Abstract
Snake envenomation is an important medical problem. One of the hurdles in antivenom development is the in vivo assay of antivenom potency which is expensive, gives variable results and kills many animals. We report a novel in vitro assay involving the specific binding of the postsynaptic neurotoxins (PSNTs) of elapid snakes with purified Torpedo californica nicotinic acetylcholine receptor (nAChR). The potency of an antivenom is determined by its antibody ability to bind and neutralize the PSNT, thus preventing it from binding to nAChR. The PSNT of Naja kaouthia (NK3) was immobilized on microtiter wells and nAChR was added to bind with it. The in vitro IC50 of N. kaouthia venom that inhibited 50% of nAChR binding to the immobilized NK3 was determined. Varying concentrations of antisera against N. kaouthia were separately pre-incubated with 5xIC50 of N. kaouthia venom. The remaining free NK3 were incubated with nAChR before adding to the NK3 coated plates. The in vitro and in vivo median effective ratio, ER50s of 12 batches of antisera showed correlation (R 2) of 0.9809 (p < 0.0001). This in vitro assay should be applicable to antisera against other elapid venoms and should reduce the use of live animals and accelerate development of life-saving antivenoms.
Similar content being viewed by others
Introduction
Snake envenomation is an important medical problem especially in the developing world. It has been estimated that around 421,000–2.5 million people are envenomed annually with about 20,000–94,000 fatalities1. Antivenoms (AVs) are the rationale and the most effective therapy of snake envenomation. However, this serious public health problem has so far been neglected and effective, affordable antivenoms remain unavailable in many parts of the developing world. Recently, efforts from a number of research institutions are underway to solve this problem2.
In the development and production of an AV, at least two major steps are involved: an effective immunization program and the pre-clinical testing to assess the neutralizing potential of the AV against the lethal effects of homologous and heterologous venoms. The accepted AV potency assay is the standard murine lethality assay to determine the median lethal dose (LD50) that estimates the lethality of the venom and the median effective dose (ED50) of the AV3, 4. For this in vivo assay, three to five mice per venom dose are used and the total of about 6 different doses are tested. Thus, about 30 mice are needed for the determination of the LD50 of a venom. Similarly, about 30 mice are needed for the ED50 determination of an AV against a venom. Therefore, a large number of mice will be used for the in vivo neutralization assays. For example, the number of mice required by European Pharmacopoeia using this method to test the activity of one European viper venom antiserum (LD50 and ED50 tests combined) against five venoms, is 374 mice per batch of antivenom5. Using this figure, testing a pan-specific AV against 27 different venoms6 would require about 2,020 mice. The assay is very costly, laborious and can give highly variable results. Moreover, some lethality studies have been shown to be inconsistent, suggesting that rodent death may not measure relevant efficacy outcomes in humans7. Lastly, witnessing the suffering and death of a large number of animals is the most difficult part of the experiment for many. In Buddhist countries like Thailand, most, if not all, laboratory personnel and graduate students refuse to do such experiments. Thus, it is becoming increasingly difficult to perform the in vivo assay for ethical and religious, as well as regulatory reasons.
For the above reasons, various types of in vitro neutralization assays have been developed to be used in place of, or to reduce the number of the in vivo assay. It has been reported that venom toxicity and effectiveness of AV can be studied using the chick biventer cervicis preparation8,9,10. This assay was used for screening AVs against the neurotoxic effects of venoms11. However, this in vitro assay requires the preparation of chick biventer cervicis muscles and the assay is laborious and time-consuming. In vitro neutralization of some venom enzymatic activities have been studied for use in AV potency assay12. It was shown that the neutralization of phospholipase A2 activities by antivenom against Micrurus nigrocinctus highly correlated with the in vivo neutralization activity. Also, the inhibition of indirect hemolytic activity induced by phospholipase A2 was also shown to correlate well with the in vivo potency of a polyspecific antivenom13. However, these assays are applicable only to antivenoms directed against venoms with enzymatic activities that parallel the lethality of the venoms.
