Abstract
Investigating samples of the cancellothyridid brachiopod Terebratulina collected during the IceAGE (Me85/3) expedition of RV METEOR at the continental shelf around Iceland with both morphometrical and molecular methods, we were for the first time able to detect a hybridization event between brachiopod sister species, which are thought to have separated 60 MYA. Terebratulina retusa and T. septentrionalis can clearly be distinguished on the basis of consistent species-specific molecular signatures in both mitochondrial and nuclear markers, whereas morphometrical analyses proved to be less reliable for species determination than previously thought. Two out of 28 specimens were identified as offspring of a one-way hybridization event between T. retusa eggs and T. septentrionalis sperm. Whereas the fossil record of Terebratulina in the North Atlantic region is too fragmentary to reconstruct the history of the hybridization event, the different life history traits of the two species and current oceanographic conditions around Iceland offer plausible explanations for the occurrence of crossbreeds in this common brachiopod genus.
Similar content being viewed by others
Introduction
Hybridization has traditionally been regarded as rare in animals but its importance and commonness may have been underrated. With ca. 10% of species being affected1, it seems to be a rather widely spread phenomenon in the animal kingdom including marine lophotrochozoans2,3,4,5. Hybridization is also discussed as a potentially important evolutionary process that drives speciation6, but its prevalence remains controversial. In this study, for the first time to our knowledge, we present evidence for hybridization in two brachiopod sister species.
The brachiopod genus Terebratulina comprises more than 30 extant species, two of which, Terebratulina retusa and T. septentrionalis, are common members of invertebrate benthic shelf communities in the temperate to boreal North Atlantic. According to molecular phylogenies T. retusa and T. septentrionalis in a comprehensive study on molecular systematics of cancellothyridid brachiopods7 were found to be sister species resulting from an allopatric speciation event during the Early Paleogene about 60 MYA, which became possible after the opening of the North Atlantic. Whereas T. retusa was originally described from Scandinavian waters by Linné8, the morphologically similar T. septentrionalis was first mentioned in Couthouy’s account on new molluscs from New England’s coast9. Ever since Couthouy’s description the sister species were traditionally attributed to either European (T. retusa) or North American (T. septentrionalis) clades, although several descriptions of T. septentrionalis from Northern Scandinavia, Greenland and Iceland existed in the literature10,11,12,13,14,15. Because determining the distribution of either species was severely hampered by their morphological similarity, Curry & Endo16 used principal component analyses of shell characters to discriminate between the two. Their more than 2000 specimens clearly fell into two distinct groups mainly distinguishable by the width of their shell ribs. Ribs occur in higher numbers in T. septentrionalis and were already used by Davidson17 for a separation of Atlantic Terebratulina species. Additionally, Curry & Endo16 were interested to see whether they would be able to identify intermediate forms, which had been described e.g. from the Danish Gothaab- and Ingolf-Expeditions by Elise Wesenberg-Lund13,14,15 and which she could not attribute to either species, due to a “confusion of characters of both of them, which really made specific identification and description impossible” (ref. 15: p. 9). This potential cline linking the two species could either be interpreted as the morphospace of a single Atlantic species of Terebratulina with its two extremes erroneously described as different species (see refs 18–20) or as the product of hybridization15. Curry & Endo16, unable to find such cline in their data, concluded that hybrids do not exist and that their morphometric approach clearly discriminated between two Atlantic species of Terebratulina. This was also confirmed by allozyme data and mitochondrial RFLP analysis21 and by sequence analyses of mitochondrial DNA7.
The geographical distribution of both species as reconstructed by Curry & Endo16 provided another interesting aspect: whereas T. retusa was restricted to the East Atlantic and the Mediterranean in their analysis, T. septentrionalis seemed to be more widespread from North America to Greenland, Iceland and the Norwegian coast with a possible ice age relict population in Finnmarken (North Norway). Despite partial sympatry no morphological overlap existed in the data of Curry & Endo16 between T. retusa and T. septentrionalis supporting the hypothesis of two valid species of Terebratulina inhabiting the North Atlantic. However, the intermediates observed by Wesenberg-Lund13,14,15 remained elusive and her dubious specimens have never been subjected to a rigorous analysis.
