Abstract
We obtained hydrophobic barite (BaSO4) and rutile titanium dioxide (TiO2) particles (as raw materials) by organic surface modification. Subsequently, TiO2-coated barite composite pigments were prepared via the hydrophobic aggregation of heterogeneous particles in a water medium. The pigment properties of the TiO2-coated barite composite pigments were characterized and evaluated by determining their hiding power, oil absorption value and whiteness. The optical properties were determined by obtaining their UV-vis diffuse reflectance spectra and using the CIE-L*a*b* method. The morphology and bonding properties were investigated using scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and infrared spectroscopy (IR). The results show the similarity between the composite pigment and pure rutile TiO2: when the mass ratio of rutile TiO2 in the composite pigment was 60%, the hiding power of the TiO2-coated barite composite pigment was 90.81% of that of pure rutile TiO2. Moreover, the surfaces of the barite particles were uniformly and firmly coated by TiO2, with a hydrophobic association occurring between the hydrophobic carbon chains on the surfaces of barite and TiO2 particles.
Similar content being viewed by others
Introduction
As a transition metal oxide with excellent properties, TiO2 has demonstrated utility in many applications, including solar energy harvesting, photocatalytic hydrogen production1, 2 and white pigment. A series of TiO2 species with different morphology, structure and properties have been prepared, such as TiO2 nanotubes3, 4 TiO2 nanosheets, and TiO2 microparticles. Among these species, TiO2 nanotube-based materials have great potential in various applications as photocatalysts5, 6 and sensors because of their outstanding optical7, 8 and electrical properties. Additionally, in the pigment field, TiO2 microparticles have advantages for certain applications because of their appropriate particle size, which locates them within the best range for scattering visible light. Titanium dioxide pigment is a functional powder material consisting of the TiO2 crystal phase with a particle size of 200–300 nm. The high refractive index of TiO2 (2.7 of rutile and 2.3 of anatase) endows it with excellent properties, such as a strong hiding power, a high tinctorial strength, a high glossiness, and a strong whiteness. Therefore, titanium dioxide has become the best white pigment and is applied in numerous fields, for example, in paints, plastics, paper, inks and rubber products9, 10. However, there are several problems with the application of TiO2, such as particle aggregation and poor compatibility with organic matrices. Because of these hindrances, the pigment properties of TiO2 are often exerted ineffectively, causing a reduction in its efficiency and an increase in the amount used. Consequently, concerns regarding the environment and increased resource use during the production of TiO2 have arisen11, 12. To improve the dispersion of TiO2 and the compatibility of TiO2 with organic matrices, researchers have focused on developing new inorganic pigments comprising inorganic particles uniformly and firmly coated with TiO2 13. In these research, many inorganic non-metallic minerals have been used as substrates, including calcined kaolin14, sericite15, and silica16. Methods such as homogeneous hydrolysis17 and mechanochemistry have also been applied to the preparation of TiO2-coated inorganic composite particles.
Barite is an inorganic non-metallic mineral with a chemical composition of BaSO4. Traditionally, the barite industry mainly produces primary products of low-added value, including petroleum, weighting material for drilling mud in natural gas operations, and barium-containing chemical products18. Recently, a series of studies on new functional barite materials have been conducted to exploit the excellent optical properties, good dispersibility, chemical stability, and anti-radiation properties of fine barite particles and to explore synergistic effects19. Barite has a strong whiteness, a low oil absorption and a similar density to TiO2. Therefore, composite pigments with a similar density to TiO2 can be prepared using barite and TiO2 as raw materials. In this way, the mixed homogenization of a composite pigment and a matrix material can be improved, resulting in an improvement in production. Zhou20 has prepared barite-TiO2 composite particles by hydrolysing TiOSO4 on the surfaces of barite particles. Wang21 and Zhou22 have separately used the mechanochemistry method to prepare anatase and rutile TiO2-coated barite composite pigments with pigment properties are similar to those of TiO2.
By contrast, to prepare TiO2-coated barite composite pigments via the hydrolysis of TiOSO4 20 the precipitation product is amorphous TiO2, which should be calcined at 800–900 °C for conversion to crystalline TiO2. Inevitably, the calcination consumes a large amount of energy, and converting the TiO2•nH2O on the surface of barite into the rutile single-crystal phase is difficult. The mechanochemistry method21, 22 also consumes considerable energy because the composite pigments are prepared using a grinding mill with high power. Meanwhile, TiO2-coated barite composite pigments prepared by the two aforementioned methods have a hydrophilic surface, leading to their poor compatibility with organic matrices and a reduction in their usage efficiency. For the above reasons, a hydrophobic aggregation method has been used in this study in order to obtain uniformly and firmly TiO2-coated barite composite pigments with a hydrophobic surface while consuming less energy.