Numerous investigators have studied and reported the use of ELISA for AV potency determinations together with the correlation between the results of the in vitro and in vivo neutralization assays14,15,16,17,18,19,20. However, the “neutralizing potency” described as “in vitro ELISA titer” occasionally did not correlate well with the in vivo neutralizing activity. For example, Ibrahim and Farid21 studied the lethality-neutralizing potency, ELISA antibody level and the avidity indexes of a polyvalent antivenom against seven snake venoms. They showed poor correlation between the in vivo and in vitro assays with the in vitro assays always giving high values. ELISA has at times been criticized on the grounds that the antigen-antibody ‘binding’ reaction measured cannot be assumed to be the same as the ‘neutralization’ reaction of the antigen.
Elapid snakes (cobras, kraits and mambas) produce the lethal postsynaptic neurotoxins (PSNTs) which bind specifically and quasi-irreversibly with nicotinic acetylcholine receptor (nAChR) at the muscle end-plate22, 23. This binding results in the inhibition of neuromuscular transmission which can lead to respiratory arrest and death23. Thus, it should be possible to develop an in vitro functional assay to test antivenom against elapid venoms based on the ability of the antivenom antibodies to inhibit the binding of PSNTs to nAChR. Such an in vitro assay would closely mimic the lethality reactions of PSNTs of the elapids in vivo, in particular cobras (Naja sp.). Cobras are in general listed as Category 1 of medically important snakes throughout most parts of Asia and Africa4, 24.
An in vitro potency assay based on PSNT binding to nAChR was previously studied for the venom of coral snake Micrurus nigrocinctus 12. It was found that, the ED50 of the horse antisera against M. nigrocinctus in neutralizing the lethal effect of the venom did not correlate with the antivenom ability to inhibit the nAChR-binding activity but correlated well with the inhibition of the venom phospholipase A2 activity.
By using a different reaction scheme and conditions from that studied above12, we report here the development of a novel in vitro potency assay of monospecific antisera against the venom of the Thai monocled cobra Naja kaouthia based on nAChR binding. The assays gave excellent correlation (R 2 = 0.9809; p < 0.0001) with the corresponding in vivo assay using mice. This in vitro assay should be useful in reducing or partially replacing the in vivo assays used to test antivenoms against N. kaouthia and other elapid venoms.
Results
Development of the in vitro neutralization assay using nAChR-PSNT binding
The optimal concentrations of NK3, nAChR, rat anti-nAChR antibody and goat-anti-rat HRP conjugate in the in vitro potency assay
The optimal concentrations of NK3, nAChR, rat anti-nAChR antibody and goat-anti-rat HRP conjugate used in the in vitro potency assay were studied as described in Materials and Methods (step: pre-incubation 2). The results are shown in Fig. 1. As the concentration of NK3 used to coat the microtiter plate increased, the signal as measured by the OD450nm increased. This was also observed when the concentration of nAchR used to bind the immobilized NK3 was increased. To economize on the nAChR available while obtaining reasonably high OD450nm signal, it was decided to use 15 µg/ml of NK3 for coating the plates and 0.707 µg/ml of nAChR for binding to the NK3 coated plate. A 1:1600 dilution of rat anti-nAchR serum and a 1:4500 dilution of goat anti-rat-IgG conjugated HRP were used.
Inhibition of nAChR binding to NK3-coated plate by N. kaouthia venom or N. kaouthia cytotoxin
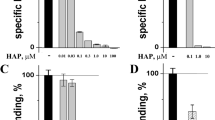
Crude N. kaouthia venom was used to determine the 50% inhibition of nAChR binding (in vitro IC50). In the first step, crude N. kaouthia venom at various concentrations was incubated with the purified nAChR (0.707 µg/ml) for 1 hr at 25 °C. The solution was then transferred to NK3 coated plates. The concentration of the crude venom that reduced nAchR binding by 50% was defined as the IC50. The results (Fig. 2) showed that the IC50 of N. kaouthia was 0.0281 µg/ml.
To study the specificity of the nAChR binding, N. kaouthia cytotoxin I at various concentrations (0.4875 to 0.0152 µg/ml) was tested as described above. It was shown (Fig. 2) that the cytotoxin had no effect on the nAChR binding. Thus, the inhibition reaction was specific to the postsynaptic neurotoxins.
