Abstract
The expression of legumain which has been shown overexpressed in patients with metastatic gastric cancer is positively correlated to both disease progression and outcome, and negatively correlated to microRNA (miR)−3978 expression. The RNA-binding protein, poly r(C) binding protein 1 (PCBP1) was the most downregulated protein in the metastatic tissue specimens. Quantitative real-time PCR showed that PCBP1 expression is transcriptionally downregulated in peritoneal metastasis tissues. RNA immunoprecipitation experiments showed that PCBP1 and miR-3978 are sequestered in normal peritoneal tissue, but the complex is disrupted following metastatic progression. PCBP1 expression mimicked miR-3978 expression across gastric cancer patients. Finally, replenishment of PCBP1 or miR-3978 expression in the peritoneal metastasis cell line MKN45 decreased legumain protein expression and chemosensitized the cells to treatment with docetaxel. However, replenishment of one and concomitant depletion of the other failed to induce chemosensitivity to docetaxel. Replenishment of miR-3978 also resulted in induction of PCBP1 protein expression, potentially indicating that miR-3978 expression might downregulate a negative regulator targeting PCBP1. Our current study reveals PCBP1 as an additional biomarker in peritoneal metastasis. PCBP1 and miR-3978 expression were correlated and suggests a potential interplay of differential miRNA biogenesis and RNA binding protein during metastatic progression.
Similar content being viewed by others
Introduction
Approximately 300,000 patients with gastric cancer are projected to die annually in China1, 50% of which is due to peritoneal metastasis2. Even though radical resection is the accepted mode of treatment in these patients, peritoneal metastasis remains the single largest cause of mortality in patients with gastric cancer3,4,5,6. Even though most patients already have regional or distant metastasis in patients with gastric cancer, radical resection without knowledge of potent diagnostic and prognostic markers is not highly efficient in preventing progression to peritoneal metastasis7,8.
Legumain or asparaginyl endopeptidase (AEP), a member of the lysosomal cysteine endopeptidase, has been found to be overexpressed in patients with metastatic gastric cancer9,10,11,12. Legumain potentiates metastatic progression by augmenting epithelial to mesenchymal transition by activation of MAPK and Akt signaling pathways13. In addition, proteolytic activation of other zymogens mediated by legumain is also a pathway leading to both tumor development and progression in vivo14,15,16.
We have earlier shown that microRNA-3978 (miR-3978) regulates legumain expression in normal peritoneum. In gastric cancer patients with peritoneal metastasis legumain expression is induced because miR-3978 expression is suppressed17. MiRNAs are evolutionarily conserved 21–23 nucleotides RNAs that regulate post-transcriptional gene expression either by blocking translation or degrading target messenger RNAs (mRNAs) and have been increasingly shown to function as tumor suppressors or oncogenes 18,19. MiRNAs can function in both normal and transformed cells and have even been shown to play a role in metastasis20,21,22,23.
Not much is known about role of miR-3978 in gastric cancer or in normal physiology. There has been one report that suggested differential expression of miR-3978 in lung cancer patients24. Given that legumain has been shown to be overexpressed in different metastatic cancers13,14,15,16,25,26,27, it is imperative to define precise mechanism that regulates miR-3978 expression in gastric cancer patients with peritoneal metastasis. Our findings in the current study cumulatively indicate that the RNA binding protein, poly r(C) binding protein 1 (PCBP1) or heterogeneous nuclear ribonucleoprotein E1 (hnRNPE1) expression correlates withmiR-3978 expression and that hnRNP E1 itself functions as a suppressor of tumor progression.
Materials and Methods
Patient samples
The study protocol was approved by the Institutional Review Board of the China-Japan Union Hospital of Jilin University and all experiments were performed in accordance with relevant guidelines and regulations. A total of 20 patients (12 men and 8 women) who had surgery for gastric cancer between 2014 and 2015 at the China-Japan Union Hospital of Jilin University were recruited in this study. Signed informed consent was obtained from all patients before enrolling for the current study. The mean age of the patients was 61.34 years (range, 39–78 years). The inclusion criteria were presence of peritoneal metastasis at the time of initial presentation as confirmed by two independent pathologists. Paired tumor tissue and normal gastric tissue specimens were obtained from all patients included in the study.
