37–45 (2002).
Caproni, M. et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin. Immunol. 114, 284–92 (2005).
Hennino, A. et al. Pathophysiology of urticaria. Clin. Rev. Allergy. Immunol. 30, 3–11 (2006).
Grattan, C. E., Wallington, T. B., Warin, R. P., Kennedy, C. T. & Bradfield, J. W. A serological mediator in chronic idiopathic urticaria–a clinical, immunological and histological evaluation. Br. J. Dermatol. 114, 583–90 (1986).
Ziolkowska, M. et al. High Levels of IL-17 in Rheumatoid Arthritis Patients: IL-15 Triggers In Vitro IL-17 Production Via Cyclosporin A-Sensitive Mechanism. The Journal of Immunology. 164, 2832–2838 (2000).
Liu, Z. J., Yadav, P. K., Su, J. L., Wang, J. S. & Fei, K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World. J. Gastroenterol. 15, 5784–8 (2009).
Zhang, Q., Putheti, P., Zhou, Q., Liu, Q. & Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine & Growth Factor Reviews. 19, 347–356 (2008).
Rabenhorst, A. & Hartmann, K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr. Allergy. Asthma. Rep. 14, 423 (2014).
Raap, U. et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy. Clin. Immunol. 122, 421–3 (2008).
Kasraie, S., Niebuhr, M. & Werfel, T. Interleukin (IL)-31 induces pro-inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins. Allergy. 65, 712–21 (2010).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA 95, 6930–5 (1998).
Trajkovic, V., Sweet, M. J. & Xu, D. T1/ST2–an IL-1 receptor-like modulator of immune responses. Cytokine. Growth. Factor. Rev. 15, 87–95 (2004).
Palmer, G. & Gabay, C. Interleukin-33 biology with potential insights into human diseases. Nat. Rev. Rheumatol. 7, 321–9 (2011).
Savinko, T. et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J. Invest. Dermatol. 132, 1392–400 (2012).
Dos Santos, J. C. et al. Increased circulating pro-inflammatory cytokines and imbalanced regulatory T-cell cytokines production in chronic idiopathic urticaria. Int. Immunopharmacol. 8, 1433–40 (2008).
Atwa, M. A., Emara, A. S., Youssef, N. & Bayoumy, N. M. Serum concentration of IL-17, IL-23 and TNF-alpha among patients with chronic spontaneous urticaria: association with disease activity and autologous serum skin test. J. Eur. Acad. Dermatol. Venereol. 28, 469–74 (2014).
Grzanka, A., Damasiewicz-Bodzek, A. & Kasperska-Zajac, A. The relationship between circulating concentrations of interleukin 17 and C reactive protein in chronic spontaneous urticaria. Allergy. Asthma. Clin. Immunol. 13, 25 (2017).
Chen, Q. et al. Different expression patterns of plasma Th1-, Th2-, Th17- and Th22-related cytokines correlate with serum autoreactivity and allergen sensitivity in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol (2017).
Bando, T., Morikawa, Y., Komori, T. & Senba, E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 142, 1263–71 (2006).
Sonkoly, E. et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy. Clin. Immunol. 117, 411–7 (2006).
Grimstad, O. et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp. Dermatol. 18, 35–43 (2009).
Ruzicka, T. et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 376, 826–835 (2017).
Raap, U. et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp. Dermatol. 19, 464–6 (2010).
Altrichter, S. et al. Successful omalizumab treatment in chronic spontaneous urticaria is associated with lowering of serum IL-31 levels. J. Eur. Acad. Dermatol. Venereol. 30, 454–5 (2016).
Kay, A. B., Clark, P., Maurer, M. & Ying, S. Elevations in T-helper-2-initiating cytokines (interleukin-33, interleukin-25 and thymic stromal lymphopoietin) in lesional skin from chronic spontaneous (‘idiopathic’) urticaria. Br. J. Dermatol. 172, 1294–302 (2015).
Zheng, D. & Yang, X. Clinical observation on the therapeutic effect of desloratadine citrate disodium in the treatment of chronic urticaria and changes in IL4, IL18, IL23 and IL-33 levels before and after treatment. Pak. J. Pharm. Sci. 30, 1139–1142 (2017).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 23, 479–90 (2005).
Allakhverdi, Z., Smith, D. E., Comeau, M. R. & Delespesse, G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J. Immunol. 179, 2051–4 (2007).
Azor, M. H. et al. Statin effects on regulatory and proinflammatory factors in chronic idiopathic urticaria. Clin. Exp. Immunol. 166, 291–8 (2011).
Daschner, A. et al. Different serum cytokine levels in chronic vs. acute Anisakis simplex sensitization-associated urticaria. Parasite. Immunol. 33, 357–62 (2011).
Tamagawa-Mineoka, R., Okuzawa, Y., Masuda, K. & Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatol. 70, 882–8 (2014).
Puxeddu, I. et al. Free IL-18 and IL-33 cytokines in chronic spontaneous urticaria. Cytokine. 61, 741–3 (2013).
Vocca, L. et al. IL-33/ST2 axis controls Th2/IL-31 and Th17 immune response in allergic airway diseases. Immunobiology. 220, 954–63 (2015).
Nygaard, U. et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 30, 1930–1938 (2016).
Meephansan, J. et al. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J. Dermatol. Sci. 71, 107–14 (2013).
Hsu, C. L., Neilsen, C. V. & Bryce, P. J. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 5, e11944 (2010).
Silver, M. R. et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflamm. Res. 59, 207–18 (2010).
Jung, M. Y. et al. IL-33 induces a hyporesponsive phenotype in human and mouse mast cells. J. Immunol. 190, 531–8 (2013).
Acknowledgements
This work was supported by the grant of Social Development of Science and Technology Project of Ningbo (No. 2014C50086, Suling Xu).
Author information
Authors and Affiliations
Contributions
W.L., Q.Z. and S.X. designed the experiments. Q.Z., C.L., M.Y., and S.X. recruited patient samples and carried out the experiments. W.L. analyzed the data. W.L., Q.Z. and S.X. wrote and edited the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, W., Zhou, Q., Liu, C. et al. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci Rep 7, 17797 (2017). https://doi.org/10.1038/s41598-017-18187-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18187-z
This article is cited by
-
Current and Emerging Therapies for Chronic Spontaneous Urticaria: A Narrative Review
Dermatology and Therapy (2023)
-
Systemic and local cytokine profile and risk factors for persistent allergic airway inflammation in patients sensitised to house dust mite allergens
BMC Pulmonary Medicine (2021)
-
A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis
Cell Death & Disease (2021)
-
Role of TF-Triggered Activation of the Coagulation Cascade in the Pathogenesis of Chronic Spontaneous Urticaria
Current Treatment Options in Allergy (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.