Abstract
There are two basic stages of fine motor learning: performance gain might occur during practice (online learning), and improvement might take place without any further practice (offline learning). Offline learning, also called consolidation, has a sleep-dependent stage in terms of both speed and accuracy of the learned movement. Sleep spindle or sigma band characteristics affect motor learning in typically developing individuals. Here we ask whether the earlier found, altered sigma activity in a neurodevelopmental disorder (Williams syndrome, WS) predicts motor learning. TD and WS participants practiced in a sequential finger tapping (FT) task for two days. Although WS participants started out at a lower performance level, TD and WS participants had a comparable amount of online and offline learning in terms of the accuracy of movement. Spectral analysis of WS sleep EEG recordings revealed that motor accuracy improvement is intricately related to WS-specific NREM sleep EEG features in the 8–16 Hz range profiles: higher 11–13.5 Hz z-transformed power is associated with higher offline FT accuracy improvement; and higher oscillatory peak frequencies are associated with lower offline accuracy improvements. These findings indicate a fundamental relationship between sleep spindle (or sigma band) activity and motor learning in WS.
Similar content being viewed by others
Introduction
Motor learning, particularly explicit learning of fine motor sequences has multiple phases. Within-session (online) gains occur during practice, both in accuracy and in speed. The online phase is followed by a consolidation phase in which motor performance improves offline, without any further practice (Fig. 1). Offline consolidation is brain-state dependent: time spent awake results in retention of performance acquired during practice, while off-line gains are sleep-dependent both in speed and in accuracy in adults1,2,3. Changes in delta, sigma and beta activity in NREM sleep, and in REM characteristics have been found following practice4,5. There is a positive correlation between offline gains in the explicit sequential finger tapping (FT) task and sigma band NREM activity in the 13–15 Hz spindle range (in spindle amplitude, number and density)4,6,7,8,9,10,11,12. Post-sleep improvement in FT is correlated with spontaneous delta and fast-sigma oscillations in the supplementary motor area, contralateral to the trained hand6,7,13. Taken together, recent evidence seems to confirm that sleep spindles, especially fast sleep spindles contribute to the activation of the neural network involved in offline consolidation of fine motor sequences4,7,11,14,15,16.
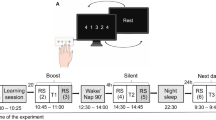
Motor learning task. (A) A four-element FT sequence, practiced with the non-dominant hand. Thumb is touched with the index, ring, middle and little fingers in this predetermined order. A “data glove” consisting of metal ring electrodes placed on each fingertip detects the order and timing of taps. (B) Each practice block is composed of 16 repetitions of the four-element practice sequence, and followed by a self-paced rest period. Beginning and end of a practice block is signalled by a computer-generated “beep” sound. (C) 10 practice blocks are carried out on Day 1. The mean of the first two practice blocks is considered as baseline performance. Online improvement is defined as the difference between the baseline and the mean of the last two practice blocks. Offline improvement is defined as the difference between the mean of the last two blocks on Day 1 and the mean of the first two blocks on Day 2.
In addition to post training sleep, sleep quality before learning also affects motor learning capacity in healthy individuals17. Interestingly, sleep spindle characteristics preceding learning are related to baseline performance and offline improvement in children when learning motor finger-sequences18. As a general trait, sleep dependent learning is correlated with EEG activity in the 8–16 Hz band during NREM sleep, which is genetically determined and stable within individuals, and across nights19,20.
As we have shown earlier, the majority of the above mentioned, motor learning related sleep characteristics are altered21, and the capacity to improve during long-term FT learning is significantly limited in Williams syndrome (WS)22. WS is a genetically determined neurodevelopmental disorder due to a microdeletion on chromosome 7 in the q11.23 region, and it is characterized by mild to moderate intellectual disability, hypersociability, attention deficits, and problems with visuospatial processing23,24,25. Delayed motor development, gross and fine motor deficits throughout the lifespan are common findings in WS25,26. With respect to sleep, an atypical sleep pattern, including prolonged sleep latency, sleep maintenance problems and fragmented sleep27,28,29, decreased total sleep time30,31, increased slow wave sleep (non-REM stage 3 and 4 sleep)28,30,31,32; decreased REM sleep percentage and reduced cyclicity in the sleep architecture31 has been found in WS. We have found WS-related alterations in the broadband sigma (8–16 Hz) NREM sleep EEG spectral profiles: decreases and increases in low (<13 Hz) and high (>13 Hz) sigma power, respectively, as well as increased oscillatory sigma peak frequencies21. This pattern has a striking stability in time, suggesting the acceleration of thalamocortical oscillatory dynamics during NREM sleep in WS21 (Fig. 2).