Hydrophobic aggregation refers to the aggregation of fine particles that attract each other because of their hydrophobic surfaces23. The attraction occurs at a particle distance ranging from 2 to 25 nm in a water medium, and the attraction strength is 10–100 times stronger than that of the electrical double layer force and Van der Waals force24. In particular, when the hydrophobic surfaces of particles are induced by surface modification, a strong aggregation occurs among the particles because of the association between the hydrocarbon chains on the surfaces. The abovementioned condition results in the particles combining firmly. The hydrophobic aggregation phenomenon of particles was first observed in the mineral flotation process, which has been improved and applied constantly. However, the existing studies mainly focus on the hydrophobic aggregation of homogeneous mineral particles in raw ore. For example, Song25, 26 has studied the hydrophobic aggregation and separation of fine rutile, haematite and hydrophobic coal particles. Furthermore, research regarding the hydrophobic aggregation and flotation of galena, sphalerite, rhodochrosite and other fine-grained minerals has been reported27. By contrast, there are fewer studies on the hydrophobic aggregation of heterogeneous particles, especially for the preparation of composite pigments through the hydrophobic aggregation of TiO2 and mineral particles. Because the hydrophobic aggregation of TiO2 and mineral particles needs to be based on the selective recognition among particles, performing this work is a significant challenge. In this study, TiO2-coated barite composite pigments were prepared via the hydrophobic aggregation of barite and TiO2 particles in a water medium. Meanwhile, their morphology, structure, and bonding properties were studied. Their pigment properties were also characterized and evaluated.
Experimental
Materials
The barite raw material used as the substrate in this study was produced in Hubei Province, China, and had a purity of 100%, a whiteness of 89.40%, a hiding power of 155.00 g/m2 and an oil absorption of 11.40 g/100 g. Figure 1(a) shows the morphology of the barite raw material, as determined by scanning electron microscope (SEM), wherein the barite particles were well dispersed and exhibited plate- and lump-like shapes. The particles possessed a uniform size distribution.
The rutile TiO2 raw material was produced in Henan Province, China, and had a whiteness of 94.40%, a hiding power of 10.97 g/m2, and an oil absorption of 24.26 g/100 g. Figure 1(b) shows the granular rutile TiO2 with an average particle size of 300 nm.
Analytical-grade sodium stearate (CH3(CH2)16COONa), sulfuric acid (H2SO4) and sodium hydroxide (NaOH) were used in the experiments. Meanwhile, we used chemically pure linseed oil and distilled water.
Preparation method
The TiO2-coated barite composite pigments were prepared via the hydrophobic aggregation method, which is illustrated in the flowchart in Fig. 2. The modified TiO2 slurry (MTS) was obtained through the following way: rutile TiO2 powder was dispersed in water, mixed with 3% mass ratio modifier (CH3(CH2)16COONa) and stirred for approximately 45 min at 60 °C. And for comparison, the unmodified TiO2 slurry (UTS) was prepared via the same process without the addition of modifier. Similarly, the modified barite slurry (MBS) was prepared: barite powder was dispersed in water, mixed with 1% mass ratio modifier (CH3(CH2)16COONa) and stirred for approximate 45 min at 70 °C, and the unmodified barite slurry (UBS) was also prepared via the same way without the addition of modifier. Afterwards, the composite process was conducted: the MBS was mixed with different mass ratios of the MTS. After stirring for 60 min at a pH value of 9, we obtained a barite-TiO2 slurry. Finally, the barite-TiO2 slurry was dried at 60 °C, and then the TiO2-coated barite composite pigments were prepared.
As a comparison, composite particles fabricated using UBS and UTS as raw materials were also prepared (discussed in 3.4.1). For Sample 1, the composite materials were prepared using UBS and UTS as the raw materials, the Sample 2 used MBS and UTS as raw materials, and the Sample 3 used UBS and MTS as raw materials. For all the three samples, the mass ratio of TiO2 to barite was 6:4, and the composite process was carried out through the way described in the previous paragraph. Subsequently, all the prepared slurries were dried at 60 °C to obtain the composite particles.
