Abstract
The aim of the present study is to investigate the role and potential mechanisms of peripheral serotonin in postoperative intra-abdominal adhesion formation in mice. The caecum-rubbing operations were conducted for intra-abdominal adhesion formation modelling in wild-type and Tph1−/− mice. The deficiency of serotonin significantly decreased the adhesion scores, weight loss, and adhesion thickness as well as levels of collagen fibres and hydroxyproline in the adhesive tissues. The Tph1−/− mice exhibited a milder inflammatory response and oxidative stress in the adhesive tissues than did the wild-type mice. Moreover, the deficiency of serotonin reduced the levels of PAI-1 and fibrinogen, and raised the t-PA and t-PA/PAI levels in the peritoneal fluids. Moreover, the expressions of CD34, VEGF, TGF-β and 5-HT2B receptor in the adhesive tissues were significantly decreased in the Tph1−/− group mice. Furthermore, the Tph1−/− +5-HTP group showed more severe adhesions than did the Tph1−/− group mice, and the p-chlorophenylalanine (PCPA) could markedly alleviated the adhesion formation in the WT mice. In conclusion, the present study showed that peripheral serotonin regulated postoperative intra-abdominal adhesion formation by facilitating inflammation, oxidative stress, disorder of the fibrinolytic system, angiopoiesis and TGF-β1 expression via the 5-HT2B receptor in the adhesive tissues.
Similar content being viewed by others
Introduction
Postoperative intra-abdominal adhesion formation is the most common complication after intra-abdominal surgery. The incidence rate reaches 90% to 95% with limited preventive measures1. The adhesions may cause intestinal obstruction, intra-abdominal pain, infertility and increase subsequent operation difficulty2, 3. The pathogenesis of postoperative intra-abdominal adhesion formation is limited. The most acceptable opinion is that the injury of the peritoneum or tissues and the subsequent wound healing processes generate the adhesion4. Numerous studies have verified disorders of the fibrinolytic process, such as the imbalance of tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1), which contribute to adhesion formation5, 6. Angiogenesis-related factors, such as vascular endothelial growth factor (VEGF), CD31, and CD34, have been demonstrated to be highly expressed in adhesive tissues7, 8. Moreover, the inflammatory process plays a vital role in the pathogenesis of postoperative intra-abdominal adhesions9. Several anti-inflammatory drugs have been developed to alleviate postoperative intra-abdominal adhesion formation10,11,12. Additionally, hypoxia performs an important role in adhesion formation after injury. Hypoxia stimulates a cascade of events that leads to oxidative stress, nitrative stress and lipid peroxidation, which further fuel inflammation and cause direct injury to aggravate adhesion formation13, 14. In addition, several proteins including transforming growth factor β1 (TGF-β1), cyclo-oxygenases (COXs), matrix metalloproteases (MMPs), and tissue inhibitors of metalloproteases(TIMPs) are associated with adhesion formation15.
Serotonin, or 5-hydroxytryptamine (5-HT), is a vital vasoactive substance and regulatory factor in the gastrointestinal (GI) tract in addition to its role as a brain neurotransmitter. Tryptophan hydroxylase 1 (Tph1) is the rate-limiting enzyme in peripheral serotonin synthesis. According to Bader et al., Tph1 knockout mice maintained approximately a 4% serotonin level in the duodenum; however, they maintained other normal conditions similar to the wild-type C57BL/6 mice16, 17. Peripheral serotonin participates in the regulation of platelet aggregation, cardiac function, metabolism, immune responses, and other functions18,19,20,21.
Peripheral serotonin plays important roles in many diseases. It has been verified that serotonin has marked pro-inflammatory and pro-oxidation functions in inflammation-linked diseases22,23,24. Serotonin facilitates tissue remodelling and fibrosis by up-regulating TGF-β1 expression25, 26. In addition, the dysregulation of peripheral serotonin is involved in the pathogenesis of cardiac remodelling, sepsis, diabetes, colitis, pulmonary arterial hypertension, ischaemia reperfusion injury, liver regeneration, tumourigenesis and other conditions27,28,29,30. However, there was no evidence in previous studies that serotonin regulates postoperative intra-abdominal adhesion formation. The purpose of the present study was to explore the impact of serotonin on tissue fibrosis, the fibrinolytic system, angiogenesis and the inflammatory response in postoperative intra-abdominal adhesion formation in mice.
Results
Tph1−/− mice exhibited a milder extent of postoperative intra-abdominal adhesion formation induced by caecum rubbing
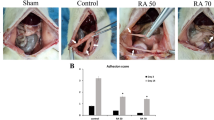
On days 1, 3 and 7 after caecum rubbing, the Tph1−/− and wild-type mice were sacrificed to observe the macroscopic adhesion conditions and assess the adhesion scores. The mice that underwent the caecum-rubbing procedure displayed varying degrees of adhesion that involved the peritoneum, omentum, epididymal fat pad, caecum, small intestine, liver and other structures. It was noteworthy that the Tph1−/− mice exhibited a milder extent of adhesion formation (Fig. 1A). Furthermore, the Tph1−/− mice received significantly lower scores than the WT mice on the 1st, 3rd and 7th days postoperatively (p < 0.05) (Fig. 1B). Additionally, the percentage of weight loss in the Tph1−/− group was less than that in the WT group on the 3rd and 7th day postoperatively (p < 0.05) (Fig. 1C).