Focus of the present study: the broadband sigma range (8–16 Hz) in NREM sleep. Normalized sigma power as expressed in z-scores of EEG activity in WS subjects and group averages (WS and TD) at derivation Cz. Spectral power densities of artifact-free, Hanning-tapered 4 second EEG epochs were calculated via the Fast Fourier Transformation method and averaged for all-night NREM sleep (data from15)/TD data shown as a reference from21. The 8–16 Hz range was normalized in a derivation- and individual-specific manner by z-transformation19. TD sigma activity typically has two peaks (it is also true for the individual subjects) which could be referred to as the slow and fast sleep spindle peak frequencies, correspondingly. The slow spindle peak is usually missing or greatly reduced in WS patients and generally the second (fast spindle) peak is at a higher frequency in WS than in TD subjects21.
Based on the above findings, here we investigate the hypothesis that the reduced capacity to improve in a motor learning task is related to the altered neural activity during sleep in WS. Specifically, we test the relationship between motor learning and 8–16 Hz NREM sleep in WS. We employed a two-day practice version of the sequential FT task in WS and TD groups, and we compared their learning capacities. The same WS individuals participated in whole night ambulatory polysomnographic recordings earlier, and we analysed the relationship between polysomnographic and FT measures. Our main assumption is that the peculiar sleep characteristics of WS (namely, decreased low sigma, increased high sigma, as well as increased sigma peak frequency Fig. 2) will influence learning capacity in terms of both accuracy and speed in a sleep-dependent learning task. More specifically, we predict lower offline motor memory improvement when WS-specific alterations in the NREM sleep EEG sigma spectral profiles are pronounced.
Results
Motor learning performance
Accuracy
A significant main effect of group (WS < TD: F(1,30) = 23.939; p = 0.00003), a significant main effect for baseline/online/offline improvement (F(2,60) = 48.614; p = 2.8098 × 10−13, and a significant group × baseline/online/offline interaction (F(2,60) = 7.465; p = 0.001) was present. Baseline was significantly lower in WS than in TD (p < 0.05), but no significant difference in online and offline improvement was found between WS and TD groups (p > 0.05). Therefore, in terms of accuracy, baseline performance was different, while online and offline improvement in the sequential FT task was comparable in WS and TD (Fig. 3A).
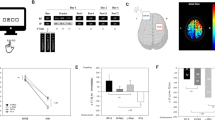
(A) Online and offline improvement in accuracy in the sequential FT task. (B) Online and offline improvement in speed in the sequential FT task in WS and in TD subjects. Baseline performance is significantly different, while improvements are comparable in WS and TD with respect to accuracy and online improvement in speed. Error bars show standard deviation.
Speed
There was a significant main effect for WS and TD groups (F(1,30) = 71.648; p = 1.9077 × 10−9), with a significant main effect of baseline/online/offline improvement (F(2,60) = 113.894; p = 3.7359 × 10−21) and with a significant group × baseline/online/offline interaction (F(2,60) = 18.605; p = 5.1657 × 10−7). Baseline was significantly lower in WS than in TD (p = 0.05), and there was no significant difference in online improvement (p > 0.05). Offline improvement showed a marginal difference between the two groups (p = 0.065) (Fig. 3B).
Lower baseline performance predicted higher online (practice-dependent) gains in terms of both accuracy (r = −0.74, p = 0.001) and speed (r = −0.59, p = 0.015) of motor learning in TD, but not in WS participants. Similar negative correlations between offline and online improvements were detected in both groups (TD r = −0.63, p = 0.01, WS r = −0.67, p = 0.005) with respect to speed, and in TD with respect to accuracy (r = −0.74, p = 0.001).
Correlations between sleep parameters and motor learning performance
Correlations between z-scores of the NREM sleep EEG 8–16 Hz spectra and motor learning in WS subjects are reported in terms of significant Rüger’s areas: continuous zones of descriptive (p < 0.05) significances in the frequency (8–16 Hz with 0.25 Hz resolution) × EEG location (10–20 system) matrices for which the area as a whole can be considered as significant (see details in Methods). No significant Rüger’s areas were revealed for baseline motor accuracy and speed performances. Bins between 11 and 13.5 Hz correlated positively with offline accuracy improvement, forming a continuous Rüger’s area involving all EEG derivations. Maximal effect occurred at derivation F4 and the 12.25 Hz frequency bin: r = 0.69, p = 0.004 (see Supplement 1 and Fig. 4A).