Characterization
Pigment properties
The pigment properties of the TiO2-coated barite composite pigments were evaluated by determining the oil absorption value, hiding power, relative hiding power and whiteness. The test methods were as follows.
Oil absorption is an important index of pigment properties, this value refers to the minimum amount of varnish (linseed oil) that can wet 100 g of the pigment completely. The oil absorption of a pigment can be tested basing on the China National Standard GB/T5211.15-2014.
Hiding power is another important and insightful index of pigment properties. It refers to the minimum amount of pigment that can completely cover a black and white checkerboard. The hiding power of a pigment can be tested using the National Industry Standards HG/T3851-2006 (the test method of pigment hiding power).
The relative hiding power (R) indicates the hiding property ratio of the composite pigment to a pure TiO2 pigment according to the definition of pigment hiding power. The R value can be calculated using Equation (1)
where HT (g/m2) and HCT (g/m2) are the hiding powers of TiO2 and the TiO2–coated barite composite pigments, respectively.
The value of ΔR calculated by Equation (2) represents the magnitude of the increase in the hiding power of TiO2 upon forming its composite with barite.
where R0 is the mass ratio of TiO2 to the composite pigment.
The whiteness was tested using a whiteness meter (SBDY-1, Shanghai Yuet Feng Instrument Co. Ltd., China).
Optical properties
The optical properties of the TiO2-coated barite composite pigments were characterized by acquiring their UV-vis diffuse reflectance spectra and using the CIE-L*a*b* method. The UV-vis spectra of the prepared composite pigments were obtained between 200 and 800 nm on a TU-1901 double beam spectrophotometer. The L*a*b* parameters of representative specimens were measured using a portable integrating sphere spectrophotometer (X-Rite Sp60, X-Rite (Shanghai) International Trade Co., Ltd., China). The CIE-L*a*b* colourimetric method recommended by the CIE (Commission Internationale de l’Éclairage) was followed. In this colour system, L* is the colour lightness (L* = 0 for black and 100 for white), a* is the green (−)/red (+) axis, and b* is the blue (−)/yellow (+) axis28.
Morphology and structure
The hydrophobic properties of the particles were evaluated based on the wet contact angle obtained by contact angle measurement (JC2000D, Shanghai Zhongchen Digital Technic Apparatus Co. Ltd., China). First, the powder samples were pressed into a thin sheet with a smooth surface by a tablet pressing machine. Next, the contact angle of the samples was measured three times. Then, the results were averaged. The images of TiO2-coated barite particles dispersed in an organic medium were obtained by an image analyser (BT-1600, Bettersize Instruments Ltd., China)
We observed the morphology of barite, rutile TiO2, and TiO2-coated barite composite pigments by SEM (S-3500N, HITACHI, Japan). The surface functional groups were examined by infrared spectroscopy (Spectrum 100, PerkinElmer Instruments (Shanghai) Co. Ltd., China) using KBr as the medium. Further analysis was carried out using X-ray photoelectron spectroscopy (XPS, Escalab 250xi, Thermo Fisher Scientific USA) and X-ray diffraction (D/MAX2000, Rigaku Corporation, Japan).
Results and Discussion
Pigment properties of TiO2-coated barite composite pigments
Barite and rutile TiO2 particles were modified using sodium stearate. Based on the induced surface hydrophobicity of the modified particles (the wetting contact angles of barite and TiO2 particles are 128.5° and 114.2°, respectively), TiO2-coated barite composite pigments with different TiO2 mass ratios were prepared via the hydrophobic aggregation method. Table 1 shows the pigment properties of the TiO2-coated barite composite pigments and raw materials.
The results show that the hiding power of the barite raw material is 155.00 g/m2, which indicates no hiding properties. However, the hiding power clearly improved after the barite particles were coated by TiO2 particles and increased as the TiO2 mass ratio increased. When the TiO2 mass ratio increased to 60%, the hiding power and relative hiding power (R) of the TiO2-coated barite composite pigments were 12.08 g/m2 and 90.81%, respectively, nearly reaching the levels observed for pure rutile TiO2, for which the R value exceeds 30.81% (ΔR) compared with the mass ratio of TiO2 (R0). In addition, the TiO2-coated barite composite pigments had a lower oil absorption value than that of TiO2 and a similar whiteness to TiO2. Additionally, the contact angle of the composite particles was more than 100°. The above results show that the TiO2-coated barite composite pigments possessed pigment properties that were completely different from those of barite but similar to those of TiO2. Furthermore, the composite pigments exhibited hydrophobicity. It can be concluded that the barite particles were uniformly and firmly coated by TiO2 particles.