Tph1−/− mice exhibited a milder extent of postoperative intra-abdominal adhesion formation. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 1st, 3rd and 7th days after the operation. (A) Representative images of intra-abdominal adhesions on postoperative day 7; (B) The intra-abdominal adhesion scores based on the Nair’s criteria on postoperative days 1, 3 and 7; (C) The percentage of weight loss on postoperative days 1, 3 and 7. n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Deficiency of serotonin decreased the collagen deposition in postoperative intra-abdominal adhesions in mice
Picrosirius red staining was conducted to detect the collagen fibres in the adhesive tissues. The operation of caecum rubbing generated thick and dense collagen fibres in the abdominal cavity in the WT mice. Nevertheless, the Tph1−/− mice appeared to have thinner and looser collagen fibres ((Fig. 2A). The percentage of the area that stained positive with the picrosirius red stain area in the adhesive tissues of the Tph1−/− mice was significantly lower than that in the WT mice on the 1st, 3rd and 7th days postoperatively (p < 0.05) (Fig. 2B). The thickness of the adhesion layers was less in the Tph1−/− group in contrast to the WT group (p < 0.05) (Fig. 2C). Hydroxyproline is a type of characteristic amino acid in collagen that can reflect the level of collagen expression. The hydroxyproline contents were significantly increased after caecum rubbing, and the mice in the Tph1−/− group dramatically exhibited lower levels of hydroxyproline than did the mice in the WT group (p < 0.05) (Fig. 2D). The results indicated that the deficiency of serotonin decreased collagen deposition in the adhesive tissues.
Deficiency of serotonin decreased collagen deposition in postoperative intra-abdominal adhesions in mice. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 1st, 3rd and 7th days after the operation. The adhesive tissues in each group were removed and processed for analysis. (A) Representative images of picrosirius red staining showing collagen fibres in the adhesive tissues (magnification ×200); (B) Positively stained areas in the adhesive tissues using picrosirius red was calculated by ImagePro Plus 5.0 software; (C) The thickness of the collagen deposition in the adhesive tissues; (D) The hydroxyproline content in the adhesive tissues. n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Deficiency of serotonin attenuated inflammation in postoperative intra-abdominal adhesion formation in mice
To determine the role of serotonin in the inflammatory response, the HE staining of the adhesive tissues and serum inflammatory cytokines were detected. The WT mice that underwent caecum rubbing exhibited massive inflammatory cell infiltration, including giant cells, lymphocytes, plasma cells, eosinophils and neutrophils, and even microabscess formation in the adhesive tissues. In contrast, the Tph1−/− mice only showed a mild inflammatory response that involved giant cells, lymphocytes, plasma cells, and a small number of eosinophils and neutrophils (Fig. 3A). The inflammation scoring based on the inflammatory cell infiltration and microabscess formation verified that the Tph1−/− group mice achieved markedly lower scores compared with the WT group on postoperative days 1, 3 and 7 (p < 0.05) (Fig. 3B). TNF-α and IL-6 are the initial and pivotal inflammatory cytokines that trigger the inflammatory cascades and promote postoperative intra-abdominal adhesion formation. A significant increase in serum TNF-α and IL-6 was observed after the cecum rubbing operation (p < 0.05). Additionally, the Tph1−/− group had significantly lower levels of serum TNF-α and IL-6 compared with the WT group (p < 0.05) (Fig. 3C,D).
Deficiency of serotonin attenuated inflammation in postoperative intra-abdominal adhesion formation in mice. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 1st, 3rd and 7th days after the operation. The adhesive tissues and blood samples in each group were harvested for analysis. (A) Representative images of haematoxylin-eosin staining of adhesive tissues (magnification ×200); (B) The inflammation scores were based on inflammatory cell infiltration and microabscess formation in the adhesive tissues; The levels of serum (C) TNF-α and (D) IL-6; n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Deficiency of serotonin alleviated oxidative stress in postoperative intra-abdominal adhesion formation in mice
Oxidative stress participates in postoperative intra-abdominal adhesion formation with its direct damage to reactive oxygen species and indirect function of aggravating inflammation31. The Tph1−/− group mice had lower levels of MDA and MPO compared with the WT group (p < 0.05) (Fig. 4A,B). The protective indicators, SOD and GSH, were significantly decreased after the creation of the intra-abdominal adhesion model; however, the deficiency of serotonin exhibited certain degrees of reversal (p < 0.05) (Fig. 4C,D).
Deficiency of serotonin alleviated oxidative stress in postoperative intra-abdominal adhesion formation in mice. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 1st, 3rd and 7th days after the operation. The adhesive tissues in each group were harvested to evaluate the oxidative stress. The levels of (A) MDA, (B) MPO, (C) SOD and (D) GSH in the adhesive tissues; n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Deficiency of serotonin relieved the disorders of the fibrinolytic system in postoperative intra-abdominal adhesion formation in mice
The levels of t-PA in the peritoneal fluids were increased after adhesion modelling occurred. However, the Tph1−/− group mice had higher levels of t-PA compared with the WT group mice on postoperative days 1 and 3 (p < 0.05). The PAI-1, the t-PA antagonist, exhibited the opposite changes (Fig. 5A,B). The ratio of t-PA/PAI-1 was higher in the Tph1−/− group than in the WT group (p < 0.05) (Fig. 5C). The fibrinogen levels in the peritoneal fluids were increased after the caecum-rubbing procedure. The Tph1−/− group mice exhibited lower levels of fibrinogen compared with the WT group on the 1st and 3rd days postoperatively (p < 0.05) (Fig. 5D).