(A) Positive correlation between z-scores of 12.25 Hz NREM sleep EEG power, and Day 1 to Day 2 offline motor accuracy improvement in WS participants. The z-score of the 12.25 Hz power is based on the individual-specific normalization of 8–16 Hz spectra20 in the right frontal derivation (F4). Offline FT accuracy improvement is expressed in terms of percent change from Day 1 to Day 2. Light and dark grey indicates ±1 and ± 2SD of the corresponding variable in TD participants, respectively. SD of TD spectral data are from ref.21. Note that higher 12.25 Hz power is associated with higher offline FT accuracy improvement. (B) Negative correlation between parietal sigma peak frequency and Day 1 to Day 2 offline motor accuracy improvement in WS participants. Highest parietal sigma peak frequency is the frequency at which the highest observable local maxima are found in the 8–16 Hz NREM sleep EEG power spectra of WS participants. Offline FT accuracy improvement is expressed in terms of percent change from Day 1 to Day 2. Light and dark grey indicates ±1 and ±2 SD of the corresponding variable in TD participants, respectively. SD of TD spectral data are from ref.21. Note that higher oscillatory frequencies are associated with lower offline accuracy improvements.
Bins in a similar, but somewhat lower frequency range (however still consistent with the low sigma range: 9.75–12.25 Hz) correlated positively with online (practice-dependent) motor speed improvement. The Rüger’s area involved the majority of EEG locations (F3, F4, Fz, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, O1, O2, Oz), except the anterior frontal ones (Fp1, Fp2, Fpz, F7, F8). No significant Rüger’s areas were revealed for baseline motor accuracy and speed performance.
Further characteristic features of the NREM sleep EEG broadband sigma spectral profiles are the oscillatory peak frequencies. Decreased offline accuracy gains in WS participants with higher oscillatory peak frequencies are indicated by the negative correlation between the highest parietal sigma peak frequency and offline motor accuracy improvement in the FT task (r = −0.52, p = 0.03, (Fig. 4B). We found no other significant correlations between sigma peak frequencies and motor learning performances in WS participants.
Discussion
The purpose of the present work was to contribute relevant evidence on the relationship between sleep and motor learning. As it has been shown in typically developing subjects, sleep spindles contribute to the activation of the neural network involved in offline consolidation of fine motor sequences4,6,7,8,9,10,11,12. Here we studied a group of subjects whose spindle activity is modulated at the group level (a single and shifted peak in the sigma domain, see Fig. 2), and the peak frequency modulation has a great individual variability (also Fig. 2). These two factors together gave us a unique possibility to test whether spindle modulation results in altered learning performance. Our results seem to attest to that, providing unique evidence for a relevant relationship between spindles and motor learning: sigma activity closer to the TD range is associated with better offline learning in WS.
We tested whether the specific alterations of broadband sigma (8–16 Hz) activity in WS21 are associated with deficits in offline fine motor learning. We found that two of these alterations are indeed related to affected offline motor learning phase in the FT task. The previously reported decrease in low sigma range of the z-normalized spectra of WS participants is involved in the efficiency of achieving offline performance gains. WS participants, characterized by a smaller amount of decrease in z-normalized low sigma power show the highest offline gains in motor accuracy (Fig. 4A). The second finding is related to the accelerated sigma peak frequency in WS, which correlates negatively with offline motor accuracy gains. In other words, WS participants, characterized by more accelerated sigma peak frequencies exhibit less effective offline consolidation (Fig. 4B). We also report an unpredicted finding, namely, a positive correlation between the individual values of z-normalized low sigma power and online (instead of offline) gains in motor speed.
In terms of motor learning, WS participants show significantly lower baseline performance as compared to TD participants with respect to both accuracy and speed. On the other hand, low baseline in WS is not associated with sigma band activity as found previously in typically developing children, where, e.g., more slow sleep spindles are associated with lower baseline when polysomnography is administered during a three-day-learning session18. Our results indicate that WS and TD participants are not different in terms of online improvement at the group level in speed or accuracy in the initial phase of motor learning. Similarly, intact online learning performance has been found in other studies that focused on populations with sleep alterations. When individuals with obstructive sleep apnoea were compared to healthy controls, there was no difference in improvement during first day practice in a finger sequence task33. Moreover, schizophrenia patients were not different from healthy controls in accuracy34,35, and subjects with attention-deficit hyperactivity (ADHD) disorder were not different in speed from TD controls36 in terms of within-session improvement.