Neutralization of N. kaouthia venom by horse monospecific antisera as determined by nAChR binding to the NK3-coated plate
Twelve horse monovalent anti-N. kaouthia sera were serially diluted 2-fold from 1:500 to 1:512,000 and the dilutions were separately incubated with 5xIC50 of N kaouthia venom (1.4029 µg/ml) in the ‘Pre-incubation 1’ experiment. After ultrafiltration and ‘Pre-incubation 2’, the reaction mixtures were added to the NK3-coated plates. The binding of the free nAChR to the plate was measured at OD450nm and the results are shown in Fig. 3.
The in vitro median effective ratio (ER50s), expressed as µg of venom neutralized per µl of antiserum, and the in vivo ER50s (mg of venom neutralized per ml of antiserum) of the 12 horse antisera are shown in Table 1. The correlation coefficient, R, between the in vitro ER50s and the in vivo ER50s was 0.9904, and the coefficient of determination for the regression model was R 2 = 0.9809 (p < 0.0001), as shown in Fig. 4.
Regression between the nicotinic binding efficacy (Log2 [in vitro ER50]) and the lethality neutralization efficacy (in vivo ER50). R 2: Coefficient of determination. In vitro ER50 values were expressed as mean ± S.D. (μg venom/μl antiserum) of 4 determinations. In vivo ER50 values were expressed as median dose ± 95% C.I. from serial dose-response study in mice (n = 4–5 mice per dose). Footnote: For each batch of antiserum, the in vitro ER50 was mean ± S.D. from 4 determinations while the in vivo ER50 was median ± 95% C.I. (C.I. = confidence level).
Discussion
It is reported here the first successful development of an in vitro potency assay for antiserum against an elapid snake based on nicotinic acetylcholine receptor binding. The reactions employed in the in vitro assay closely mimicked those of the in vivo toxicological reactions of the elapid postsynaptic neurotoxins. Unlike the ELISA which at times gave poor correlation with in vivo assay and has often been criticized in that the antibody binding did not necessarily result in toxin neutralization, the present in vitro assay involved the binding and neutralization by the antisera antibodies of the lethal snake toxins thus preventing them from binding to nAchR. The correlation between the in vivo assay using mice and the developed in vitro assay was very high as supported by the correlation coefficient of R = 0.9904.
An in vitro assay based on PSNT binding to nAchR was studied by Stiles25, 26 and Alape-Giron et al.12, 27. Using an ELISA format, the bindings of purified nAchR from T. californica to the immobilized long and short PSNTs were shown to be specific. Furthermore, these researchers showed that horse antivenom against M. nigrocinctus nigrocinctus venom contained antibodies that inhibited the binding of the venom α-neurotoxins to purified nAchR, and reversed the binding of the toxins already complexed with the receptor12. The antivenom ED50 in neutralizing the lethal effect of the venom was shown not to correlate with the antivenom’s ability to inhibit the nAchR-binding activity (r = 0.34; p > 0.05) but correlated well with the inhibition of phospholipase A2 activity. From these results, they concluded that the lethality of the venom was the result of the combined actions of various toxins12 and recent proteomics results have shown that the short-chain α-neurotoxins are likely to play a leading role in the lethality induced by this venom28.
The in vitro assay reported here involved reaction schemes that were different from those reported by Alape-Giron et al.12. Two salient features of the present assay protocol were as follows.
First, the two crucial reactions i.e., the antibody-venom toxins reaction, and the toxin-nAChR reaction, were carried out in solution rather than on solid surface. This was to allow for total exposure of the reactants’ surface residues resulting in more complete binding with their counterparts, and also to avoid any possible steric interactions between the two high molecular weight reactants (IgG and nAchR) which are likely to be more pronounced on solid surface.
Second, after the venom-antibody reaction in ‘Pre-incubation 1’, the antibodies, free or toxin bound, were removed from the reaction mixture by ultrafiltration. This was important in that if these antibodies were not removed, any free excess antibody remaining in the ‘Pre-incubation 1’ reaction could react with the immobilized toxins in the final reaction resulting in reduced binding of nAChR (added in the later step) to the immobilized toxins. Furthermore, since the dissociation constant of toxin-antibody complexes are usually in the micromolar range while the toxin-nAchR dissociation constant is closer to nanomolar range29, it is conceivable that, without the ultrafiltration to remove the antibodies, the antibody-bound toxin might dissociate and form tighter complex with nAChR. These reactions would shift the equilibrium of the toxin-antibody reaction, and the measured amount of nAChR bound to the immobilized toxin would also be reduced.