Figure 3 shows the morphology of the TiO2-coated barite composite pigments with different TiO2 mass ratios. Figure 3a,b and c show that the number of TiO2 particles and the coated area of the barite surface increased remarkably as the TiO2 mass ratio increased from 20% to 60%, giving a uniform coating. Particularly, when the TiO2 mass ratio increased to 60%, the surfaces of the barite particles were nearly completely covered by the TiO2 particles. The results in Table 2 show the atomic percentages of different samples obtained from XPS analysis. Clearly, as the mass fraction of TiO2 increases, the percentage of Ti atoms on the surface of the barite particles increased, whereas the number of Ba atoms decreased. Meanwhile, the value of Ti/Ba increased as the TiO2 mass fraction increased. The above results show that the TiO2 coated on the barite surface becomes more compact as the TiO2 content increases. The SEM and XPS results are consistent with the results in Table 1.
Optical properties of TiO2-coated barite composite particles
There are several methods for measuring the colour of pigments, and the CIE-L*a*b* values were used to specify and compare the colour of the TiO2-coated barite composite pigments with different TiO2 mass ratios. Table 3 shows the results.
The L* value of the TiO2-coated barite composite pigment gradually increased with the TiO2 mass ratio until it was close to that of rutile TiO2. This observation indicates that the lightness of the composite pigments improved because of the coating of TiO2 particles on the barite particles surface. Similarly, the a* and b* values of the TiO2-coated barite composite particles are also close to those of rutile TiO2 at increased TiO2 mass ratios. Meanwhile, the total colour difference (∆E) between the TiO2-coated barite composite pigments and rutile TiO2, which was used to compare the colour of two samples, ranges from 0.66 to 1.93. The ∆E value decreased as the TiO2 mass ratio increased. These results indicate that the TiO2-coated barite composite pigments display a similar visual colour to rutile TiO2 because of the coating of TiO2 particles on the barite particle surface.
The UV-vis absorption spectra of TiO2-coated barite composite pigment (TiO2 mass ratio was 60%), barite and TiO2 were obtained and compared (See Fig. 4). There was less light absorbed by barite between 300 and 400 nm, whereas TiO2 absorbed light of wavelengths below 400 nm. The TiO2-coated barite composite pigments exhibited light absorption in a wavelength range from 200 to 400 nm. These pigments also show high light absorption at wavelengths of 300–400 nm, in complete contrast to barite but similar to TiO2. The results indicate that the TiO2-coated barite composite pigment has equivalent UV absorption and anti-UV properties, confirming that the barite is coated by TiO2 particles with similar optical properties to TiO2.
Compatibility with an organic matrix
To investigate the compatibility of the TiO2-coated barite composite particles with an organic matrix, a dispersion experiment was performed. In this experiment, 1 g of composite particles was dispersed in 100 mL of kerosene to prepare a suspension, which was stirred for 30 min at a speed of 800 rpm. After stirring, a small amount of the suspension was taken to prepare samples. Images of the composite pigments in the kerosene medium are shown as Fig. 5. There are two types of particles in this experiment: the TiO2-coated barite composite particles in Fig. 5(a), which were prepared by the mechanochemical method21, 22 and the particles in Fig. 5(b), which were prepared by the hydrophobic aggregation method.
Clearly, the particle clusters in Fig. 5(a) are significantly larger than those in Fig. 5(b). In the kerosene medium, the TiO2-coated barite composite particles prepared by the mechanochemical method agglomerate to form big aggregate particles with a size of 20–50 μm, which reflects their poor compatibility with the organic matrix. However, the particles observed in Fig. 5(b) possess a smaller particle size and exhibit good dispersion, indicating that the TiO2-coated barite composite particles with a hydrophobic surface have good compatibility with the organic matrix.
Bonding properties of barite and TiO2 particles
Effect of barite and TiO2 particles’ surface hydrophobicity
To investigate the effect of surface hydrophobicity and the carbon chain of the modifier on TiO2 and barite particles and to further elucidate the bonding mechanism, TiO2-coated barite composite pigments (the TiO2 mass ratio was 60%) were prepared under the following conditions: The barite raw material had a contact angle of 22.5°, the modified barite material had a contact angle of 128.5°, the TiO2 raw material had a contact angle of 33.2°, and the modified TiO2 had a contact angle of 114.2°. The preparation details of the four samples are described in experiment section, Table 4 shows the pigment properties.