Surprisingly, online improvement in speed is correlated with low sigma power in WS in our present study. Given that most of the studies in the field focus on the relationship between offline gains and changes in post-training sleep, the studies on the interrelation between sleep characteristics and baseline and/or online improvement are scarce. A recent study of King et al.37 shows that cerebral activation during initial online learning predicts sleep dependent motor performance improvements in the elderly. They hypothesize that a given level of activation in motor networks involving the putamen, cerebellum and parietal cortex is required to induce sleep related consolidation. In healthy adults38, performance changes are related to the increased activation of putamen and medial temporal lobe including the hippocampus. During the initial online phase of motor sequence learning, performance improvement as measured by consistency is correlated with the interaction between the striatal and hippocampal systems that in turn predicts offline improvement in typically developing adults. That is, brain activation associated with performance gains during online learning may trigger and, in turn, predict offline performance changes. Since the above studies did not administer polysomnographic recordings, only an indirect relationship may be hypothesized between online improvement and low sigma band activity in the present study. Since brain activation during motor learning seems to be disease specific in spite of similar behavioural characteristics39, further studies need to clarify the functional relevance of given brain areas and their polysomnography correlates in motor learning in WS.
With respect to offline improvement, a pattern of dissociation is frequently observed in age related changes or in disrupted sleep: while online performance is retained, offline improvement is compromised9,33,34,35,36,40. Unlike in these previous studies, offline learning in WS was comparable to that of the TD participants from Day 1 to Day 2 in our study (Fig. 3). Individual differences in offline accuracy gains were correlated with WS-specific NREM sleep EEG sigma activity features (low sigma power and highest peak frequency) (Fig. 4B). These results are consistent with findings in recent study of individuals with ADHD. Participants with ADHD had lower baseline, but similar performance on Day 2 post-test compared to TD. Authors found positive correlation between offline improvement and slow sleep spindle activity (12–13.5 Hz) during the night after training in ADHD, but not in TD controls. Participants with higher relative power in the frequency band related to slow sleep spindles, the frequency band that is in turn the more altered in ADHD, had better overnight improvement41. Similarly, in patients with major depression, overnight learning was associated with slow frequency spindle activity (10.5–12.5 Hz), and this relationship was true for healthy control participants too42. Furthermore, typically developing children with lower initial performance but more slow spindles and slower slow waves improved more in accuracy overnight18.
In our study, we found no association between sigma band activity and offline gain in speed in WS. We analysed speed and accuracy measures separately, since our previous study showed a lower baseline and impaired learning in WS with respect to speed during a five day motor training22. Here we analyse an initial phase of learning, and we find only a marginal difference between WS and TD groups in terms of offline improvement in speed, not reaching significance, and not correlated with sigma activity in WS. Other studies allowing for a dissociation between speed and accuracy with respect to online and offline improvement show an incoherent picture. In TD children, improvement in accuracy in a FT task was sleep-dependent, while improvement in speed was not. In conditions with disrupted sleep, e.g., when individuals with ADHD were trained on a sequential FT task, they expressed delayed gains at 24 h and at 2 weeks retention in speed, but did not improve compared to baseline in accuracy36. On the other hand, in schizophrenia patients, while sleep dependent learning was compromised both in speed and accuracy34,35, offline performance gains were correlated only with the stage 2 NREM sleep length but not with spindle activity when measured in the 4th quarter of the night in schizophrenia patients35.
In spite of the exciting findings, our study also has several limitations. The number of available participants is relatively small since WS is a rare disorder. Furthermore, the difficulty of the motor task for young individuals with WS, and the presence of motor symptoms (such as tremor) in adult individuals with WS also put restriction on the number of participants. Consequently, the relatively large age-range in the study is a possible limitation of the results. While the identification of fast spindles in children is controversial43 we think that the IAM method used for spindle detection is able to detect fast spindles also in children44.It might occur as another limitation that data submitted to correlation analysis were not obtained at the same time point. However, since the studied sleep characteristics are genetically determined and stable within individuals, and across nights, this factor may not affect the interpretation of our data. Another limitation is that are not presenting a direct comparison in terms of sleep and behavioural data in typically developing control participants. However, the aim of the present study is to reveal the relationship between a known and confirmed alteration in sigma activity in NREM sleep and motor learning capacity in WS, and the studied sleep alteration is not present in TD subjects.
Our results are consistent with studies indicating the importance of sigma band activity in NREM sleep in motor memory consolidation, where it has been shown that among individuals with a neurodevelopmental disorder, characteristics of sigma band activity closer to that of TD subjects results in superior learning, with retained/higher slow sigma activity being associated with higher offline improvements41,42. We have also found a more general effect by demonstrating that trait-like NREM sigma activity characteristics (not associated with the motor training) may influence motor memory consolidation in addition to changes in post training sleep. Further studies are necessary to explore the more detailed relationship between sigma band activity and online learning, and the dissociation between speed and accuracy during motor learning. Notwithstanding that IQ is not a direct predictor of learning in the motor domain12, interpretation of data in relation to IQ may also give new insights. The WS-specific alterations of sigma band activity are of potential interest for those aiming to unravel the neural roots of the individual differences in motor learning performance in participants with WS or other neurodevelopmental disorders.