The described assay procedure was thought to improve the reactions involved and to eliminate or minimize any inaccuracy of reactant concentrations measured; leading to highly correlated in vitro and in vivo results.
It should be noted in Fig. 3 that at higher concentrations of the antisera Nk-A Nk-B and Nk-I, the nAChR bindings were decreased. This phenomenon, often observed in immunoassays, is known as the ‘prozone phenomenon’ or ‘hook effect’ where, at excess concentration of antibody, immunochemical reactions e.g., hemagglutination, were inhibited or become less pronounce30, 31.
The in vitro potency assay described here should be applicable to antivenoms against most, if not all, elapids whose venoms contain mainly or exclusively postsynaptic neurotoxins as major lethal components. However, the usefulness of the assay for some elapids producing other lethal toxins e.g., some Bungarus venoms may contain, in addition to PSNTs, presynaptic neurotoxins (β-neurotoxins) which are highly lethal32, 33. Thus, this in vitro nAchR binding assay which worked well with antisera against the Naja venoms might not work as well with some of the Bungarus venoms, depending on the abundance of the β-neurotoxins present in the particular venom and their role to the overall neurotoxicity.
Since effective, affordable antivenoms against snake venoms remain unavailable in many parts of the world2, studies were being made to produce pan-specific antivenoms that cover multiple snake venoms from wide geographical areas6. Such a pan-specific antivenom could be produced in large volume and, due to the economy of scale, could be produced at low cost. However, in the development of such pan-specific antivenoms, a large number of mice would be needed to assay its efficacy against many homologous and heterologous venoms, and over the years, the cumulative number of mice used will be even more perturbing considering the need to repeat the assay from batch to batch of antivenom. With the developed in vitro assay described here, the development and production of poly-specific or pan-specific AVs should become easier and simpler. This should eventually result in saving the lives of mice and the victims of snake envenomation.
In conclusion, the assay should reduce the use of mice for potency assays for example, during the immunization program and/or fractionation process of antivenom production. In some cases, it may even replace the in vivo assay. The in vitro assay is less expensive, less biologically variable and could avoid the ethical and religious issues involved. The in vitro assay could facilitate the development and production of new and effective antivenoms, especially the pan-specific antivenoms which usually employ a large number of mice. The availability of new antivenoms combined with the reduction in production cost could, in turn, save the lives of more snakebite victims, which are mostly from the poorer regions of the world34.
Materials and Methods
Materials
Electroplaque tissue from Torpedo californica (Pacific electric ray) was obtained from Dr. Charles Winkler, Aquatic Research Consultants (San Pedro, CA, USA). Naja kaouthia (NK, formally known as Naja naja siamensis) venom from pool of several adult snakes of Thai origin and horse monovalent antisera against N. kaouthia was purchased from Queen Soavabha Memorial Institute (QSMI). Benzoquinonium dibromide was purchased from Santa Cruz (Dallas, TX, USA). Goat against rat IgG conjugated with horse radish peroxidase (HRP) was purchased from Abcam (SF, USA). N. kaouthia postsynaptic toxin 3 (NK3, formally known as N. n. siamensis toxin 3) was purified as described by Karlsson et al.35. N. kaouthia cytotoxin (CTX-I) was purified as described by Tan et al.36. N-hydroxysuccinamide-Sepharose (NHS-Sepharose) was from GE Health Care. All other reagents were from Sigma Chemical, St Louis. Missouri, unless stated otherwise.
Methods
Purification of nAChR and production of anti-nAChR antibody in rats
Purification of nAChR from T. californica electroplaque was carried out as described by Lindstorm et al.37. The purified receptor (10 µg) in 0.1 ml phosphate buffer saline (PBS) pH 7.4 was emulsified with Complete Freund adjuvant and injected subcutaneously into each of the eleven Wistar rats. The second and third immunizations were carried out using the receptor emulsified in Incomplete Freund adjuvant and alum as the adjuvant, respectively. Blood of each rat was collected from the heart at the end of the experiment.