In 2011 the IceAGE project collected marine benthos around Iceland with the German research vessel METEOR. Among the samples were 28 specimens of Terebratulina suitably preserved for molecular analysis from the geographical region where the unidentifiable specimens of Wesenberg-Lund15 had been collected. Sitting half way between mainland Europe and Greenland on top of the mid-Atlantic ridge, Iceland is crucial to understand the biogeographical distribution of T. septentrionalis because it represents the most prominent contact zone with its East Atlantic sister taxon T. retusa. The IceAGE material enables us to show not only that T. retusa in Iceland is much more variable in shell ornamentation than Curry & Endo16 suggested, but also that Wesenberg-Lund legitimately struggled to identify her confusing specimens. In particular, we found molecular evidence for hybridization between T. retusa and T. septentrionalis.
Results
Morphology
As in the study of Curry & Endo16, length, width and dorso-ventral height of the Terebratulina shells as well as ratios between the three size measurements did not discriminate the species because these data formed a gradient from small to large specimens simply reflecting changing size with age (not shown). However, the average rib width over a defined transect (see below and16) showed a discontinuity in the resulting plot (Fig. 1A) at about 0.35 mm seemingly reflecting the species boundary between T. septentrionalis and T. retusa. When displayed as a box-and-whisker plot (Fig. 1B) it was even more obvious that the 40 included specimens (27 IceAGE, 13 MfN brachiopod collection) fell into two significantly different groups, thereby corroborating previous results. The rib width mean value of 0.298 mm representing the group with narrower ribs (=the putative T. septentrionalis specimens) was almost identical with the mean value given in Curry & Endo’s study for this species. However, the rib width mean value of the group with broader ribs (=the putative T. retusa specimens) was conspicuously smaller than that given by these authors. Nevertheless, the rib width of all our putative T. retusa specimens identified by morphology only fell into the variability range of the specimens assigned to T. retusa by Curry & Endo, i.e. the distribution of our specimens within this variability range was slightly shifted towards smaller rib widths.
Measurements of shells (rib width) of Terebratulina specimens examined in this study. Boxes in (B–D) depict data between the 25th and the 75th percentiles, vertical lines illustrate the full range of data. (A) Mean width of shell ribs of all investigated specimens (n = 39; 23 IceAGE samples supplemented by 16 shells from the brachiopod collection of the Museum für Naturkunde, Berlin). Note the discontinuity between two clusters at about 0.35 mm (red arrow). (B) The same data set depicted as box-and-whisker plots showing a significant difference between two groups (A: mean rib width 0.416 mm ± 0.078 SD, and B: 0.289 mm ± 0.037 SD; Mann-Whitney U-test: p ≤ 0.0001), which according to Curry & Endo16 should represent the two Atlantic species T. retusa (group A) and T. septentrionalis (group B). (C) Rib widths of T. retusa (0.345 mm ± 0.05 SD, n = 15) and T. septentrionalis (0,282 mm ± 0.038 SD, n = 10) are still significantly different (Mann-Whitney U-test: p = 0.003), when species are identified based on mitochondrial sequence data (12 S and 16 S rRNA), but the difference is less obvious than in (B ). Same as in (C), but species identification based on nuclear sequence data (28 S rRNA) leading to non-significant differences (Mann-Whitney U-test: p = 0.154) between rib-widths of T. retusa (0.335 mm ± 0.049 SD, n = 12) and T. septentrionalis (0.298 mm ± 0.059 SD, n = 12).