Among the four samples, Sample 4 exhibited the best pigment properties with a hiding power and a relative hiding power (R) of 12.08 g/m2 and 90.81%, respectively. By contrast, the other three samples exhibited poor hiding properties with ∆R values ranging only from 6.04 to 14.04%, this result indicates the poor compositing of these samples. Based on these findings, it can be concluded that the composite performs effectively only when both the barite and TiO2 surfaces exhibit hydrophobicity. Because the hydrophobicity of TiO2 and barite particles is obtained via modification with sodium stearate, the organic carbon chains of the modifier on the particle surface play an important part in the composite process. These observations demonstrate the hydrophobic interaction of barite and TiO2 particles.
Figure 6 shows SEM images of the four samples. The clear view of the exposed surface of barite in Samples 1, 2, and 3 indicate that the barite particles in these samples have not been well coated by the TiO2 particles. By contrast, the barite-TiO2 composite particles in Sample 4 show an excellent coating morphology, where the barite particles are thoroughly coated by the TiO2 particles. Figure 7 shows the composite model. The microstructure of the composite particles in the four samples can be used to explain the results in Table 4.
Bonding strength analysis of barite and TiO2 particles
The bonding strength between barite and TiO2 particles is undoubtedly another key factor influencing the composite pigment properties. To evaluate the bonding strength between the barite and TiO2 particles, an experiment based on the ultrasonic treatment of the TiO2-coated barite composite pigments was carried out. First, the as-prepared TiO2-coated barite composite pigments were mixed with ethanol-water (the mass ratio of ethanol to water was 1:4) to prepare a suspension, and then the suspension was ultrasonicated for 10 min at different powers with an ultrasonic oscillator. Finally, the samples were obtained after drying. Figure 8 shows the SEM images of the samples.
The barite particles were still coated by TiO2 particles after the TiO2-coated barite composite pigments were ultrasonicated at powers of 100 and 400 W. When the power increased to 800 W, only a small amount of the TiO2 particles were removed from the barite particle surface. These results indicate that the bonding between barite and TiO2 particles is strong enough, as the energy of ultrasonic vibration is substantially stronger than the Van der Waals forces of barite-TiO2 particles21. This phenomenon can account for the similarity in pigment properties between the composite pigment and TiO2. The aforementioned results also indicate that the bonding between TiO2 and barite in the TiO2-coated barite composite pigments is chemical or another physical combination with relatively large bonding energy.
XRD analysis
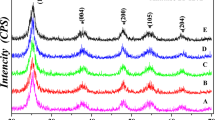
The XRD patterns of TiO2, barite and TiO2-coated barite composite pigments (see Fig. 9) show that the raw materials used in this study comprise pure barite (JCPDS 24–1035) and rutile crystal phase (JCPDS 21–1276). Only the diffraction peaks of barite and rutile appear at Fig. 9c, indicating that there is no new phase produced in the process of preparing the composite particles. Meanwhile, the barite and TiO2 raw materials remain firmly in their complete crystal phases. Therefore, it can be inferred that the barite and TiO2 particles form a mixed phase rather than undergoing a chemical reaction during the preparation process.
XPS analysis
The XPS analysis was carried out to prove the presence of TiO2 particles in the TiO2-coated barite composite pigment (the TiO2 mass ratio is 60%), and also to elucidate the bonding mechanism. Figure 10 shows the wide-scan XPS spectra of the modified barite, modified rutile TiO2, and TiO2-coated composite pigments.
The spectrum of the modified barite contains Ba 3d, S 2p and O 1s peaks, whereas the spectrum of modified TiO2 shows Ti 2p and O 1s peaks, consistent with the composition of the raw materials. In contrast to the modified barite, the spectrum of the TiO2-coated barite composite pigment contains the Ti 2p peaks, suggesting that the barite surface was indeed coated with rutile TiO2.
Figure 11 shows the narrow-scan spectra of Ba 3d, S 2p, and Ti 2p in the modified barite, rutile TiO2 and composite particles. In Fig. 11(a), the Ba 3d peaks appear at 780.40 eV and 780.75 eV, with the two peaks nearly overlapping. This finding indicates that the chemical state of barite was not changed during the compositing. The S 2p peaks in Fig. 11(b) appear at 169.10 eV and 168.95 eV and are assigned to SO4 2−. This result indicates that SO4 2− is not involved in any chemical reaction29. Meanwhile, the peaks observed at 458.55 and 458.40 eV in the XPS spectrum of Ti 2p (see Fig. 11(c)) represent the Ti 2p3/2 species of Ti4+ in TiO2 30, 31, confirming that the chemical state of TiO2 was not changed. The abovementioned results show that no chemical reaction occurred between the barite and TiO2 particles.