In vivo neutralizing activity of horse monospecific antisera against N. kaouthia venom
The intravenous median lethal dose, LD50, of N. kaouthia venom, 0.18 (0.12–0.27) µg/g, was adapted from Tan et al.38 of the same laboratory using the same batch of venom as with the current work. Neutralization of lethality was conducted as described by Ramos-Cerrillo et al.39. Briefly, a challenge dose of the venom constituting 5 LD50 in 50 μl saline was pre-incubated at 37 °C for 30 min with varying dilutions of the pooled horse sera in normal saline, to give a total volume of 250 μl. The venom-antiserum mixture was subsequently injected into the caudal vein of the mice. The mice were allowed free access to food and water ad libitum and the number of survival after 48 h was recorded. The effective dose-50 (ED50) was determined as the volume of antiserum that protected 50% of the challenged mice from death using probit analysis. The neutralizing efficacy of the antiserum was also expressed as median effective ratio (ER50 ± 95% C.I. where C.I. is confidence interval) in mg venom/ml antiserum that gave 50% survival of the mice tested.
Development of the in vitro neutralization assay using nAChR-PSNT binding
Optimal conditions of nAChR, rat anti-nAChR antibody and goat-anti-rat HRP conjugate binding to NK3 coated microtiter plate
This assay was the basic assay format for in vitro binding of solubilized, purified nAChR to the elapid PSNTs immobilized on the microtiter plate. Briefly, purified NK3 at various concentrations were coated to the microtiter wells (Polystylene High Binding 3590, Costar). After washing with 0.05% TWEEN 20 in phosphate buffered saline (PBST), the plate was blocked with 200 µl/well of PBST and 1% BSA for 2 hr. The purified nAChR (in PBS containing 0.05% Tween 20 and 0.15 BSA) at various concentrations were added to bind the immobilized NK3 by incubation at 25 °C for 1 hour. After 3 time washings to remove the unbound nAChR, rat anti-nAChR serum at various concentrations was added and incubated at 25 °C for 1 hr; this was followed by addition of goat-anti-rat-HRP conjugate (ab7097, Abcam) and incubated for 1 hr at room temperature. After 4 washes with PBST, 100 µl/well of freshly prepared substrate solution (0.01% w/w 3,3′,5,5′-tetramethyl benzidine and 0.003% hydrogen peroxide in 0.075 M citrate buffer, pH 5.0) was added. The plate was allowed to stand in the dark for 30 min at 25 °C and the reaction was stopped by adding 25 µl of 4 N sulfuric acid. The absorbance of 450 nm was read against blank using an ELISA reader (Multiskan Go, Thermo Scientific). Optimal concentrations of NK3 (used for coating the plate), nAChR, rat anti-nAChR antibody and goat-anti-rat-HRP conjugate were estimated and used in the experiments that followed.
Inhibition of nAChR binding to the NK3 coated plate by N. kaouthia venom
The ability of an elapid venom (N. kaouthia) which contains PSNTs to inhibit the binding of nAChR to NK3 immobilized plate was studied and was expressed as IC50 (venom concentration inhibiting 50% of the nAChR binding). In this assay, N. kaouthia crude venom at various concentrations was pre-incubated (25°C for 1 hr) with a fixed and optimal concentration of nAChR before the mixture was added to the NK3-coated plate and incubated at 25°C for 1 hour. This was followed by additions of rat anti-nAChR serum at 1:1600 dilution and incubated at 25°C for 1 hr, followed by 1:4500 diluted goat-anti-rat-HRP conjugate (Abcam) and incubated for 60 min at 25 °C. A parallel experiment using purified NK3 as the reference standard in place of the venom was also carried out. The concentration of the tested venom used in the pre-incubation step that inhibited 50% of the nAChR binding to the immobilized NK3 was the median inhibitory concentration (IC50) of that venom.