Molecular analysis
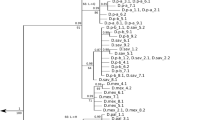
All Terebratulina specimens collected during the IceAGE expedition yielded suitable amounts of DNA for sequence analysis. The analysed mitochondrial markers (n = 26) resulted in 6.07% (12 S) and 8.10% (16 S) sequence divergence between two groups of specimens clearly defining them as separate taxa. As we were interested in testing the IceAGE specimens for signs of hybridization, we needed an additional nuclear marker (28 S rRNA) in which potential recombinations may have happened in the past leading to conserved discordancies between the mitochondrial and nuclear genotypes. Thus, we analyzed a 1020 bp long fragment of this marker (n = 25) showing a much lower, but still measurable sequence divergence of 2.25% between the same two groups of specimens. These results confirm that T. septentrionalis and T. retusa both have species-specific nucleotide signatures, which are intra-specifically conservative and allow for species discrimination on the basis of molecular markers alone.
The critical question now was whether all molecular identifications agree with the clear morphological differentiation on the basis of rib width measurements or whether single specimens showed a combination of sequence identity with one species and morphological affinity to the other species. A second aspect was to look at the combination of both mitochondrial and nuclear markers to check for potential hybrids with discordant species-specific signatures. The sequence alignments of all three markers are given as additional files [see Supplementary alignments S1–S3].
Morphological and molecular data combined
When combining all results from morphological and molecular analyses (Table 1) it became clear that four different combinations of characters exist among the 25 Terebratulina specimens from Iceland successfully sequenced for all three markers in this study. The majority of specimens clearly belonged to either T. septentrionalis (first cluster) or T. retusa (second cluster) being consistent in all molecular and morphological characters for one or the other species. These were four T. retusa (one from station 1034, three from station 1047) and eleven T. septentrionalis (one from station 1034, four from station 1047, and six from station 1213). The third cluster comprised 8 specimens (two from station 1034, six from station 1047) which in both mitochondrial and nuclear markers were clearly T. retusa, but which showed very narrow shell ribs shifting them into the T. septentrionalis morphospace. These specimens, on the basis of morphological characters alone, would certainly have been misidentified in a morphometrical analysis. The fourth cluster is the most interesting one, represented by only two specimens. The rib width of these specimens would clearly identify them as T. retusa, as do the mitochondrial markers. But the nuclear marker clearly has the T. septentrionalis signature. This mixture of the otherwise highly conserved and species-specific molecular sequences in both mitochondrial and nuclear markers can only be explained by interspecific hybridization. Both specimens with this character mix were homozygous for T. septentrionalis in their 28 S rRNA, i.e. they almost certainly do not represent the F1 of a recent hybridization event, but are the result of backcrossings of a hybrid with a parental species, in this case with T. septentrionalis. As mitochondrial genes are inherited from the female parent we can even conclude that the original hybridization event happened when a T. retusa egg was fertilized by a T. septentrionalis sperm. The mitochondrial T. retusa signature was then passed on to following generations through the female line.
Consequently, molecular data enable us to divide the samples into bona fide T. septentrionalis versus T. retusa, which partly contrasts with the previous grouping of specimens “with narrow shell ribs” versus those “with broad shell ribs”. Once the species are identified on the basis of their molecular sequence markers, the resulting box-and-whisker plots of rib widths show that the clear difference between the two morphologically defined clusters becomes much smaller and when using the nuclear sequences for species identification this significant morphological difference even collapses (Fig. 1C,D). This means that the difference between narrow-ribbed and broad-ribbed Terebratulina specimens from Iceland is arbitrary and does not reflect a significant difference between T. septentrionalis and T. retusa. T. retusa around Iceland seems to be more variable in this shell character than elsewhere, i.e. some individuals have narrower ribs than the typical T. retusa from North Atlantic coasts of mainland Europe.
Discussion
Species identification and the hybridization event
As has been shown in a previous study7, the molecular markers used here are highly conserved intraspecifically, i.e. identification of either T. retusa or T. septentrionalis is possible based on sequence information alone. Even seemingly small genetic differences (e.g. 2.25% as in the 28 S rRNA fragment) are sufficient to tell the species apart. The occurrence of sequence information of both species in one individual can be explained if a mixture of these otherwise highly conserved sequences happened in the past. In nature this can be achieved either vertically through hybridization or horizontally through lateral gene transfer. Analyses of full mitochondrial genomes of brachiopods showed no signs of lateral gene transfer22,23,24,25, so that our observations can only be explained by hybridization between the two species.