Deficiency of serotonin relieved the disorder of the fibrinolytic system in postoperative intra-abdominal adhesion formation in mice. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 1st, 3rd and 7th days after the operation. The peritoneal fluids were harvested to evaluate the fibrinolytic system. The levels of (A) t-PA and (B) PAI-1 in the peritoneal fluids; (C) t-PA/PAI-1ratio in the peritoneal fluids; (D) The fibrinogen content in the peritoneal fluids; n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Serotonin accelerated angiogenesis in postoperative intra-abdominal adhesion formation by up-regulating VEGF expression
CD34 is a marker of vascular endothelial cells and VEGF plays a pivotal role in angiogenesis. Immunohistochemistry verified that the expression levels of CD34 and VEGF in the Tph1−/− mice were lower than those in the WT mice, which was also confirmed by semi-quantitation (p < 0.05) (Fig. 6A,B). The results certified that serotonin accelerated angiogenesis in postoperative intra-abdominal adhesion formation by up-regulating the VEGF expression.
Serotonin accelerated angiogenesis in postoperative intra-abdominal adhesions by up-regulating VEGF expression. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 7th day after the operation. The adhesive tissues were harvested to detect the angiogenesis. (A,B) Immunohistochemistry for evaluating the levels of CD34 and VEGF in the adhesive tissues (magnification ×200); n = 6, mean ± SEM, *P < 0.05 vs. the WT group.
Serotonin facilitated postoperative intra-abdominal adhesion formation via the 5-HT2B receptor and up-regulating TGF-β1 expression
To study the potential mechanisms of serotonin in facilitating postoperative intra-abdominal adhesion formation, the related proteins including TGF-β1, COX-2, MMP-2 and TIMP-1 were detected. Immunohistochemistry and its semi-quantitation proved that the Tph1−/− group had a lower level of TGF-β1 in contrast to the WT group (Fig. 7A,B). Additionally, western blot analysis also revealed that the 5-HT2B receptor was more highly expressed in the WT mice compared with the Tph1−/− mice (Fig. 7C,D).
Serotonin facilitated postoperative intra-abdominal adhesion formation by up-regulating TGF-β1 expression. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 7th day after the operation. The adhesive tissues were harvested. (A,B) Immunohistochemistry was performed to evaluate the levels of TGF-β1, COX-2, TIMP-1 and MMP-2 in the adhesive tissues (magnification ×200); (C,D) Western blot analysis was conducted to evaluate the levels of 5-HT2B receptor in the adhesive tissues; n = 6, mean ± SEM, #P < 0.05 vs. the WT sham group, *P < 0.05 vs. the WT group.
Inhibition of serotonin synthesis alleviated postoperative intra-abdominal adhesion formation in mice
To validate the role of serotonin in adhesion formation, 5-hydroxytryptophan (5-HTP, the precursor of serotonin) was applied to replenish serum serotonin in the Tph1−/− mice. The reloading of serotonin significantly increased the adhesion scores and weight loss (p < 0.05). Meanwhile, 5-HTP pretreatment aggravated the collagen fibre deposition and was associated with more serious inflammation than the Tph1−/− group mice (p < 0.05).
To explore whether the intervention of serotonin synthesis could alleviate postoperative intra-abdominal adhesion formation, p-chlorophenylalanine (PCPA,the Tph1 inhibitor) was pretreated in the WT mice. The WT + PCPA group mice showed a milder extent of adhesion formation, lower adhesion score and weight loss compared with the WT group (p < 0.05) (Fig. 8A–C). The picrosirius red staining proved that the PCPA pretreatment significantly decreased the collagen fibre deposition and thickness of the adhesion layers (p < 0.05) (Fig. 8D,E). In addition, PCPA markedly quenched the inflammatory response in the adhesive tissues (Fig. 8F). The results indicated that the suppression of serotonin synthesis might be a new method for prevention of postoperative intra-abdominal adhesion formation.
Inhibition of serotonin synthesis alleviated postoperative intra-abdominal adhesion formation in mice. The WT mice were pretreated with PCPA and the Tph−/− mice were pretreated 3 days before modelling. Postoperative intra-abdominal adhesion formation was induced by caecum rubbing, and the mice were sacrificed on the 7th day after the operation to detect the severity of adhesion formation. (A) Representative images of adhesive bands, picrosirius red staining and haematoxylin-eosin staining of adhesive tissues; (B) Representative intra-abdominal adhesion scores based on the Nair, s criteria; (C) Percentage of weight loss, (D) Positive area according to picrosirius red staining; (E) Thickness of the collagen deposition and (F) Inflammation scores were assessed in the adhesive tissues; n = 6, mean ± SEM, *P < 0.05 vs. the WT sham group, #P < 0.05 vs. the Tph1−/− sham group.