Inhibition of the N. kaouthia venom PSNTs from binding to nAChR by horse antisera
Using a format similar to that described above, an in vitro assay of horse antiserum potency (in vitro ED50) was carried out. Horse sera at various amounts (0.94 nl–0.96 µl) were pre-incubated at 37 °C for 1.5 hr with a fixed amount (5 × IC50) of N. kaouthia venom in 137 mM NaCl, 2.68 mM KCl, 8.10 mM Na2HPO4, 1.47 mM KH2PO4, 0.05% TWEEN20, 0.1% w/v BSA in a total volume of 480 µl. This was referred to as ‘Pre-incubation 1’. The mixture was then filtered through a 100 kDa MWCO ultrafiltration membrane (Amicon®) to remove antibody-toxin complexes, free antibodies and some other high molecular weight horse serum proteins. The filtrates (126 µl) containing the remaining free venom PSNTs were then incubated with an optimal amount of nAChR (14 µl in the same buffer) at 25o C for 1 hr as described above and this was referred to as ‘Pre-incubation 2’. The mixtures containing any remaining free nAchR were then added to the microtiter wells immobilized with NK3, followed by the rat anti-nAChR antibody, goat-anti-rat HRP conjugate, etc. The reaction products were then processed as described above. Wells incubated with a non-immune horse serum in place of antisera were included as background control.
The percentage of nAChR binding was then determined using the following formula:
‘OD max’ represented the binding of nAChR (optimal amount) which was not pre-incubated with the venom or antiserum.
‘OD Ag control’ represented the binding of nAChR after being pre-incubated with 5 folds of IC50 of N kaouthia venom (and without antiserum in ‘Pre-incubation 1’).
‘OD sample’ represented the binding of nAChR after nAChR (optimal amount) was pre-incubated with filtrate from ‘Pre-incubation 1’ (where 5 folds of IC50 of N. kaouthia venom was pre-incubated with various amount of antiserum).
From the results, dose–response curves of horse sera volumes vs percents of nAChR binding were constructed. The in vitro neutralizing activities (ED50s) represented the horse antiserum volumes at which the nAChR binding was inhibited by 50 percent compared to wells incubated with buffer in place of antisera. The in vitro median effective ratio, ER50, represented µg venom/µl antiserum that the nAChR binding was inhibited by 50% was calculated. The results of the in vitro study on nAChR binding for every batch of the horse antisera (Nk-A to Nk-L) were presented as means ± S.D. of 4 determinations.
Ethics approval
Experiment involving rats was reviewed and approved by the Animal Care and Use Committee of the Faculty of Veterinary Science, Mahidol University, Protocol no. MUVS-2014–29 in accordance with the Guidelines of the National Research Council of Thailand. The protocol of animal study on mice was based on the guidelines given by the Council for International Organizations of Medical Sciences (CIOMS) and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Malaya (Ethical clearance No. 2014-09-11/PHAR/R/TCH).
Miscellaneous procedures
Protein concentration was determined by the procedure described by Lowry et al.40 and by BCA Protein assay Kit (PierceTM) using bovine serum albumin as the standard. IC50 and ED50 values were determined using GraphPad Prism 5.0 program and BioStat 2009 version 5.8.3.0, respectively. The correlation analysis was evaluated by linear regression using GraphPad Prism 5.0 software. In brief, the correlation coefficient R was determined from the linear regression model, and R 2 (coefficient of determination) is the square of the correlation coefficient. An R 2 of 0.8–1.0 indicates that the regression line well fits the data in correlation. The statistical significance of the correlation test was set at p < 0.05.
References
Kasturiratne, A. et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLOS medicine 5, 218 (2008).
Williams, D. J. et al. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J Proteomics 74, 1735–1767 (2011).
WHO. Progress in the characterization of venoms and standardization of antivenoms (World Health Organization, Geneva, 1981).
WHO. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins (World Health Organization, Geneva, 2010).
Weisser, K. et al. Animal welfare aspects in the quality control of immunobiologicals: a critical evaluation of the animal tests in pharmacopoeial monographs. (FRAME for ECVAM and PEI, 1997).
Ratanabanangkoon, K. et al. A Simple and Novel Strategy for the Production of a Pan-specific Antiserum against Elapid Snakes of Asia. PLOS neglected tropical diseases 10, 1–20 (2016).
Maduwage, K., Silva, A., O’Leary, M. A., Hodgson, W. C. & Isbister, G. K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: lethality studies or clinically focussed in vitro studies. Sci Rep 6, 26778 (2016).