The role of temperature preferences and taxon sampling
The failure to discover the hybrid zone of T. retusa and T. septentrionalis on the southwestern shelf off Iceland in the otherwise meticulous and well-structured study of Curry & Endo16 is simply due to bad luck as their impressive number of specimens analysed supposedly did not contain enough specimens from this critical region. However, their Iceland specimens coded as “b” in their principal component analysis (Fig. 1 in ref. 16) were all identified as T. retusa and cluster conspicuously close to the border between the two clouds identified in their data set. This may be interpreted as a hint that T. retusa in Iceland has no “typical” morphology, something we could clearly show for at least some of the specimens collected at Meteor stations #1034 and #1047 in the southwest of Iceland. But why is this region so critical? When looking at the temperature regimes around Iceland it is obvious that the southwestern region is influenced by the warm North Atlantic Current leading to temperatures of 7–10 °C throughout the year. In contrast, the water in the northeast of Iceland is much colder due to the East Icelandic Current carrying polar waters southward, leading to annual temperatures of about 5–7 °C (temperature measurements for both regions at 50 m depth by the Icelandic Marine Research Institute in 201126,27,28). According to Curry & Endo16 T. retusa prefers temperate water conditions (see also ref. 29), whereas T. septentrionalis prefers colder climate (but see ref. 30), the latter being in line with our results that station #1213 revealed only T. septentrionalis specimens. Potential hybridization can only occur at water temperatures, which are suitable for both species and this seems to exclude the northeastern region off Iceland. According to the reconstruction of water currents around the island31 current mediated transport of T. septentrionalis sperm along the clockwise running Iceland coastal current towards the southwestern region may be possible, but seems unlikely due to generally low sperm survival rates in open waters32 and the low salinity and variable flow velocity of this coastal current27. Rather, our results show that adults of T. septentrionalis were found at Meteor stations #1034 and #1047 in the southwestern region, albeit in low frequency, offering the opportunity of crossbreeding through sympatric distribution of the two species.
The potential influence of life history traits on hybridization
Apart from their specific temperature preferences (see above), T. retusa and T. septentrionalis also differ in their reproductive biology. T. septentrionalis is a brooder, retaining its embryos within the mantle cavity until they have reached an advanced stage of development33, 34 (see also ref. 35 for the related Pacific species T. unguicula). In contrast, T. retusa is a free spawner with both sexes shedding their gametes into the surrounding water. Thus, for a T. retusa egg the probability is rather high to be hit by a T. septentrionalis sperm, especially if in sympatric populations in Europe the temperature dependent reproductive season of both species is isochronic. On the other hand, larval brooding in the mantle cavity as in T. septentrionalis is only possible if (i) sperm is washed into this cavity by the adult’s inhalant feeding current and (ii) spawned eggs ready to be inseminated are retained in the same place. As has been shown in several studies, the inhalant current enters the mantle cavity from left and right sides of the articulate brachiopod shell36,37,38, leading the water through the network of tentacles of both lophophoral arms to filter planktonic particles. Only the filtered and clean water passes through the tentacle network into the mantle cavity and leaves the animal as the exhalant current at the mediofrontal margin of the shell. The brooding female of T. septentrionalis must have a sorting mechanism to differentiate between sperm and food as the sperm has to pass the lophophoral tentacles to enter the mantle cavity for inseminating the ripe eggs. This sorting may be accomplished by size selection of the captured particles, as the maximum efficiency for particle capture in T. retusa applies to food particles sized 7-8 µm39, whereas sperm diameter in Terebratulina does not exceed 1.5 µm40. If in addition this sorting mechanism is able to differentiate between conspecific and other gametes, T. retusa sperm randomly entering the inhalant current of a T. septentrionalis female may be doomed. This could be an explanation for finding only descendents of a hybridization event between a T. retusa egg and a T. septentrionalis sperm in our data. One-way hydridization as assumed here on the basis of different life history traits may be characteristic for this species pair. However, as we have found only two hybrids among our samples, this prediction needs future verification.