Western blotting
Cells were lysed in buffer containing 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol supplemented with complete, Mini protease inhibitor cocktail (Roche Diagnostics, Shanghai, China). Whole cell lysate was resolved on SDS-PAGE and probed with anti-PCBP1 antibody (clone E-2, sc-137249; Santa Cruz Biotechnology, USA) or anti-Legumain antibody17. All blots were subsequently stripped, and re-probed with GAPDH (ThermoFisher Scientific, Shanghai, China) to confirm equal loading.
Mass spectrometry analysis
Tissue (tumor and adjacent normal) obtained from 5 patients were washed thrice with ice-cold PBS before being lysed using NET buffer (50 mmol/L Tris (pH, 7.4), 150 mmol/L NaCl, 0.1% NP40, 1 mmol/L EDTA, 0.25% gelatin, 0.02% sodium azide) supplemented with protease and phosphatase inhibitor cocktail (ThermoFisher Scientific, Shanghai, China). Lysates were centrifuged at 10,000 rcf for 20 minutes at 4 °C and the supernatant were stored at −80 °C until further use. The lysates were dialyzed against PBS before being reduced and alkylated using 5 mM Tris 2-carboxyethyl phosphine (TCEP) and 10 mM iodoacetamide, respectively. Peptides were desalted, lyophilized, before being re-dissolved in 20 µl of HPLC-grade water containing 0.1% formic acid. Mass spectrometry analysis was performed on a linear ion trap LTQ mass spectrometer (Life Technologies, Shanghai, China) as per manufacturer’s guidelines. Of note, the instrument was operated in positive ion mode and MS full scans were recorded over a mass range of 400–1600 m/z. Post-acquisition of data, ReAdW was used to convert files to mzXML, which were used to query the human IPI database using the Sequest platform. False discovery rate was determined using the Trans-Proteomic Pipeline TPP, with a FDR set at approximately 5%. Finally, relative enrichment or depletion in tumor group was normalized to control group.
Isolation of miRNA, mRNA and qRT-PCR
RNA was isolated using Trizol (Life Technologies, Shanghai, China) as per manufacturer instructions. The expression level of PCBP1 mRNA and GAPDH mRNA were detected by TaqMan miRNA assays (Life Technologies, Shanghai, China). MiRNA from cells and tissues were extracted by the mirVana miRNA isolation kit (ThermoFisher Scientific, Shanghai, China) according to the manufacturer instructions. The expression levels of indicated miRNAs and RNU6B were detected by TaqMan miRNA assays (Life Technologies, Shanghai, China). The −ΔΔCt method was used to analyze the data in each case and normalization was done to GAPDH and RNU6B expression for mRNA and miRNA, respectively.
RNA immunoprecipitation
Tissue specimens from 5 patients were lysed for 15 minutes on ice in a lysis buffer containing 100 mM KCl, 5 mM MgCl2, 10 mM HEPES, pH 7.0, 0.5% NP-40 detergent supplemented with fresh 1 mM DTT, 1000 units/ml RNasin (Promega, Madison, WI, USA), and Mini protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The post-nuclear fraction was collected by spinning the cells at 15,000 rcff for 15 minutes at 4 °C, and 10% (v/v) was removed for input sample. Four milligrams of lysate was utilized for immunoprecipitation using 10 µg of mouse monoclonal anti-PCBP1 antibody (clone E-2, sc-137249; Santa Cruz Biotechnology, USA) or 10 µg of mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using Pierce Crosslink IP Kit (Life Technologies, Shanghai, China). Aliquots were sequestered to confirm successful immunoprecipitation via SDS-PAGE. The rest of the immunoprecipitated complex was digested with 30 μg of proteinase K to release the ribonucleoprotein complex. TRIzol LS reagent (Life Technologies, Shanghai, China) was subsequently used to extract miRNA from the immunoprecipitates and the input samples following manufacturer’s recommendations. The miRNAs isolated above were templated for qRT-PCR as described above. Fold enrichment above the sample specific background (linear conversion of the first ΔΔCt) was calculated using the formula: Fold Enrichment = 2(−ΔΔCt [RIP/NS]). Data are presented as mean ± SD.