Discussion
In the present study, the model of postoperative intra-abdominal adhesions was established by caecum rubbing in the WT and Tph1−/− mice. We verified that serotonin regulated postoperative intra-abdominal adhesion formation in mice. The potential mechanisms might be related to serotonin’s role in facilitating inflammation, oxidative stress, disorder of the fibrinolytic system, angiogenesis, and TGF-β1 expression (Fig. 9). In addition, the exogenous antagonist of the serotonin synthesis enzyme significantly alleviated postoperative intra-abdominal adhesion formation in mice.
Peripheral serotonin regulated postoperative intra-abdominal adhesion formation in mice. After the surgery, serum serotonin was substantially released. Serotonin facilitated the following functions: recruitment of inflammatory cells and fibroblasts to the injury site, damage related to oxidative stress to the endotheliocyte, and the disorder of the fibrinolytic system, all of which resulted in collagen deposition, angiogenesis via the up-regulation of VEGF expression, and the secretion of extracellular matrix (ECM) via the up-regulation of TGF-β1 expression. The above mechanisms contributed to postoperative intra-abdominal adhesion formation.
Inflammation on a large scale occurs when the peritoneum is injured32. Inflammatory cells, such as giant cells, lymphocytes, and plasma cells, as well as fibroblasts, are recruited to the injured area33. Moreover, the inflammatory cytokines are substantially released. These processes lead to the development of fibrous adhesions4. In addition, the oxidative stress and nitrative stress play important roles in adhesion formation34. The ROS, MPO and NO directly damage the mesothelial cells and suppress fibrinolytic activity in the injured peritoneum31. Several studies have demonstrated that serotonin has pro-inflammatory and pro-oxidative functions in many diseases22, 24. Our study verified that the deficiency of serotonin markedly decreased inflammation scores and the levels of TNF-α and IL-6. Moreover, the oxidative stress indicators, MDA and MPO were suppressed in the tissues with adhesions. The results demonstrated that serotonin might aggravate postoperative intra-abdominal adhesions in mice by facilitating inflammation and oxidative stress.
The fibrinolytic system is a complex and dynamic variation in the process of intra-abdominal adhesion formation. Fibrinous exudation is an essential physiological activity of tissue repair. However, the persistent fibrin deposition promotes fibroblast recruitment, new blood vessel formation and tissue remodelling10. Plasminogen activators (PAs) including t-PA and u-PA are serine proteases that convert plasminogen to plasmin. Both t-PA and u-PA can be inhibited by PAI-16. The previous studies showed that the levels of t-PA and PAI-1 in the peritoneal fluids were markedly elevated after the surgery35, 36. Furthermore, it has been verified that serotonin content has a positive correlation with the PAI-1 levels in a concentration- and time-dependent manner in rat aortic endothelial cells37. In this study, we discovered that the concentrations of t-PA and PAI-1 in peritoneal fluid were markedly increased after the caecum-rubbing operation. However, the deficiency of serotonin significantly increased the t-PA level and t-PA/PAI-1 ratio and reduced the PAI-1 and fibrinogen contents in peritoneal fluid compared with the WT mice in the adhesion model on postoperative days 1 and 3. The study proved that serotonin might facilitate the disorder of the fibrinolytic system in postoperative intra-abdominal adhesions.
Angiogenesis is an important pathological process in adhesion formation38. CD34 is a marker of vascular endothelial cells, and VEGF plays a pivotal role in angiogenesis. One study demonstrated that VEGF is up-regulated in the adhesion formation39. Anti-angiogenesis drugs significantly alleviated postoperative intra-abdominal adhesion formation40, 41. Furthermore, the previous studies indicated that serotonin facilitates angiogenesis by accelerating VEGF expression in several diseases42, 43. In our study, we verified that a deficiency of serotonin could markedly decrease the elevation of CD34 and VEGF expressions in the adhesion model. Additionally, studies have shown that the TGF-β1 is over-expressed in adhesions44, 45.
TGF-β1 is responsible for the secretion of extracellular matrix (ECM) proteins, tissue remodelling and angiogenesis in peritoneal fibroblasts12, 46. COX-2, the rate-limiting enzyme of prostaglandin (PG) synthesis, is increased in adhesive tissues47. The MMPs, known as zinc-dependent proteolytic enzymes, can degrade specific proteins in the extracellular matrix48. The MMPs are decreased, while the TIMPs, inhibitors of MMPs, are highly expressed in adhesive tissues49. It has been verified that peripheral serotonin facilitates tissue remodelling and fibrosis by up-regulating TGF-β1 expression. Moreover, serotonin, which binds to its 5-HT2A receptor, enhanced the secretion of TGF-β1 and MMPs in cardiac fibroblasts50. In addition, a significant positive correlation of 5-HT2B receptor expression with MMPs was observed in canine dilated cardiomyopathy51. Furthermore, serotonin has a close relationship with COX-2 expression52. In the present study, we discovered that the expressions of TGF-β1, COX-2 and TIMPs were markedly increased after the caecum-rubbing operation. There were no differences in the levels of COX-2, MMPs and TIMPs between the WT mice and Tph1−/− mice. However, the serotonin knockout mice exhibited significantly lower levels of TGF-β1 in the adhesive tissues compared with the WT mice. Serotonin might exert its role of facilitating adhesions by up-regulating TGF-β1 expression.