Does the fossil record help?
The oldest fossil brachiopods attributed to the genus Terebratulina date back to the Late Jurassic of Europe, California, and New Zealand41, 42 basically representing terebratulids with shell ornament. Fossil specimens similar to or identified as T. retusa have been reported only from Upper Oligocene to Pleistocene strata in France, Italy, Hungary, Rhodes, and Algeria16, 43,44,45. As described above, extant specimens of T. retusa and T. septentrionalis are difficult to tell apart on the basis of shell morphology alone, i.e. distinguishing these species as fossils may be even more problematic. This might be the reason for T. septentrionalis being absent altogether from the records in the Paleobiology Database46. With regard to the unusually high longevity of 60 MY of the two species according to a previous molecular clock approach7, the fossil record is, therefore, not suitable to reconstruct the speciation history and palaeogeographic distribution of Terebratulina species in the North Atlantic.
Conclusions
Our results provide the first evidence of hybridization in brachiopods, i.e. between the species T. retusa and T. septentrionalis. We demonstrated that T. retusa in Iceland is much more variable in its shell ornament than previously thought, blurring the clear morphological disparity between the two species assumed by Curry & Endo16. The intermediate specimens of Wesenberg-Lund13,14,15 although triggering the search for true hybrids do not necessarily represent descendants of a hybridization event between the two species of Terebratulina, but may just reflect the morphological variability of T. retusa in Iceland. The scarcity of fossil representatives of the two species and the demonstrated difficulties to identify T. retusa and T. septentrionalis beyond doubt on the basis of morphometrical characters alone hampers the reconstruction of the speciation event leading to the extant Terebratulina species in the North Atlantic. Whereas the genus Terebratulina based on the oldest known fossils is of Tethyan origin, its modern representatives in the North Atlantic almost certainly came into being through a vicariance event caused by the opening of the Atlantic Ocean. However, the origin of the Norwegian Finnmarken specimens described as T. septentrionalis (see Fig. 3 in ref. 16) remains elusive until molecular data for this isolated population are available.
Methods
The investigated 28 specimens of Terebratulina from Iceland were collected during the IceAGE expedition (RV METEOR Me85/3) in September 2011 at three Stations (#1034, #1047, and #1213) from depths of 209–320 m (Supplementary Fig. S1, Supplementary Table S1). They were collected with Agassiz trawls, picked and preserved in 96% ethanol upon arrival on deck of the vessel. From all samples collected during the cruise, the brachiopods were separated at DZMB Wilhelmshaven and sent to us for further investigation.
Morphological examination
To maximize comparability with the study of Curry & Endo16 we adopted their morphometrical methods and determined shell length, width, and dorso-ventral height with a digital caliper. After dissection of soft tissue for molecular analysis (see below) the 28 Iceland specimens were immersed in 6% sodium hypochlorite and subsequently rinsed in distilled water to get rid of organic tissues and epibionts potentially masking the shell ribs. For photography, shells were mounted dorsal side up in a bed of glass beads and imaged under a Leica Z16 APO Zoom Microscope. Z-Stacks of 12 to 19 photos of the dorsal valve were combined in Auto Montage Essentials v. 5.03 to produce sharp composite images of the dorsal valves. These composites were used for measurements of the rib width in ImageJ, version 1.47. In accordance with Curry & Endo16 rib width was measured over a 4 mm transect, lying 4 mm anterior of the dorsal umbo. Ribs were also counted along this transect (Supplementary Fig. S2). For each shell, number of ribs and rib width within the 4 mm transect were measured ten times and mean/standard deviation was calculated. To enlarge the morphometrical dataset we additionally measured 16 Terebratulina specimens from the brachiopod dry collection of the Museum für Naturkunde (acronym: ZMB) covering the biogeographical distribution of both, T. retusa and T. septentrionalis (Supplementary Table S2).