Immunohistochemistry
Tissue sections (5 μm) were de-paraffinized and rehydrated through a graded alcohol series. Antigen retrieval was performed using 1X Target Retrieval Solution (Dako, Carpinteria, CA, USA). Endogenous peroxidase was blocked using 3% hydrogen peroxide for 10 minutes. Sections were incubated with primary antibody against PCBP1 (ab133421; Abcam, Waltham, MA, USA) (1:250 dilution) at 4 °C for overnight. Following wash and incubation with anti-goat secondary antibody at room temperature for 1 hour, immunoreactivity was detected using diaminobenzidine (Innovex Biosciences, Richmond, CA, USA) as a chromogen. Slides were counter-stained with hematoxylin before imaged with microscope at different magnifications.
Cell culture and treatment
MKN45 cell lines were purchased from the cell bank of Chinese Academy of Sciences. Cells were maintained in RPMI1640 medium (Life Technologies, Shanghai, China) supplemented with 20% FBS (Lonza, Germany). All cells were cultured in a 5% CO2 humidified atmosphere and at 370 C. Where indicated cells were treated with indicated dose of Docetaxel.
Plasmids and transfection
PCBP1 plasmid was obtained from Open Biosystems. The miR-3978 mimic was bought from ThermoFisher Scientific. Where indicated cells were either transfected with PCBP1 overexpression construct or the miR-3978 mimic or miR-6124 mimic for 12 hours using Lipofectamine 3000 (ThermoFisher Scientific, Shanghai, China). In indicated cases, cells were transfected with both miR-3978 mimic and either siRNA targeting Luciferase or PCBP1 (Sigma-Aldrich, Shanghai, China).
MTT assay
The MTT assay kit (Sigma-Aldrich, Shanghai, China) was used to measure cell proliferation rates. The relative optical density (OD) was measured at 570 nm and data is represented as mean ± standard deviation from at least three independent experiments.
Statistical analyses
All analyses were done by using the SPSS statistical software program version 18.0 (IBM Corporation, NY, USA). P-values < 0.05 were considered statistically significant.
Results
Given that miR-3978 expression is downregulated during peritoneal metastasis, we wanted to determine the underlying mechanism of altered miR-3978 expression. Mass spectrometry analysis of protein expression from tissues obtained from patients with metastatic gastric cancer and tumor adjacent normal tissue revealed that whereas five proteins (PCBP1, DSP, FZD7, ITGA5, and MMP2) were downregulated at least 3 folds (log2), four proteins (IGFBP4, KRT19, NODAL, and PRP4A1) were upregulated at least 3 folds (log2) in metastatic tumor tissue specimens compared to control tissue specimens. Poly r(C) binding protein (PCBP1), also known as heterogeneous nuclear ribonucleoprotein E1 (hnRNP E1), was the most downregulated protein in the metastatic samples (−8.54 ± 1.12 folds) (Fig. 1a). Given that PCBP1 has been indicated in the pathogenesis of a wide variety of cancers28,29,30,31,32,33,34, we decided to further pursue the role of PCBP1 in peritoneal metastasis of gastric cancer. Since, PCBP1’s tumor suppressor role can be attenuated either by its loss of expression or Akt2-mediated phosphorylation29, we next evaluated expression of PCBP1 messenger RNA (mRNA) in metastatic gastric cancer patient samples. As shown in Fig. 1b, PCBP1 mRNA was downregulated 12.2 ± 1.55 folds in metastatic tissue compared with normal peritoneum, indicating that loss of PCBP1 expression might be the pervasive mechanism dictating its attenuation of tumor suppressor role in the context of peritoneal metastasis in gastric cancer.
PCPB1 is differentially expressed in gastric cancer patients with peritoneal metastasis. (a) Differential protein expression determined by mass spectrometry in metastatic versus adjacent control tissue specimens (n = 5). Shown are log2 ratio of expression of normalized values compared to the control group. Data are mean + standard deviation of 5 pairs of patient specimens. (b) Evaluation of PCBP1 mRNA expression in the five patients groups. Data was normalized to GAPDH expression and expressed as compared to the corresponding control group. Data are mean ± standard deviation of three independent replicates.