The previous study showed that 5-HT2 receptors blockade decreased the formation of intra-abdominal adhesions in a dose-dependent manner in a rat caecum serosal denuding procedure53. Clara Dees et al. verified that 5-HT/5-HT2B signalling links vascular damage and platelet activation to tissue remodelling25. In the current study, we discovered that 5-HT2B receptors were highly expressed in the WT mice after the caecum-rubbing operation, and maintained a low level in the Tph1−/− mice. Serotonin might facilitate postoperative intra-abdominal adhesion formation via its 5-HT2B receptor.
This study’s findings indicated that serotonin was involved in postoperative intra-abdominal adhesion formation in mice. Serotonin facilitated inflammation, oxidative stress, disorders of the fibrinolytic system, angiogenesis, and TGF-β1 expression via the 5-HT2B receptor in the adhesive tissues. Moreover, PCPA pretreatment could alleviate adhesion formation. Serotonin may be a new target for adhesion prevention and therapy. However, the present study was only performed in mice. Further in vitro studies are needed to investigat deeper mechanisms. Moreover, prospective clinical studies are needed to evaluate whether the level of 5-HT relates to the process of postoperative intra-abdominal adhesion formation.
Materials and Methods
Experimental animals
The research was conducted using male wild-type C57BL/6 J mice (the Experimental Animal Center of Xi’an Jiaotong University) and male Tph1−/− mice (4–5 weeks of age, 20–26 g). The Tph1−/− mice were described previously16. The mice were fed in the Animal Feeding Center of Xi’an Jiaotong University Health Science Centre. The mice were housed (5 mice per cage) at 23 °C, 50% humidity, 12 h of light daily and free access to food and water. All animals were euthanized using isoflurane gas for tissue collection. The animal protocol was designed to minimize pain and discomfort to the animals. All animal experiments were performed in accordance with the guidelines of the China Council on Animal Care and Use. All animal procedures performed in this study were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee of the Ethics Committee of Xi’an Jiaotong University Health Science Centre, China.
Experimental design
Mice were randomly allocated into the following groups: (1) WT sham group: wild-type C57BL/6 mice undergoing sham operation; (2) Tph1−/− sham group: Tph1 knockout mice undergoing sham operation; (3) WT group: wild-type C57BL/6 mice undergoing the caecum rubbing model; (4) Tph1−/− group: Tph1 knockout mice undergoing the caecum rubbing model; (5) Tph1−/− + 5HTP group: Tph1 knockout mice pretreated with 5-hydroxytryptophan (5-HTP, precursor of 5-HT, replenishing the serum serotonin content, 75 mg/kg/day for 3 days by subcutaneous injection) before the caecum rubbing model as previously reported54, 55; (6) WT + PCPA group: wild-type C57BL/6 mice pretreated with p-chlorophenylalanine (PCPA, Tph1-inhibitor, 150 mg/kg/day for 3 days by subcutaneous injection) before the caecum rubbing model as previously reported55, 56. The experiment was designed (6 mice per group) to assess adhesion formation on postoperative days 1, 3 and 7. To assess the inflammatory factors, mouse blood samples were collected from the periorbital plexus after the mice were anaesthetized with isoflurane gas. Following blood collection, the mice were euthanized. The peritoneal fluids and adhesive tissues were obtained to assess the extent of the intra-abdominal adhesions and determine the potential mechanisms.
Postoperative intra-abdominal adhesions model
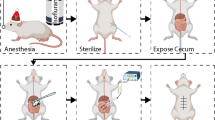
The postoperative intra-abdominal adhesion model was induced by laparotomy with caecum rubbing, which was validated by previous studies57, 58. The mice fasted 12 h and were forbidden to drink for 6 h before the operation. The mice were anaesthetized with chloral hydrate (10 ml/kg, 4%). The abdomen was disinfected with iodine volts cotton swabs, and an approximate 1.5-cm midline abdominal incision was performed. The caecum was exposed and rubbed gently with 2 dry gauze pads on the ventral and dorsal surfaces until the shine was lost and haemorrhagic points became visible. Next, the caecum was placed back in its original place and closed in layers. The sham operation was conducted abdominal incision only, which was exposed for 3 minutes and subsequently closed in layers. The mice were given 30 ml/kg saline for recovery from anaesthesia and were returned to their cages after a full recovery.
Adhesion scoring system
After euthanasia, the extent of the postoperative intra-abdominal adhesions was assessed according to the adhesion score criteria reported by Nair, et al.59 (Table S1). The score criteria were as follow: 0, no adhesion band; 1, one filmy adhesion band between the viscera or between the viscera and abdominal wall; 2, two thin bands between the viscera or between the viscera and abdominal wall; 3, more than two moderate bands between the viscera or between the viscera and the abdominal wall, or the whole intestine formed a mass without adhering to the abdominal wall; and 4, very thick adhesion band between the viscera and the abdominal wall. The scores were assessed in a blind manner by two researchers.