Molecular analysis
Tissue (lophophore, gonad or musculature) of all 28 Iceland specimens was dried and dissolved in a CTAB mastermix (0.5% 2-mercaptoethanol and 3% proteinase K in CTAB buffer) to extract the mitochondrial and nuclear DNA. Proteins were precipitated with chloroform/isoamyl alcohol, and nucleic acids were precipitated in EtOH with sodium acetate, dried and re-dissolved in 0.1x TE buffer. Mitochondrial sequences were amplified with PCR (GenAmp® PCR system 2700) using primers 12SF1091, 12SR1478, 16SF2510 and 16SR308047, 48, which after sequencing and editing yielded 357 bp fragments of the 12 S rRNA and 429 bp fragments of the 16 S rRNA (n = 26, two samples did not amplify), respectively. All mitochondrial sequences were aligned against the full mt-genome of T. retusa (Genbank acc. no. NC_000941.1)22. Additionally, nuclear sequences were obtained with specific primers 28SF680, 28SF700, 28SF1062, 28SR1460 and 28SR1797 (see refs 49 and 50 and B.L. Cohen pers. comm.) resulting in an edited alignment of 920 bp (n = 25, with three samples not amplifying) against the 28 S sequence of T. retusa published in ref. 50. PCR products were purified using a Nucleospin Kit (Macherey-Nagel, Düren) and commercially sequenced at Services in Molecular Biology GmbH, Rüdersdorf. Sequencing results were edited with BioEdit sequence alignment editor v7.0.0. Sequence alignments of both mitochondrial and nuclear markers were compared between specimens based on single bp comparison across their entire length. This method yielded robust data for species identification even when comparing Terebratulina populations across large spatial scales7. For PCR primers used see Supplementary Table S3. All sequences obtained were submitted to NCBI and can be identified by their respective Genbank accession numbers according to Supplementary Table S4.
Statistics
To test for significant differences between morphometric measurements (length, width, thickness and rib width) we used the nonparametric Mann-Whitney U-test (threshold: 5% with p ≤ 0.05) which is appropriate to compare differences between two independent groups when the dependent variable is not normally distributed. Calculations were done with XLSTAT, ver. 2013.5.05 (Addinsoft 1995–2013).
References
Mallet, J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 20, 229–237 (2005).
Gardner, J. P. A. Developmental stability is not disrupted by extensive hybridization and introgression among populations of the marine bivalve molluscs Mytilus edulis (L.) and M. galloprovincialis (Lmk.) from south-west England. Biol. J. Linn. Soc. 54, 71–86 (1995).
Rawson, P. D., Agraval, V. & Hilbish, T. J. Hybridization between the blue mussels Mytilus galloprovincialis and M. trossulus along the Pacific coast of North America: evidence for limited introgression. Mar. Biol. 134, 201–211 (1999).
Wilding, C. S., Butlin, R. K. & Grahame, J. Differential gene exchange between parapatric morphs of Littorina saxatilis detected using AFLP markers. J. Evol. Biol. 14, 611–619 (2001).
Boon, N. A. M. et al. Detecting hybridization in African schistosome species: does egg morphology complement molecular species identification? Parasitology 144, 954–964 (2016).
Abbott, R. et al. Hybridization and speciation. J. Evol. Biol. 26, 229–246 (2013).
Lüter, C. & Cohen, B. L. DNA sequence evidence for speciation, paraphyly and a Mesozoic dispersal of cancellothyridid articulate brachiopods. Mar. Biol. 141, 65–74 (2002).
Linné, C. Systema Naturae. 10 th ed. (Holmiae, 1758).
Couthouy, J. P. Descriptions of new species of Mollusca and shells, and remarks on several Polypi, found in Massachusetts Bay. J. Nat. Hist. 2, 53–111 (1838).
Arndt, W. & Grieg, I. A. Die Brachiopoden des arktischen Gebietes. Fauna Arctica 6, 477–488 (1933).
Helmcke, J.-G. Die Brachiopoden des Zoologischen Museums zu Berlin. Sbr. Ges. Naturf. Freunde Jg. 1938 (Okt.-Dez.), 221–268 (1939).