Immunohistochemical staining of PCBP1 in representative tissue specimens obtained from metastatic tissue or tumor adjacent normal tissue exhibited robust PCBP1 staining in the control peritoneal tissues, which was significantly attenuated in the tumor tissues (Fig. 2a–d). PCBP1 staining in metastatic tumor tissue was observed at comparatively much less intensity (Fig. 2c,d), confirming in vivo loss of PCBP1 expression might play a role in promoting peritoneal metastasis.
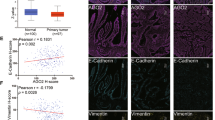
PCBP1 immunoprecipitates from gastric cancer patients with peritoneal metastasis are enriched in miR-3978. (a–d) Immunohistochemical staining of PCBP1 expression in representative normal (a,b) and tumor tissue sections (c,d). Scale bar, 100 µm. (e) Shown are relative enrichment of indicated miRNA in PCBP1 or mouse IgG immunoprecipitates as determined by qRT-PCR of lysates made from metastatic and normal adjacent tissue specimens. Data shown are mean ± standard deviation of three independent replicates.
To determine if altered PCBP1 expression was a coincidental occurrence or was also correlated to differential miR-3978 expression in these patients, we performed RNA immunoprecipitation using anti-PCBP1 antibody. Immunoprecipitation with anti-PCBP1 antibody, but not a control mouse IgG, showed significant enrichment of miR-3978 and miR-6124, but not miR-3148 or miR-390 in normal tissue compared to tumor tissue (Fig. 2e). Each of the miRNA tested for enrichment were predicted to be putative ones regulating legumain expression17. Cumulatively, this suggested that PCBP1 expression levels (high in normal and low in peritoneal metastatic tissues) correlate directly with miR-3978 expression levels in these tissues. Our results are indicative of a potential common regulatory mechanism underlying PCBP1 and miRN-3978 expression.
Given that our experiments indicated that miR-3978 and PCBP1 expression are correlated, we hypothesized that suppression of PCBP1 expression might be an underlying feature of peritoneal metastasis pathogenesis, much like what is observed for miR-397817. We determined miR-3978 and PCBP1 expression in 20 patients. Our results indicated a dynamic and direct correlation between expression of miR-3978 and PCBP1 (Fig. 3) (P < 0.005, Pearson correlation r = 0.9361).
We next determined if rescue of miR-3978 and PCBP1 expression can function as chemosensitizer. We evaluated the effect of miR-3978 mimic-mediated and ectopic PCBP1 overexpression-mediated replenishment on the cytotoxicity of docetaxel on the well-differentiated cell line mimicking peritoneal metastasis, MKN45 cells. Interestingly, miR-3978 mimic, but not miR-6124 mimic, induced PCBP1 protein expression in the MKN45 cells (Fig. 4a). Legumain expression was suppressed following miR-3978 mimic transfection. Transfection of miR-3978 mimic, but not miR-6124 mimic, significantly chemosensitized MKN45 cells to docetaxel treatment (p < 0.05) (Fig. 4b). Similarly overexpression of PCBP1 also suppressed legumain expression in MKN45 cells and significantly increased chemosensitivity of the cells to docetaxel treatment (p < 0.05) (Fig. 4b). Cumulatively, these results indicate that miR-3978 or PCBP1 replenishment sensitizes peritoneal metastatic cells to the cytotoxicity of docetaxel.
Ectopic expression of either miR-3978 or PCBP1 levels increased sensitivity to docetaxel. MKN45 cells were either not transfected or transiently transfected with either miR-3978 mimic, miR-6124 mimic, or PCBP1 for 12 hours. The cells were then treated with indicated doses of docetaxel for 72 hours. Post-treatment the cells were either processed for western blot or MTT assay was used to determine cell viability. (a) Immunoblot analysis of PCBP1 and legumain expression and (b) proliferation under the aforementioned conditions are shown. Data in (b) represent mean ± SD of three independent replicates. Please refer to Supplementary Figure 1a,b for full-length blots shown in (a). Please note PCBP1 and GAPDH were from the same gel, whereas Legumain was from a different gel. Samples ran were from experiments processed at the same time.