Picrosirius red staining for collagen
On days 1, 3, and 7 following the caecum-rubbing modelling, the adhesive tissues were collected, fixed in 10% formalin at 4 °C for 48 h, and then embedded in the paraffin. Serial 5-μm-thick sections were obtained, and 0.1% picrosirius red (Sigma-Aldrich, St. Louis, MO, USA) was used for staining. The picrosirius red staining was evaluated by two researchers in a blind manner using a microscope and a representative field was chosen for application. The percentage of the adhesion area that stained positive using picrosirius red was calculated using ImagePro Plus 5.0 software.
Hematoxylin and Eosin (HE) Staining and inflammation score
The 5-μm-thick sections were obtained from the paraffin. The hematoxylin and eosin (HE) staining of adhesive tissues was performed. Next, the histological changes were assessed in a blind manner by two researchers using a light microscope, and a representative field was chosen for application. The inflammation score was determined using the following classical criteria described previously60 (Table S2). 0, no inflammation; 1, mild inflammation: infiltration of giant cells, lymphocytes, and plasma cells; 2, moderate inflammation: infiltration of giant cells, plasma cells, eosinophils, and neutrophils; 3. severe inflammation: inflammatory cell infiltration and microabscess formation.
Enzyme-linked immunosorbent assays (ELISA)
The levels of serum TNF-a, IL-6 as well as t-PA, PAI-1 and fibrinogen in the peritoneal fluid were measured using commercial ELISA kits according to the instructions of the manufacturer (Dakewe, Shenzhen, China).
Measurement of adhesion oxidative stress
The concentrations of malonaldehyde (MDA), superoxide dismutase (SOD), myeloperoxidase (MPO) and glutathione (GSH) in the peritoneal adhesive tissues were measured as markers of oxidative stress. The adhesive tissues were homogenized, and measured using the activity assay kit (NanJing JianCheng Bioengineering Institute). All of the procedures were conducted according to the instructions of the manufacturer.
Hydroxyproline determination
The content of hydroxyproline was measured using a Hydroxyproline Assay Kit (Sigma-Aldrich) according to the manufacturer’s instructions. The results were expressed in μg/mg of protein.
Western blot analysis
The peritoneal adhesive tissues were collected and stored at −80 °C after the mice were sacrificed. The proteins were extracted from the adhesive tissues using RIPA lysis buffer. BCA protein assay kit was used for detecting the concentration of extracted proteins. The proteins were separated by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis, and transferred onto poly-vinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% skim milk and incubated with primary antibodies, anti-5-HT2B antibody (bs-1892R, BIOSS, China, 1:500 dilution), and anti-β-actin antibody (sc-47778, Santa Cruz Biotechnology, USA, 1:1000 dilution). The membranes were incubated with secondary antibodies for 2 h at room temperature. The protein expressions were detected using a chemiluminescence system (BIO-RAD) according to the manufacturer’s specifications. The protein quantification was performed using ImageJ2x software. The results are expressed as relative intensity of protein/β-actin.
Immunohistochemistry
The VEGF, CD34, TGF-β1, COX-2, TIMP-1 and MMP-2 expressions in peritoneal adhesive tissues were detected by immunohistochemical staining. The detailed procedures were conducted as previously described61. The primary antibodies anti- CD34 antibody (bs-9752R, BIOSS, China, 1:300 dilution), anti- VEGF antibody (bs-0279R, BIOSS, China, 1:300 dilution), anti-TGF-β1 antibody (sc-146, Santa Cruz Biotechnology, USA, 1:400 dilution), anti-COX-2 antibody (bs-10411R, BIOSS, China, 1:300 dilution), anti-TIMP-1 antibody (bs-4600R, BIOSS, China, 1:300 dilution) and anti-MMP-2 antibody (bs-4599R, BIOSS, China, 1:300 dilution) were incubated overnight at 4 °C, and a secondary antibody was incubated for 1 h at room temperature. The staining was assessed in a blind manner using a light microscope, and a representative field was chosen for as application. The semi-quantitation of immunohistochemical staining was performed, and the intensity was scored as follows: 0, negative; 1, mild; 2, moderate; and 3, severe. The extent of staining was assessed based on the percentage of positive cells and was scored as follows: 0, negative; 1, 1–25%; 2, 26–50%; 3,51–75%; and 4,76–100%. The staining scores were the sum of the intensity and extent scores, and ranged from 0 to 7. The final staining score was represented as the mean in each group.
Statistical analysis
All the measurement data are expressed as the means ± SEM. The t-test or one way ANOVA was applied to analyse the differences between groups, using data statistics software SPSS 18.0. P < 0.05 represents a significant difference.
References
ten Broek, R. P. et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. Bmj. 347, f5588 (2013).
Ward, B. C. & Panitch, A. Abdominal adhesions: current and novel therapies. The Journal of surgical research. 165, 91–111 (2011).
Ellis, H. et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 353, 1476–1480 (1999).
diZerega, G. S. & Campeau, J. D. Peritoneal repair and post-surgical adhesion formation. Human reproduction update. 7, 547–555 (2001).
Di Filippo, C. et al. Plasma levels of t-PA and PAI-1 correlate with the formation of experimental post-surgical peritoneal adhesions. Mediators of inflammation. 2006, 13901 (2006).
Saed, G. M. & Diamond, M. P. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertility and sterility. 79, 164–168 (2003).