IR analysis
FTIR was used to illustrate the characteristic groups of sodium stearate, the modified rutile TiO2, the modified barite, and the TiO2-coated barite composite pigment (TiO2 mass ratio is 60%). The results of FTIR analysis are shown in Fig. 12. In Fig. 12(a), the absorption peaks induced by the stretching vibrations of C-H bonds in the –CH3 and –CH2- groups appear at 2,917 and 2,850 cm−1. Figure 12(b) displays peaks located at below 1,000 cm−1, which are induced by the stretching vibrations of Ti-O-Ti bonds. These peaks are a characteristic rutile band32, 33. The absorption peak of hydroxyl groups (O-H) appears at 3,420 cm−1 because of the intense hydration of unsaturated Ti4+. The absorption bands appearing at 2,917 and 2,849 cm−1 are induced by the vibrations of C-H bonds34. The aforementioned findings are attributed to the adsorption of the sodium stearate group (C15H35COOH or C15H35COO−) on the surface of the TiO2 particles. Because of the existence of the O-H groups in TiO2, it can be inferred that sodium stearate was adsorbed on the TiO2 particle surface via its reaction with O-H. In Fig. 12 (c), the absorption peaks of -CH3 and -CH2- at 2,917 and 2,850 cm−1 are present, indicating that the modifier was adsorbed on the surface of the barite particles. In addition, the absorption peaks appearing in the range from 900 to 1,200 cm−1 are typical SO4 2− bands35, 36 and the absorption bands of a terminal hydroxyl group (O-H) at 3,420 cm−1 are induced by the hydration of SO4 2 37, 38. Based on the aforementioned results, sodium stearate was adsorbed on the surface of barite through an adsorption mechanism similar to that of TiO2.
In Fig. 12(d), the characteristic adsorption bands of TiO2 appear, and there are no new absorption peaks. This observation indicates that the TiO2 coated the surface of the barite without chemical bonding. Meanwhile, the absorption bands of -CH3 and -CH2- also appear with a higher intensity; thus, the composite of TiO2 and barite particles is induced by the hydrophobic interaction between the organic carbon chains on their surfaces. Since the interaction can induce the strong intertwining of the extended organic carbon chains on the surface of the particles, the particles are combined firmly with a binding energy that is much stronger than the Van der Waals force24.
Composite mechanism and model
Figure 13 shows the composite model of the TiO2-coated barite composite pigment. Based on the aforementioned research and analysis, the composite mechanism of the TiO2-coated barite composite pigment prepared by hydrophobic aggregation can be described as follows:
First, as Fig. 13(a) and (b) show, the sodium stearate used as a modifier is adsorbed on the barite and TiO2 particle surfaces via the reaction between C15H35COOH and the -OH groups on the surfaces of the TiO2 and barite particles. The surfaces of the barite and TiO2 particles are then covered by the organic groups of the modifier, and the hydrophobic groups are directed outward, thus increasing the hydrophobicity of particles. Second, the modified TiO2 and barite are mixed and stirred in water, and the energy produced by stirring promotes the collision of the particles. Therefore, the distance between particles is reduced to such a range that the carbon chains adsorbed on the surfaces of particles are in contact. Then, the composite of barite and TiO2 particles is formed by the interaction of the organic carbon chains (See Fig. 13(c)). Finally, the TiO2-coated barite composite pigments with good pigment properties and hydrophobicity are prepared.
Several reasons may account for the good pigment properties and hydrophobicity of the TiO2-coated barite composite pigments. Because of the TiO2 coating on the surface of barite, the hydration hydroxyl groups on the surface of barite are covered. Meanwhile, the amount of -CH3 and -CH2- groups increase with the incorporation of the organic carbon chains on the surface of the composite particles.
Conclusions
Based on the surface hydrophobicity of barite and rutile TiO2 particles induced by organic surface modification, rutile TiO2-coated barite composite pigments with similar pigment properties to those of rutile TiO2 were prepared by heterogeneous particle hydrophobic aggregation in a water medium. When the mass ratio of TiO2 was 60%, the hiding power of the composite pigment was 12.08 g/m2, which was equivalent to 90.81% of that of pure rutile TiO2 pigment. Additionally, the TiO2-coated barite composite pigment exhibited a strong surface hydrophobicity and similar optical properties to those of rutile TiO2.