To determine if miR-3978 and PCBP1 interaction was important for their individual potency of sensitizing cells to docetaxel treatment, MKN45 cells were transfected with miR-3978 mimic and either a siRNA targeting Luciferase or PCBP1. Similar to what was observed in Fig. 4a, miR-3978 mimic induced PCBP1 protein expression in the MKN45 cells, which was prevented in cells transfected with siRNA-targeting PCBP1 (Fig. 5a). Transfection of miR-3978 mimic, significantly chemosensitized MKN45 cells to docetaxel treatment (p < 0.05), which was completely attenuated once PCBP1 was suppressed in these cells (Fig. 5b). Our results thus indicate that interaction or presence of both PCBP1 and miR-3978 is essential to potentiate chemosensitivity to docetaxel treatment in gastric cancer patients with peritoneal metastasis.
Both miR-3978 and PCBP1 expression is required to potentiate sensitivity to docetaxel. MKN45 cells were either not transfected or transiently co-transfected with either miR-3978 mimic and Luciferase siRNA, or miR-3978 mimic and PCBP1 siRNA for 12 hours. The cells were then treated with indicated doses of docetaxel for 72 hours. Post-treatment the cells were either processed for western blot or MTT assay was used to determine cell viability. (a) Immunoblot analysis of PCBP1 expression and (b) proliferation under the aforementioned conditions are shown. Data in (b) represent mean ± SD of three independent replicates. Please refer to Supplementary Figure 2a,b for full-length blots shown in (a).
Discussion
In the current study, our experimental results show that miRNA-3978 and PCBP1 is downregulated in gastric cancer patients with peritoneal metastasis. MiR-3978 seems to be functioning as part of PCBP1-mediated ribonucleoprotein complexes in the normal peritoneum and such enriched complexes are absent in metastatic tissue.
There are two possibilities – miR-3978 and PCBP1 are dictating each other’s expression, or they are regulating each other’s function. In the current study, we observed that in metastatic tissue, miR-3978 enrichment in PCBP1 immunoprecipitates are significantly downregulated. This can obviously stem from the downregulation of PCBP1 in the metastatic tissues. However, we have earlier shown that miR-3978 expression is also suppressed during metastatic progression. So are these related? Replenishment of miR-3978 upregulated PCBP1 protein expression in the MKN45 cells (Fig. 4a), but ectopic overexpression of PCBP1 did not increase miR-3978 expression (data not shown). Hence, at least in this context it seems that miR-3978 can regulate expression of PCBP1. However, miRNA prediction algorithms did not predict putative binding site of miR-3978 in the 3′UTR of PCBP1 mRNA (data not shown). This will indicate that miR-3978 is suppressing a negative regulator of PCBP1, and hence indirectly mimicking PCBP1 expression patterns. Such an observation is not without precedence. In fact, it has also been shown that miRNAs and their associated ribonucleoprotein complexes are amenable to various modulations that directly affect both their expression and function35,36.
The RNA binding protein, PCBP1 is well characterized for its role as a tumor suppressor. It has been shown that either loss of PCBP1 expression or phosphorylation at serine 43-mediated post translational modification has a pro-tumorigenic and pro-metastatic effect in pancreatic, ovarian, breast, lung, gastric, and Burkitt lymphoma28,29,30,31,32,33,34. The paralog of PCBP1, PCBP2, has been shown to regulate the miRNA biogenesis pathway based on cytosolic iron levels37. It was shown that PCBP2 associates with Dicer and thus regulates processing of miRNA precursors and that cytosolic iron levels determined whether PCBP2 could physically interact with Dicer and to the miRNA precursors37. Our experiments showed a direct interaction of PCBP1 and miR-3978 that are repressed during peritoneal metastasis of gastric cancer. It will be important to determine if PCBP1 dictates this miRNA modulation through a similar mechanism by interacting with Dicer and potentiating miRNA processing from the precursor pre-miRNAs. At least in the context of cellular iron homeostasis, PCBP1 was shown not to interact with Dicer37. If a similar scenario is observed in gastric cancer, it will have to be determined what alternate nodes in the miRNA biogenesis pathway are being modulated by differential PCBP1 expression, and vice versa.