Methods
Ethics statement
The present study was approved by the Social Sciences Ethical Review Board of the Budapest University of Technology and Economics and was conducted according to the approved guidelines. Informed consent was obtained from adult participants and the parents of participating children in the study.
Participants
Motor learning:16 individuals with WS (6 males, 10 females) participated in the study. The age-range was 11 to 27 years, mean age was 18,4 years (SD:5,5 years). Three participants with WS were left-handed, two were mixed handed, and eleven were right handed as measured by tool use. WS diagnosis of all WS participants was confirmed by fluorescent in situ hybridization tests showing deletion on chromosome band 7q11.23. 11 participants took part also in a previous motor learning study16. Furthermore, 16 typically developing individuals (6 males, 10 females), age range 11–29 years, mean age 18,4 years (SD:5,6 years) participated in the study. All TD participants were right handed and did not report sleep disruptions. None of the participants were professional musicians or had skeletal deformities that could influence motor performance.
Polysomnography (PSG): 20 participants with WS took part in an earlier polysomnographic study21. A subgroup of these WS participants (n = 13) also took part in the motor learning experiment. 4 males and 9 females (age range 11–27 years, mean age: 18,6 years, SD: 5,6 years; 9 right-handed, 3 left-handed, 1 mixed-handed) with WS participated both in the ML and the PSG the studies.
Participants were allowed to go to bed at will, and were not awakened during the PSG recording, and during the night following acquisition. Prior to PSG recording, an adaptation night with the same conditions served the subjects to get used to the experimental settings. Subjects were only included in the FT experiment if a minimum of 6 hours of night sleep was reported previously, and between Day 1 and Day 2 of testing. Sleep duration was assessed by self-reports of the participants. Sleep architecture of WS participants of the present experiment is shown in Table 1.
Experimental Design
Motor learning experiment
Task and design
The motor learning task was a four elements FT task performed with the non-dominant hand. Participants touched their thumb with the index finger, followed by the ring finger, middle finger and little finger, always in this same sequence (Fig. 1). Data acquisition started after participants performed three successful sequences consecutively. They practiced 10 blocks of 16 repetitions on the first day, and 2 blocks of 16 repetitions on the second day. One of the participants with WS practiced only five blocks on the first day. Participants were allowed to rest between practice blocks at their own pace to avoid fatigue45.
Data acquisition
Data were collected using a custom-made data glove including metal rings placed on each fingertip. The data glove was connected to a laptop computer where a Java based data acquisition software stored and processed the timing and order of finger taps. Since 11 out of 16 participants took part in a previous long term motor learning study22, that database was included in the present study for performing a new analysis focusing on online and offline learning in the fast phase of learning.
Dependent variables
We monitored performance in speed and accuracy during acquisition. Speed was defined as the number of finger taps in a second (taps/s). Accuracy (%) was defined as the ratio of the number of finger taps in correctly performed sequences to all sequences. Baseline was calculated as the mean performance of the first two blocks on the first practice day, and post-training performance as the mean performance of the last two blocks on the first practice day. Online (practice-dependent) improvement was calculated as a difference between the mean of the first two blocks of a daily session and the mean of the last two blocks of the same session. Offline improvement was calculated as the difference between the last two blocks of a daily session, and the first two blocks on the following day.
Baseline performance, online and offline improvement were calculated with respect to speed and accuracy.
EEG recordings and analyses
A subgroup of WS participants from our sleep EEG study21 were enrolled in the FT learning session protocol. We analysed sleep EEG data with high interindividual variability and proven intra-individual stability (the sleep EEG fingerprints) of this subgroup in relation with the FT measures of the current study. Sleep EEG data were derived from whole night ambulatory polysomnographic records as follows. Home sleep was recorded according to the participants preferred sleeping habits by using a 32 channel SD-LTM Hardware together with the BRAIN QUICK System PLUS EVOLUTION software (Micromed, Italy). We recorded EEG according to the 10–20 system (Jaspers, 1958) at 21 recording sites (Fp1, Fp2, Fpz, F3, F4, F7, F8, Fz, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, O2, Oz) referred to the mathematically linked mastoids. We also recorded bipolar EOG, ECG and submental as well as tibialis EMG. Signals were high-pass filtered at 0.15 Hz and low-pass filtered at 250 Hz by 40 dB/decade anti-aliasing hardware input filters. Data were collected with 22 bit resolution and with an analogue to digital conversion rate of 4096 Hz/channel (synchronous). The firmware applied a further 40 dB/decade anti-aliasing digital filter which low-pass filtered the data at 463.3 Hz. The digitized and filtered EEG was subsequently undersampled at 1024 Hz. We also applied a 50 Hz digital notch filter performed by the recording software. Data were exported and converted to EDF46 before further analyses.