Shavell, V. I., Saed, G. M. & Diamond, M. P. Review: cellular metabolism: contribution to postoperative adhesion development. Reproductive sciences. 16, 627–634 (2009).
Basbug, M. et al. The effect of antivascular endothelial growth factor on the development of adhesion formation in laparotomized rats: experimental study. Gastroenterology research and practice. 2011, 578691 (2011).
Holmdahl, L. et al. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 129, 626–632 (2001).
Hellebrekers, B. W. & Kooistra, T. Pathogenesis of postoperative adhesion formation. The British journal of surgery. 98, 1503–1516 (2011).
Ambler, D. R., Fletcher, N. M., Diamond, M. P. & Saed, G. M. Effects of hypoxia on the expression of inflammatory markers IL-6 and TNF-a in human normal peritoneal and adhesion fibroblasts. Systems biology in reproductive medicine. 58, 324–329 (2012).
Chegini, N. TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Seminars in reproductive medicine. 26, 298–312 (2008).
Fletcher, N. M., Jiang, Z. L., Diamond, M. P., Abu-Soud, H. M. & Saed, G. M. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free radical biology & medicine. 45, 530–536 (2008).
Saed, G. M., Zhao, M., Diamond, M. P. & Abu-Soud, H. M. Regulation of inducible nitric oxide synthase in post-operative adhesions. Human reproduction. 21, 1605–1611 (2006).
Braun, K. M. & Diamond, M. P. The biology of adhesion formation in the peritoneal cavity. Seminars in pediatric surgery. 23, 336–343 (2014).
Walther, D. J. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 299, 76 (2003).
Walther, D. J. & Bader, M. A unique central tryptophan hydroxylase isoform. Biochemical pharmacology. 66, 1673–1680 (2003).
Fligny, C. et al. Maternal serotonin influences cardiac function in adult offspring. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 22, 2340–2349 (2008).
El-Merahbi, R., Loffler, M., Mayer, A. & Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS letters. 589, 1728–1734 (2015).
Sepiashvili, R. I., Balmasova, I. P. & Staurina, L. N. Serotonin and its immune and physiological effects. Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova. 99, 17–32 (2013).
Oh, C. M. et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nature communications. 6, 6794 (2015).
Shajib, M. S. & Khan, W. I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta physiologica. 213, 561–574 (2015).
Duerschmied, D. et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 121, 1008–1015 (2013).
Nocito, A. et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 133, 608–618 (2007).
Dees, C. et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. The Journal of experimental medicine. 208, 961–972 (2011).
Ruddell, R. G. et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. The American journal of pathology. 169, 861–876 (2006).
Gershon, M. D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in endocrinology, diabetes, and obesity. 20, 14–21 (2013).
Rouzaud-Laborde, C. et al. Platelet activation and arterial peripheral serotonin turnover in cardiac remodeling associated to aortic stenosis. American journal of hematology. 90, 15–19 (2015).
Li, Y. et al. Sepsis-induced elevation in plasma serotonin facilitates endothelial hyperpermeability. Scientific reports. 6, 22747 (2016).
Lesurtel, M. & Clavien, P. A. Platelet-derived serotonin: translational implications for liver regeneration. Hepatology. 60, 30–33 (2014).
Gotloib, L., Wajsbrot, V., Cuperman, Y. & Shostak, A. Acute oxidative stress induces peritoneal hyperpermeability, mesothelial loss, and fibrosis. The Journal of laboratory and clinical medicine. 143, 31–40 (2004).
Thompson, J. Pathogenesis and prevention of adhesion formation. Digestive surgery. 15, 153–157 (1998).
Holmdahl, L. & Ivarsson, M. L. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. The European journal of surgery = Acta chirurgica. 165, 1012–1019 (1999).
Awonuga, A. O. et al. Advances in the Pathogenesis of Adhesion Development: The Role of Oxidative Stress. Reproductive sciences. 21, 823–836 (2014).
Hellebrekers, B. W. et al. A role for the fibrinolytic system in postsurgical adhesion formation. Fertility and sterility. 83, 122–129 (2005).
Tarhan, O. R. et al. Fibrinolytic responses of human peritoneal fluid in laparoscopic cholecystectomy: a prospective clinical study. Surgical endoscopy. 22, 1008–1013 (2008).
Kawano, H. et al. Serotonin induces the expression of tissue factor and plasminogen activator inhibitor-1 in cultured rat aortic endothelial cells. Blood. 97, 1697–1702 (2001).
Cahill, R. A., Wang, J. H., Soohkai, S. & Redmond, H. P. Mast cells facilitate local VEGF release as an early event in the pathogenesis of postoperative peritoneal adhesions. Surgery. 140, 108–112 (2006).
Rout, U. K., Oommen, K. & Diamond, M. P. Altered expressions of VEGF mRNA splice variants during progression of uterine-peritoneal adhesions in the rat. American journal of reproductive immunology. 43, 299–304 (2000).
Kim, S. et al. Inhibition of intra-abdominal adhesion formation with the angiogenesis inhibitor sunitinib. The Journal of surgical research. 149, 115–119 (2008).
Chiang, S. C., Cheng, C. H., Moulton, K. S., Kasznica, J. M. & Moulton, S. L. TNP-470 inhibits intraabdominal adhesion formation. Journal of pediatric surgery. 35, 189–196 (2000).