The TiO2-coated barite composite pigment was characterized as TiO2 particles uniformly and compactly coated on the barite particles surfaces. Notably, the coating structure could be formed only when both the surfaces of barite and TiO2 particles had strong hydrophobicity. The barite and TiO2 particles were combined by the association of the organic carbon chains on their surfaces without chemical reaction.
According to the reaction and bonding characteristics, we established the modification and composite model of the TiO2-coated barite composite pigments prepared by the hydrophobic aggregation method.
References
Roy, P., Berger, S. & Schmuki, P. TiO2 nanotubes: synthesis and applications. Angew. Chem. Int. Ed. 50, 2904–2939 (2011).
Chappanda, K. N. et al. TiO2–WO3 composite nanotubes from co–sputtered thin films on Si substrate for enhanced photoelectrochemical water splitting. J. Electrochem. Soc. 161, H431–H437 (2014).
Chappanda, K. N. et al. Effect of sputtering parameters on the morphology of TiO2 nanotubes synthesized from thin Ti film on Si substrate. IEEE. T. Nanotechnol. 14, 18–25 (2015).
Chappanda, K. N., Smith, Y. R., Misra, M. & Mohanty, S. K. Site-specific and patterned growth of TiO2 nanotube arrays from e-beam evaporated thin titanium film on Si wafer. Nanotechnology 23, 385601 (2012).
Smith, Y. R., Chappanda, K. N., Mohanty, S. K. & Misra, M. TiO2-WO3 nanotubular composite synthesized by anodization of simultaneous multi-target sputtered thin films characterized by laser ablation ICP-MS. ECS Trans. 58, 115–124 (2014).
Nguyen, N. T. et al. Optimizing TiO2 nanotube morphology for enhanced photocatalytic H2 evolution using single-walled and highly ordered TiO2 nanotubes decorated with dewetted Au nanoparticles. Electrochem. Commun. 79, 46–50 (2017).
Kment, S. et al. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting - superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 46, 3716–3769 (2017).
Nguyen, N. T., Yoo, J. E., Altomare, M. & Schmuki, P. “Suspended” Pt nanoparticles over TiO2 nanotubes for enhanced photocatalytic H2 evolution. Chem. Commun. 50, 9653–9656 (2014).
Morsy, F. A., El-Sherbiny, S., Samir, M. & Fouad, O. A. Application of nanostructured titanium dioxide pigments in paper coating: a comparison between prepared and commercially available ones. J. Coat. Technol. Res. 13, 307–316 (2016).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Pei, R. The Production of TiO2 by sulfuric acid process (Chemical Industry Press, 1982).
Tang, Z. Environmental management and safety in the production of titanium dioxide in Production and environmental treatment of titanium dioxide (ed. Wang, X.) 211–251 (Chemical Industry Press, 2000).
Zhao, X. et al. Preparation and mechanism of TiO2 -coated illite composite pigments. Dyes Pigments 108, 84–92 (2014).
Wang, B., Ding, H. & Deng, Y. Characterization of calcined kaolin/TiO2, composite particle material prepared by mechano-chemical method. J. Wuhan Univ. Technol. 25, 765–769 (2010).
Hou, X., Yu, S., Ding, H. & Ye, C. Preparation of sericite-TiO2 composite particle material by mechano-chemical method and its application. Adv. Mater. Res. 427, 104–109 (2012).
Sun, S. J., Ding, H., Luo, Q. & Chen, S. J. The preparation of silica–TiO2 composite by mechanochemistry method and its properties as a pigment. Mater. Res. Innovations 19, 269–272 (2015).
Zhao, X., Li, J. & Zhang, Y. Preparation of nanosized anatase TiO2 -coated illite composite pigments by Ti(SO4)2 hydrolysis. Powder Technol. 271, 262–269 (2015).
Akkurt, I., Basyigit, C., Kilincarslan, S. & Mavi, B. The shielding of γ-rays by concretes produced with barite. Prog. Nucl. Energy 46, 1–11 (2005).
Bahl, S. et al. Characterization and luminescence studies of Eu doped barite nanophosphor. J. Lumin. 149, 176–184 (2014).