We visually scored sleep recordings according to standard criteria47 in 20 s epochs. Next, we manually removed the 4 s epochs containing artifacts before further automatic analyses. Average power spectral densities were calculated by a Fast Fourier Transformation (FFT) algorithm applied to the 50% overlapping, Hanning-tapered, artifact-free 4 s epochs of whole night stages 2–4 NREM sleep. We used z-scores of 8–16 Hz spectra in the statistical analyses. We introduced z-transformation in order to follow previous approaches in the field21, as well as to control for potentially simultaneous differences in general EEG amplitude and delta power, the latter being supported by our own results31 and indirectly by the reports of an increased SWS in WS31,32. The z-transformation is individual and derivation-specific as described below. Spectral values (µV2/0.25 Hz) between 8 and 16 Hz (33 bins of 0.25 Hz each) are averaged (m) and the standard deviation calculated (σ). Then the original frequency bins were replaced by the individual- and derivation-specific z-scores by using the z = (x-m)/σ formula, where x is the actual value of the frequency bin (in µV2/0.25 Hz) to be transformed. This normalization results in series with a mean of 0 and standard deviation of 1, varying between 8 and 16 Hz, and reflecting the individual- and derivation-specific shapes of the spectra, previously termed as spectral EEG fingerprints of sleep19, characterized by strong genetic determination14 and proven reliability (repeatability) in both healthy19 and WS participants21.
Spectral peaks were processed automatically as follows: the zero-crossing points of the first order derivatives of the z-scores of 8–16 Hz spectra were considered as locations of spectral peaks if the second order derivatives were negative at these frequencies (local maxima in mathematical terms). Spectral peak processing was performed on the averaged z-scores of the left and right frontal (F3 and F4), as well as on the left and right parietal (P3 and P4) derivations. Slow peaks were defined as the slowest peak in the frontal derivations, while fast peaks as the fastest ones in parietal channels.
Statistics
Finger tapping task
We performed two-way mixed ANOVA (group × baseline/online/offline) with repeated measures on baseline/online/offline regarding all dependent variables. Mauchly’s Test of Sphericity indicated that the assumption of sphericity had not been violated, therefore correction was not performed for sphericity of the repeated measures. Post hoc test were performed by using the Fisher LSD method. To investigate the relationship between baseline, online and offline improvement, we calculated correlation coefficients between these measures both in speed and accuracy. A p-value of 0.05 was set as the significance level for all statistical tests.
Correlations between spectral values and learning performance
We calculated correlations between spectral values and learning performance as follows. We correlated individual-specific slow and fast spectral peak frequencies, as well as binwise z-scores with FT measures (Pearson product-moment correlation coefficients). The binwise calculations result in an inflation of type I statistical error, thus, we applied the Descriptive Data Analysis procedure47 to control this effect. As a first step, continuous regions of descriptive, uncorrected (p < 0.05) significances derived from binwise z-power vs motor performance correlations are defined in the frequency (between 8 and 16 Hz, with 0.25 Hz resolution) × EEG location (according to the 10–20 system) matrices. These regions are termed Rüger’s areas and are further tested in order to make global confirmatory statements with controlled uncertainty (at least one of the null hypotheses in the Rüger’s area is wrong). In order to refuse this global null hypothesis, at least half of the significant correlations in the respective Rüger’s area have to be significant at the level of 0.05/2 (0.025). As our hypotheses were directional (formerly reported WS-specific features of the 8–16 Hz spectra15 were hypothesized to negatively influence motor learning), we used one-tailed tests when assessing significances. In addition, we tested the Rüger’s areas for outliers: we visually screened the scatterplots of maximal correlational effects in the potential Rüger’s areas and removed the areas where extreme values caused spurious associations from further analyses.
References
Korman, M., Raz, N., Flash, T. & Karni, A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc. Natl. Acad. Sci. USA 100, 12492–12497, https://doi.org/10.1073/pnas.2035019100 (2003).
Walker, M. P., Brakefield, T., Hobson, J. A. & Stickgold, R. Dissociable stages of human memory consolidation and reconsolidation. Nature 425, 616–620, https://doi.org/10.1038/nature01930 (2003).
Fischer, S., Hallschmid, M., Elsner, A. L. & Born, J. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. USA 99, 11987–11991, https://doi.org/10.1073/pnas.182178199 (2002).
Morin, A. et al. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep 31, 1149–1156 (2008).
Fogel, S. & Smith, C. T. Learning-dependent changes in sleep spindles and Stage 2 sleep. Journal of Sleep Research 15, 250–255 (2006).