Wesenberg-Lund, E. The Zoology of Iceland Vol. 4 (67): Brachiopoda. 1–11 (Muunsgard, 1938).
Wesenberg-Lund, E. The Godthaab Expedition 1928: Brachiopods from the water west of Greenland. Meddelelser om Grønland 80, 1–24 (1940).
Wesenberg-Lund, E. The Zoology of East Greenland: Brachiopoda. Meddelelser om Grønland 121, 1–12 (1940).
Wesenberg-Lund, E. The Danish Ingolf-Expedition, Vol 4 (12): Brachiopoda. 3–17 (Bianco Luno, 1941).
Curry, G. B. & Endo, K. Migration of brachiopod species in the North Atlantic in response to Holocene climatic change. Geology 19, 1101–1103 (1991).
Davidson, T. A monograph of recent Brachiopoda, part I. Trans. Linnean Soc. Lond. 2nd ser; IV, 1–74 (1886).
Posselt, H. J. Brachiopoder og Bløddyr. Meddelelser om Grønland 23, 1–298 (1898).
Hägg, R. Mollusca und Brachiopoda gesammelt von der schwedischen zoologischen Polarexpedition nach Spitzbergen, dem nordöstlichen Grönland und Jan Mayen im J. 1900. Arkiv för Zoologie 2, 1–66 (1905).
Emig, C. C. Examples of post-mortality alteration in Recent brachiopod shells and (paleo)ecological consequences. Mar. Biol. 104, 233–238 (1990).
Cohen, B. L., Balfe, P., Cohen, M. & Curry, G. B. Molecular evolution and morphological speciation in North Atlantic brachiopods (Terebratulina spp.). Can. J. Zool. 69, 2903–2911 (1991).
Stechmann, A. & Schlegel, M. Analysis of the complete mitochondrial DNA sequence of the brachiopod Terebratulina retusa places Brachiopoda within the protostomes. Proc. R. Soc. Lond. B 266, 2043–2052 (1999).
Helfenbein, K., Brown, W. M. & Boore, J. L. The complete mitochondrial genome of the articulate brachiopod Terebratalia transversa. Mol. Biol. Evol. 18, 1734–1744 (2001).
Endo, K., Noguchi, Y., Ueshima, R. & Jacobs, H. T. Novel repetitive structures, deviant protein-encoding sequences and unidentified ORF’s in the mitochondrial genome of the brachiopod Lingula anatina. J. Mol. Evol. 61, 36–53 (2005).
Luo, Y.-J., Satoh, N. & Endo, K. Mitochondrial gene order variation in the brachiopod Lingula anatina and its implications for mitochondrial evolution in lophotrochozoans. Mar. Genom. 24, 31–40 (2015).
Valdimarsson, H. & Danielsen, M. Oceanographic group homepage: Hydrography, www.hafro.is/Sjora (2016) (Date of access: 07/03/2017).
Logemann, K., Ólafsson, J., Snorrason, Á., Valdimarsson, H. & Marteinsdóttir, G. The circulation of Icelandic waters – a modelling study. Ocean Sci. 9, 931–955 (2005).
Ostmann, A., Schnurr, S. & Martínez Arbizu, P. Marine environment around Iceland: hydrography, sediments and first predictive models of Icelandic deep-sea sediment characteristics. Pol. Polar. Res. 35, 151–176 (2014).
Curry, G. B. Ecology and population structure of the Recent brachiopod Terebratulina from Scotland. Palaeontology 25, 227–246 (1982).
Richardson, J. R. Ecology of articulated brachiopods in Treatise on Invertebrate Paleontology, part H: Brachiopoda, revised, Vol. 1 (ed. Kaesler, R. L.) 441–462 (The Geological Society of America and University of Kansas Press, 1997).
Vihjálmsson, H. Capelin (Mallotus villosus) in the Iceland-East Greenland- Jan Mayen ecosystem. ICES J. Mar. Sci. 59, 870–883 (2002).