Defining the mechanism(s) that regulates miR-3978 and PCBP1 expression and deciphering whether they are in the same or different regulatory lineages will be important. PCBPs are known to act as transcriptional regulators by binding to cis elements in gene promoters which in turn interact with RNA polymerase II transcription machinery38. This will explain widespread effect of suppressed PCBP1 mRNA expression on RNA polymerase II function. The PCBPs itself is also regulated by their cellular localization. It has been shown that p21-activated kinase 1 (PAK1) increases promoter activity of PCBP138. PAK1 and Akt2 both lie downstream of signaling pathways activated during metastatic progression, like TGF-beta signaling pathway. Whereas Akt2-mediated phosphorylation of PCBP1 has been shown to inhibit its RNA-binding capacity (resulting in translational activation of EMT inducer genes)29, PAK1 inactivation can lead to transcriptional downregulation of PCBP138. It will be interesting to determine if miR-3978 and PCBP1 share similar promoter architecture and whether they are both regulated by PAK1 in the context of peritoneal metastasis in gastric cancer.
Cumulatively, our findings highlight both loss of miR-3978 expression and PCBP1 as potential prognostic biomarker in gastric cancer patients. It will be interesting to investigate in future research endeavors how miR-3978, PCBP1, and legumain expression varies and correlates to disease progression in patients that had either radiation therapy or adjuvant chemotherapy post-radical resection of the primary tumor.
Change history
01 June 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Chen, W. Q. et al. Report of cancer incidence and mortality in China, 2012. China. Cancer 24, 1–10 (2015).
Cho, J. M. et al. Pattern, timing and survival in patients with recurrent gastric cancer. Hepatogastroenterology 61, 1148–53 (2014).
Tanaka, T., Kumagai, K., Shimizu, K., Masuo, K. & Yamagata, K. Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J Surg Oncol 75, 165–171 (2000).
Boku, T. et al. Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 77, 436–439 (1990).
Hippo, Y. et al. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res 61, 889–895 (2001).
Yoo, C. H., Noh, S. H., Shin, D. W., Choi, S. H. & Min, J. S. Recurrence following curative resection for gastric carcinoma. Br J Surg 87, 236–242 (2000).
Crosby, J. et al. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol 8, 50–59 (2001).
Poste, G. & Fidler, I. J. The pathogenesis of cancer metastasis. Nature 283, 139–146 (1980).
Barrett, A. J., Rawlings, N. D. & O’Brien, E. A. The MEROPS database as a protease information system. J Struct Biol 134, 95–102 (2001).
Dando, P. M. et al. Pig kidney legumain: an asparaginyl endopeptidase with restricted specificity. Biochem J 339, 743–49 (1999).
Li, N. et al. Effects of legumain as a potential prognostic factor on gastric cancers. Med Oncol 30, 621 (2013).
Guo, P. et al. Expression of legumain correlates with prognosis and metastasis in gastric carcinoma. PLoS One 8, e73090 (2013).
Cui, Y. et al. Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget. https://doi.org/10.18632/oncotarget.8879 (2016).
Zhen, Y. et al. Clinicopathologic significance of legumain overexpression in cancer: a systematic review and metaanalysis. Sci Rep 5, 16599 (2015).
Bajjuri, K. M., Liu, Y., Liu, C. & Sinha, S. C. The Legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity. Chem Med Chem 6, 54–59 (2011).
Murthy, R. V., Arbman, G., Gao, J., Roodman, G. D. & Sun, X. F. Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin Cancer Res 11, 2293–2299 (2005).
Zhang Y, et al. MiRNA-3978 regulates peritoneal gastric cancer metastasis by targeting legumain. Oncotarget (in press) (2016).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell. 136, 215–33 (2009).
Esquela-Kerscher, A. & Slack, F. J. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 6, 259–69 (2006).
Aleckovic, M. & Kang, Y. Regulation of cancer metastasis by cell-free miRNAs. Biochim Biophys Acta. 1855, 24–42 (2015).
Gaur, A. et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 67, 2456–68 (2007).