Barakat, M. et al. Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain. Mapp 34, 2918–2928, https://doi.org/10.1002/hbm.22116 (2013).
Barakat, M. et al. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav. Brain Res. 217, 117–121, https://doi.org/10.1016/j.bbr.2010.10.019 (2011).
Fogel, S. et al. Sleep spindles: a physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol. Aging 49, 154–164, https://doi.org/10.1016/j.neurobiolaging.2016.10.009 (2017).
Fogel, S. M. et al. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum. Brain Mapp. 35, 3625–3645, https://doi.org/10.1002/hbm.22426 (2014).
Nishida, M. & Walker, M. P. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One 2, e341, https://doi.org/10.1371/journal.pone.0000341 (2007).
Tamaki, M., Matsuoka, T., Nittono, H. & Hori, T. Fast sleep spindle (13–15 hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep 31, 204–211 (2008).
Tucker, M. A. & Fishbein, W. The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J. Sleep Res. 18, 304–312, https://doi.org/10.1111/j.1365-2869.2009.00740.x (2009).
Tamaki, M. et al. Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J. Neurosci. 33, 13894–13902, https://doi.org/10.1523/JNEUROSCI.1198-13.2013 (2013).
Genzel, L. et al. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology 37, 987–998, https://doi.org/10.1016/j.psyneuen.2011.11.006 (2012).
Laventure, S. et al. NREM2 and Sleep Spindles Are Instrumental to the Consolidation of Motor Sequence Memories. PLoS Biology 14, e1002429, https://doi.org/10.1371/journal.pbio.1002429 (2016).
Tamaki, M., Matsuoka, T., Nittono, H. & Hori, T. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin. Neurophysiol. 120, 878–886, https://doi.org/10.1016/j.clinph.2009.03.006 (2009).
Appleman, E. R., Albouy, G., Doyon, J., Cronin-Golomb, A. & King, B. R. Sleep quality influences subsequent motor skill acquisition. Behavioral Neuroscience 130, 290–297, https://doi.org/10.1037/bne0000131 (2016).
Astill, R. G. et al. Sleep spindle and slow wave frequency reflect motor skill performance in primary school-age children. Front. Hum. Neurosci. 8, 910, https://doi.org/10.3389/fnhum.2014.00910 (2014).
De Gennaro, L., Ferrara, M., Vecchio, F., Curcio, G. & Bertini, M. An electroencephalographic fingerprint of human sleep. Neuroimage 26, 114–122, https://doi.org/10.1016/j.neuroimage.2005.01.020 (2005).
De Gennaro, L. et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Annals of Neurology 64, 455–460, https://doi.org/10.1002/ana.21434 (2008).
Bodizs, R., Gombos, F. & Kovacs, I. Sleep EEG fingerprints reveal accelerated thalamocortical oscillatory dynamics in Williams syndrome. Res. Dev. Disabil. 33, 153–164, https://doi.org/10.1016/j.ridd.2011.09.004 (2012).
Berencsi, A., Gombos, F. & Kovacs, I. Capacity to improve fine motor skills in Williams syndrome. J. Intellect. Disabil. Res. 60, 956–968, https://doi.org/10.1111/jir.12317 (2016).
Bellugi, U., Lichtenberger, L., Jones, W., Lai, Z. & St George, M. I. The neurocognitive profile of Williams Syndrome: a complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience 12(Suppl 1), 7–29 (2000).
Donnai, D. & Karmiloff-Smith, A. Williams syndrome: from genotype through to the cognitive phenotype. American Journal of Medical Genetics 97, 164–171 (2000).
Carrasco, X., Castillo, S., Aravena, T., Rothhammer, P. & Aboitiz, F. Williams syndrome: pediatric, neurologic, and cognitive development. Pediatr. Neurol. 32, 166–172, https://doi.org/10.1016/j.pediatrneurol.2004.09.013 (2005).
Chapman, C. A., du Plessis, A. & Pober, B. R. Neurologic findings in children and adults with Williams syndrome. J. Child Neurol. 11, 63–65 (1996).
Annaz, D., Hill, C. M., Ashworth, A., Holley, S. & Karmiloff-Smith, A. Characterisation of sleep problems in children with Williams syndrome. Res. Dev. Disabil. 32, 164–169, https://doi.org/10.1016/j.ridd.2010.09.008 (2011).
Arens, R. et al. Periodic limb movement in sleep in children with Williams syndrome. J. Pediatr. 133, 670–674 (1998).
Goldman, S. E., Malow, B. A., Newman, K. D., Roof, E. & Dykens, E. M. Sleep patterns and daytime sleepiness in adolescents and young adults with Williams syndrome. J. Intellect. Disabil. Res. 53, 182–188, https://doi.org/10.1111/j.1365-2788.2008.01140.x (2009).