Zhou, H., Wang, M., Ding, H. & Du, G. Preparation and characterization of barite/TiO2 composite particles. Adv. Mater. Sci. Eng. 2015, 1–8 (2015).
Wang, B., Ding, H. & Wang, Y. Preparation of barite/TiO2 composite particle and interaction mechanism between TiO2 and barite particles. Rare Metal. Mat. Eng. S3, 193–197 (2011).
Zhou, H., Ding, H. & Yang, Q. R. Study on preparation of barite-TiO2 composite particles by mechanochemical method and its pigments performance. Ind. Miner. Process 2, 18–23 (2015).
Duzyol, S. & Ozkan, A. Role of hydrophobicity and surface tension on shear flocculation and oil agglomeration of magnesite. Sep. Purif. Technol. 72, 7–12 (2010).
Israelachvili, J. N. & Pashley, R. M. Measurement of the hydrophobic interaction between two hydrophobic surfaces in aqueous electrolyte solutions. J. Colloid Interf. Sci. 98, 500–514 (1984).
Song, S., Lu, S. & Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 15, 415–422 (2002).
Song, S. Experimental studies on hydrophobic flocculation of coal fines in aqueous solutions and flotation of flocculated coal. Int. J. Oil Gas Coal T. 1, 180–193 (2008).
Song, S., Lopez-Valdivieso, A. & Ding, Y. Effects of nonpolar oil on hydrophobic flocculation of hematite and rhodochrosite fines. Powder Technol. 101, 73–80 (1999).
Gao, Q., Wu, X., Fan, Y. & Zhou, X. Low temperature synthesis and characterization of rutile TiO2 -coated mica–titania pigments. Dyes Pigments 95, 534–539 (2012).
Paal, Z., Matusek, K. & Muhler, M. Sulfur adsorbed on Pt catalyst: its chemical state and effect on catalytic properties as studied by electron spectroscopy and n-hexane test reactions. Appl. Catal. A 149, 113–132 (1997).
Wen, Y., Ding, H. & Shan, Y. Preparation and visible light photocatalytic activity of Ag/TiO2/graphene nanocomposite. Nanoscale 3, 4411–4417 (2011).
Yu, D., Bai, J., Liang, H., Ma, T. & Li, C. AgI-modified TiO2 supported by PAN nanofibers: A heterostructured composite with enhanced visible-light catalytic activity in degrading MO. Dyes Pigments 133, 51–59 (2016).
Bezrodna, T. et al. Spectroscopic study of TiO2 (rutile)–benzophenone heterogeneous systems. J. Mol. Struct. 614, 315–324 (2002).
Bennett, R. A., Mulley, J. S., Newton, M. A. & Surman, M. Spectroscopy of ultrathin epitaxial rutile TiO2 (110) films grown on W(100). J. Chem. Phys. 127, 1–7 (2007).
Tran, H. V., Tran, L. D., Vu, H. D. & Thai, H. Facile surface modification of nanoprecipitated calcium carbonate by adsorption of sodium stearate in aqueous solution. Colloid Surface. A. 366, 95–103 (2010).
Sekar, G., Ramakrishnan, V. & Aruldhas, G. IR and polarized Raman spectra of K2Mg(SO4) 2·6H2O. J. Solid State Chem. 66, 235–241 (1987).
Bala, H. et al. Preparation of BaSO4 nanoparticles with self-dispersing properties. Colloid. Surface. A. 252, 129–134 (2005).
Lam, T. D., Hoang, T. V., Quang, D. T. & Kim, J. S. Effect of nanosized and surface-modified precipitated calcium carbonate on properties of CaCO3 /polypropylene nanocomposites. Mater. Sci. Eng. A. 501, 87–93 (2009).
Nakamoto, K. Compounds of sulfur and selenium in Infrared and Raman spectra of inorganic and coordination compounds 292–296 (John Wiley & Sons, 2009).
Acknowledgements
The authors would like to thank the Technical Institute of Physics and Chemistry for their technical assistance. This work was supported by The National Key Technology R&D Program (Grant No. 2008BAE60B06).
Author information
Authors and Affiliations
Contributions
Sijia Sun and Hao Ding wrote the main manuscript text, and Hong Zhou prepared Figures 1 and 10. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, S., Ding, H. & Zhou, H. Preparation of TiO2-coated barite composite pigments by the hydrophobic aggregation method and their structure and properties. Sci Rep 7, 10083 (2017). https://doi.org/10.1038/s41598-017-10620-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10620-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.