Kumar, M. S., Lu, J., Mercer, K. L., Golub, T. R. & Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 39, 673–677 (2007).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature. 435, 834–838 (2005).
Goto, A., Dobashi, Y., Tsubochi, H., Maeda, D. & Ooi, A. MicroRNAs associated with increased AKT gene number in human lung carcinoma. Hum Pathol. https://doi.org/10.1016/j.humpath.2016.04.011 (2016).
Liu, C., Sun, C., Huang, H., Janda, K. & Edgington, T. Overexpression of Legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res 63, 2957–2964 (2003).
Lewen, S. et al. A Legumain based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother 57, 507–515 (2008).
Wang, L. et al. Legumain: A biomarker for diagnosis and prognosis of human ovarian cancer. J Cell Biochem 113, 2679–2686 (2012).
Cho, S. J., Jung, Y. S. & Chen, X. Poly (C)-BindingProtein 1 Regulates p63 Expression through mRNA Stability. PLoS One 8, e71724 (2013).
Chaudhury, A. et al. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol 12, 286–293 (2010).
Hussey, G. S. et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell 41, 419–431 (2011).
Wang, H. et al. PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell 18, 52–62 (2010).
Liu, Y. et al. Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as a negative regulator of EMT and its clinical value. Int J Clin Exp Pathol 8, 7165–7172 (2015).
Zhang, Z. Z. et al. HOTAIR Long Noncoding RNA Promotes Gastric Cancer Metastasis through Suppression of Poly r(C)-Binding Protein (PCBP) 1. Mol Cancer Ther 14, 1162–70 (2015).
Wagener, R. et al. ICGC MMML-Seq-Project; “Molecular Mechanisms in Malignant Lymphomas” Network Project of the Deutsche Krebshilfe. The PCBP1 gene encoding poly(rC) binding protein I is recurrently mutated in Burkitt lymphoma. Genes Chromosomes Cancer. 54, 555–564 (2015).
Kim, Y. K., Heo, I. & Kim, V. N. Modifications of small RNAs and their associated proteins. Cell 143, 703–709 (2010).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10, 126–139 (2009).
Li, Y. et al. Iron homeostasis regulates the activity of the microRNA pathway through poly(C)-binding protein 2. Cell Metab 15, 895–904 (2012).
Choi, H. S. et al. Poly(C)-binding proteins as transcriptional regulators of gene expression. Biochem Biophys Res Commun 13, 431–436 (2009).
Acknowledgements
This study was supported by the Project of Jilin Provincial Development and Reform Commission (Item number: 2010018–5); Health Special Items of the Finance Department of Jilin Province (Item number: Sczsy201503); Health and Family Planning Commission of jilin Province (Item number: 2015ZC003).
Author information
Authors and Affiliations
Contributions
F.J.J. conceived and designed the experiments. Y.Y.W. and Z.A. performed the experiments. X.S.L. and J.N.J. contributed reagents, materials and/or equipment. F.F.C. and F.X.D. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ji, Fj., Wu, Yy., An, Z. et al. Expression of both poly r(C) binding protein 1 (PCBP1) and miRNA-3978 is suppressed in peritoneal gastric cancer metastasis. Sci Rep 7, 15488 (2017). https://doi.org/10.1038/s41598-017-15448-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15448-9
This article is cited by
-
Molecular and functional characterization of porcine poly C binding protein 1 (PCBP1)
BMC Veterinary Research (2024)
-
Depletion of C12orf48 inhibits gastric cancer growth and metastasis via up-regulating Poly r(C)-Binding Protein (PCBP) 1
BMC Cancer (2022)
-
The RNA-binding protein PCBP1 represses lung adenocarcinoma progression by stabilizing DKK1 mRNA and subsequently downregulating β-catenin
Journal of Translational Medicine (2022)
-
Overexpression of splicing factor poly(rC)-binding protein 1 elicits cycle arrest, apoptosis induction, and p73 splicing in human cervical carcinoma cells
Journal of Cancer Research and Clinical Oncology (2022)
-
Targets and regulation of microRNA-652-3p in homoeostasis and disease
Journal of Molecular Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.