Mason, T., Arens, R., Sharman, J., Pack, A. & Kaplan, P. In 12th International Professional Conference on Williams Syndrome.
Gombos, F., Bodizs, R. & Kovacs, I. Atypical sleep architecture and altered EEG spectra in Williams syndrome. J. Intellect. Disabil. Res. 55, 255–262, https://doi.org/10.1111/j.1365-2788.2010.01354.x (2011).
Mason, T. B. et al. Sleep in children with Williams Syndrome. Sleep Medicine 12, 892–897, https://doi.org/10.1016/j.sleep.2011.05.003 (2011).
Landry, S., Anderson, C., Andrewartha, P., Sasse, A. & Conduit, R. The impact of obstructive sleep apnea on motor skill acquisition and consolidation. J. Clin. Sleep. Med. 10, 491–496, https://doi.org/10.5664/jcsm.3692 (2014).
Manoach, D. S. et al. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol. Psychiatry 56, 951–956, https://doi.org/10.1016/j.biopsych.2004.09.012 (2004).
Manoach, D. S. et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J. Psychiatr. Res. 44, 112–120, https://doi.org/10.1016/j.jpsychires.2009.06.011 (2010).
Adi-Japha, E., Fox, O. & Karni, A. Atypical acquisition and atypical expression of memory consolidation gains in a motor skill in young female adults with ADHD. Res. Dev. Disabil. 32, 1011–1020, https://doi.org/10.1016/j.ridd.2011.01.048 (2011).
King, B. R. et al. Cerebral Activation During Initial Motor Learning Forecasts Subsequent Sleep-Facilitated Memory Consolidation in Older Adults. Cereb. Cortex. https://doi.org/10.1093/cercor/bhv347 (2016).
Albouy, G. et al. Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One 8, e59490, https://doi.org/10.1371/journal.pone.0059490 (2013).
Genzel, L. et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol. Psychiatry 77, 177–186, https://doi.org/10.1016/j.biopsych.2014.06.004 (2015).
Manoach, D. S. & Stickgold, R. Does abnormal sleep impair memory consolidation in schizophrenia? Front Hum Neurosci 3, 21, https://doi.org/10.3389/neuro.09.021.2009 (2009).
Saletin, J. M., Coon, W. G. & Carskadon, M. A. Stage 2 Sleep EEG Sigma Activity and Motor Learning in Childhood ADHD: A Pilot Study. Journal of Clinical Child & Adolescent Psychology, 1–10, https://doi.org/10.1080/15374416.2016.1157756 (2016).
Nishida, M., Nakashima, Y. & Nishikawa, T. Slow sleep spindle and procedural memory consolidation in patients with major depressive disorder. Nature and Science of Sleep 8, 63–72, https://doi.org/10.2147/NSS.S100337 (2016).
Hoedlmoser, K. et al. Slow Sleep Spindle Activity, Declarative Memory, and General Cognitive Abilities in C hildren. Sleep 37(9), 1501–1512, https://doi.org/10.5665/sleep.4000 (2014).
Ujma, P. P., Sándor, P., Szakadát, S., Gombos, F. & Bódizs, R. Sleep spindles and intelligence in early childhood-developmental and trait-dependent aspects. Dev. Psychol. 52(12), 2118–2129 (2016).
Pan, S. C. & Rickard, T. C. Sleep and motor learning: Is there room for consolidation? Psychol Bull 141, 812–834, https://doi.org/10.1037/bul0000009 (2015).
Kemp, B., Varri, A., Rosa, A. C., Nielsen, K. D. & Gade, J. A simple format for exchange of digitized polygraphic recordings. Electroencephalography and Clinical Neurophysiology 82, 391–393 (1992).
Rechtschaffen, A. & Kales, A. Manual of standardized terminology, techniques and scoring system for sleep stages of human participants. (Los Angeles: UCLA Brain Information Service/Brain Research Institute. (1968).
Acknowledgements
This work was supported by the Hungarian National Science Fund (OTKA-NF60806 and OTKA-NK104481 to I.K.). We are grateful to the Hungarian Williams Syndrome Association and to the participating families in the study.
Author information
Authors and Affiliations
Contributions
I.K. designed the study, R.B. and F.G. processed and analysed PSG data, A.B. collected and analysed FT data. A.B., R.B., I.K., F.G. wrote the article and Sz.L. prepared figures of the article with the help of A.B., R.B., F.G., I.K. All authors reviewed the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berencsi, A., Bódizs, R., Gombos, F. et al. Sigma frequency dependent motor learning in Williams syndrome. Sci Rep 7, 16759 (2017). https://doi.org/10.1038/s41598-017-12489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12489-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.