Johnson, S. L. & Yund, P. O. Remarkable longevity of dilute sperm in a free-spawning colonial ascidian. Biol. Bull. 206, 144–151 (2004).
Cloud, P. E. Notes on recent Brachiopods. Am. J. Sci. 246, 241–250 (1948).
Webb, G. R., Logan, A. & Noble, J. P. A. Occurrence and significance of brooded larva in a recent brachiopod, Bay of Fundy, Canada. J. Paleo. 50, 869–871 (1976).
Long, J. A. The embryology of three species representing three superfamilies of articulate Brachiopoda. Dissertation (University of Washington, 1964).
Rudwick, M. J. S. Filter-feeding mechanisms in some brachiopods from New Zealand. Zool. J. Linnean Soc. 44, 592–615 (1962).
Rudwick, M. J. S. Living and fossil brachiopods (Hutchinson University Library, 1970).
LaBarbera, M. Brachiopod orientation to water movement 1. Theory, laboratory behavior, and field orientations. Paleobiology 3, 270–287 (1977).
James, M. A. et al. Biology of living brachiopods. Adv. Mar. Biol. 28, 175–387 (1992).
Afzelius, B. A. & Ferraguti, M. Fine structure of brachiopod spermatozoa. J. Ultrastruct. Res. 63, 308–315 (1978).
Endo, K. & Curry, G. B. Molecular and morphological taxonomy of a recent brachiopod genus Terebratulina in Brachiopods through time (eds. MacKinnon, D. I. et al.) 101–108 (Balkema, 1991).
MacFarlan, D. A. B. Middle and Late Jurassic terebratulides from New Zealand. Palaeoworld 25, 467–495 (2016).
Bitner, M. A. & Moissette, P. Pliocene brachiopods from north-western Africa. Geodiversitas 25, 463–479 (2003).
Bitner, M. A. & Dulai, A. Revision of Miocene brachiopods of the Hungarian Natural History Museum, with special regard to the Meznerics collection. Fragm. Palaeont. Hung. 22, 69–82 (2004).
Bitner, M. A., Lozouet, P. & Cahuzac, B. Upper Oligocene (Chattian) brachiopod fauna from the Aquitaine Basin, southwestern France and its paleoenvironmental implications. Geodiversitas 35, 579–606 (2013).
Uhen, M. D. & Clapham, M. Paleobiology Database, https://paleobiodb.org (2017) (Date of access: 07/03/2017).
Palumbi, S. et al. The simple fool’s guide to PCR (University of Hawaii, 1989).
Kocher, T. D. et al. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 86, 6196–6200 (1989).
Silberman, J. D. & Walsh, P. J. Species identification of spiny lobster phyllosome larvae via ribosomal DNA analysis. Mol. Mar. Biol. Biotechnol. 1, 195–205 (1992).
Cohen, B. L. & Weydmann, A. Molecular evidence that phoronids are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic divergence of metazoan phyla began before the early Cambrian. Org. Divers. Evol. 5, 253–273 (2005).
Acknowledgements
We thank the crew and the scientific shipboard party of RV METEOR Me 85/3 “IceAGE”, and especially Saskia Brix-Elsig, for collecting and providing the Terebratulina specimens for our study. We also thank B.L. Cohen for his valuable comments on earlier drafts of this paper and two anonymous reviewers for their constructive suggestions. The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Author information
Authors and Affiliations
Contributions
C.L. designed the study and the experiments. N.E. did the morphometrical analyses, prepared the IceAGE samples, extracted DNA, performed PCR and sample preparation for commercial sequencing. Alignment and data interpretation was mainly performed by C.L. and N.E. C.L. and M.A. wrote the manuscript, with M.A. substantially contributing to its palaeobiogeographic perspective. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lüter, C., Ebeling, N.A. & Aberhan, M. Detecting hybridization between sister species of Terebratulina (Brachiopoda, Cancellothyridoidea) in the North Atlantic: morphology versus molecules. Sci Rep 7, 8845 (2017). https://doi.org/10.1038/s41598-017-09195-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09195-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.