Zamani, A. & Qu, Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS letters. 586, 2360–2365 (2012).
Gautam, J. et al. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Molecular cancer. 15, 75 (2016).
Jin, X., Ren, S., Macarak, E. & Rosenbloom, J. Pathobiological mechanisms of peritoneal adhesions: The mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-beta1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation. Matrix biology: journal of the International Society for Matrix Biology. 51, 55–64 (2016).
Wang, G. et al. Role of IL-17 and TGF-beta in peritoneal adhesion formation after surgical trauma. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 22, 631–639 (2014).
Salma, U. et al. Role of Transforming Growth Factor-beta1 and Smads Signaling Pathway in Intrauterine Adhesion. Mediators of inflammation. 2016, 4158287 (2016).
Saed, G. M., Munkarah, A. R., Abu-Soud, H. M. & Diamond, M. P. Hypoxia upregulates cyclooxygenase-2 and prostaglandin E(2) levels in human peritoneal fibroblasts. Fertility and sterility. 83(Suppl 1), 1216–1219 (2005).
Saed, G. M. & Diamond, M. P. Molecular characterization of postoperative adhesions: the adhesion phenotype. The Journal of the American Association of Gynecologic Laparoscopists. 11, 307–314 (2004).
Alpay, Z., Saed, G. M. & Diamond, M. P. Female infertility and free radicals: potential role in adhesions and endometriosis. Journal of the Society for Gynecologic Investigation. 13, 390–398 (2006).
Yabanoglu, S. et al. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. Journal of molecular and cellular cardiology. 46, 518–525 (2009).
Fonfara, S., Hetzel, U., Oyama, M. A. & Kipar, A. The potential role of myocardial serotonin receptor 2B expression in canine dilated cardiomyopathy. Veterinary journal. 199, 406–412 (2014).
Mackowiak, M. et al. DOI, an agonist of 5-HT2A/2C serotonin receptor, alters the expression of cyclooxygenase-2 in the rat parietal cortex. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 53, 395–407 (2002).
Langer, J. C., Liebman, S. M., Monk, P. K. & Pelletier, G. J. Mast cell mediators and peritoneal adhesion formation in the rat. The Journal of surgical research. 59, 344–348 (1995).
Jang, J. H. et al. Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation. Hepatology 56, 209–218 (2012).
Zhang, J. et al. Role of Serotonin in MODS: Deficiency of Serotonin Protects Against Zymosan-Induced Multiple Organ Failure in Mice. Shock. 43, 276–284 (2015).
Izikki, M. et al. Tryptophan hydroxylase 1 knockout and tryptophan hydroxylase 2 polymorphism: effects on hypoxic pulmonary hypertension in mice. American journal of physiology. Lung cellular and molecular physiology. 293, L1045–1052 (2007).
Hemadeh, O., Chilukuri, S., Bonet, V., Hussein, S. & Chaudry, I. H. Prevention of peritoneal adhesions by administration of sodium carboxymethyl cellulose and oral vitamin E. Surgery. 114, 907–910 (1993).
Peyton, C. C. et al. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. The Journal of surgical research. 178, 545–552 (2012).
Nair, S. K., Bhat, I. K. & Aurora, A. L. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Archives of surgery. 108, 849–853 (1974).
Mahdy, T., Mohamed, G. & Elhawary, A. Effect of methylene blue on intra-abdominal adhesion formation in rats. International journal of surgery. 6, 452–455 (2008).
Zhang, J. Y. et al. Protective role of hydrogen-rich water on aspirin-induced gastric mucosal damage in rats. World journal of gastroenterology. 20, 1614–1622 (2014).
Acknowledgements
We are indebted to all individuals who participated in or helped with this research project. This study was supported by funding from “the Fundamental Research Funds for the Central Universities” (Grant No. 1191320114) and “the National Nature Science Foundation of China” (Grant No. 81601672).
Author information
Authors and Affiliations
Contributions
Bi J.B. designed the experiments, performed most experiments and wrote the paper; Zhang S.M. participated in the research design and Western blot analysis. Du Z.Q., Zhang J. and Deng Y., participated in the mice modeling and IHC performance. Liu C. and Zhang J.Y. provided substantial advice in designing the study and directed the overall project.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, J., Zhang, S., Du, Z. et al. Peripheral serotonin regulates postoperative intra-abdominal adhesion formation in mice. Sci Rep 7, 10001 (2017). https://doi.org/10.1038/s41598-017-10582-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10582-w
This article is cited by
-
Evaluation of the effect of Dracocephalum kotschyi hydroalcoholic extract in preventing intra-abdominal adhesions after abdominal surgery in rats based on inflammatory factors (TNF-α and IL-1β)
Comparative Clinical Pathology (2024)
-
D-limonene inhibits peritoneal adhesion formation in rats via anti-inflammatory, anti-angiogenic, and antioxidative effects
Inflammopharmacology (2024)
-
Effects of linalool on postoperative peritoneal adhesions in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Rosmarinic acid prevents post-operative abdominal adhesions in a rat model
Scientific Reports (2022)
-
Transcriptome sequencing analysis of primary fibroblasts: a new insight into postoperative abdominal adhesion
Surgery Today (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.