Barfaraz, A. & Harvey, A. L. The use of the chick biventer cervicis preparation to assess the protective activity of six international reference antivenoms on the neuromuscular effects of snake venoms in vitro. Toxicon 32, 267–272 (1994).
Fry, B. G., Wickramaratna, J. C., Jones, A., Alewood, P. F. & Hodgson, W. C. Species and regional variations in the effectiveness of antivenom against the in vitro neurotoxicity of death adder (Acanthophis) venoms. Toxicol Appl Pharmacol 175, 140–148 (2001).
Tan, K. Y., Tan, C. H., Sim, S. M., Fung, S. Y. & Tan, N. H. Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): venom-induced neuromuscular depression and antivenom neutralization. Comp Biochem Physiol C Toxicol Pharmacol 185-186, 77–86 (2016).
Hodgson, W. C. & Wickramaratna, J. C. In vitro neuromuscular activity of snake venoms. Clin Exp Pharmacol Physiol 29, 807–814 (2002).
Alape-Giron, A., Miranda-Arrieta, K., Cortes-Bratti, X., Stiles, B. G. & Gutierrez, J. M. A comparison of in vitro methods for assessing the potency of therapeutic antisera against the venom of the coral snake Micrurus nigrocinctus. Toxicon 35, 573–581 (1997).
Gutierrez, J. M., Avila, C., Rojas, E. & Cerdas, L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 26, 411–413 (1988).
Barbosa, C. F., Rodrigues, R. J., Olortegui, C. C., Sanchez, E. F. & Heneine, L. G. Determination of the neutralizing potency of horse antivenom against bothropic and crotalic venoms by indirect enzyme immunoassay. Braz J Med Biol Res 28, 1077–1080 (1995).
Heneine, L. G., Carvalho, A. D. Jr., Barbosa, C. F. & Aravjo dos Santos, M. R. Development of an ELISA to assess the potency of horse therapeutic polyvalent antibothropic antivenom. Toxicon 36, 1363–1370 (1998).
Maria, W. S., Cambuy, M. O., Costa, J. O., Velarde, D. T. & Chavez-Olortegui, C. Neutralizing potency of horse antibothropic antivenom. Correlation between in vivo and in vitro methods. Toxicon 36, 1433–1439 (1998).
Rial, A., Morais, V., Rossi, S. & Massaldi, H. A new ELISA for determination of potency in snake antivenoms. Toxicon 48, 462–466 (2006).
Rungsiwongse, J. & Ratanabanangkoon, K. Development of an ELISA to assess the potency of horse therapeutic antivenom against Thai cobra venom. J Immunol Methods 136, 37–43 (1991).
Theakston, R. D. & Reid, H. A. Enzyme-linked immunosorbent assay (ELISA) in assessing antivenom potency. Toxicon 17, 511–515 (1979).
Leong, P. K., Fung, S. Y., Tan, C. H., Sim, S. M. & Tan, N. H. Immunological cross-reactivity and neutralization of the principal toxins of Naja sumatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Trop 149, 86–93 (2015).
Ibrahim, N. M. & Farid, N. M. Comparison between Two In Vitro ELISA-Based Assays in the Determination of Antivenom Potency. Journal of Applied Sciences Research 5, 7 (2009).
Barber, C. M., Isbister, G. K. & Hodgson, W. C. Alpha neurotoxins. Toxicon 66, 47–58 (2013).
Changeux, J. P. The TiPS lecture. The nicotinic acetylcholine receptor: an allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol Sci 11, 485–492 (1990).
WHO. Guideline for the management of snake-bites (World Health Organization, Geneva, 2016).
Stiles, B. G. A non-radioactive receptor assay for snake venom postsynaptic neurotoxins. Toxicon 29, 503–510 (1991).
Stiles, B. G., Sexton, F. W., Guest, S. B., Olson, M. A. & Hack, D. C. Characterization of monoclonal antibodies against Naja naja oxiana neurotoxin I. Biochem J 303(Pt 1), 163–170 (1994).
Alape-Giron, A. et al. Characterization of multiple nicotinic acetylcholine receptor-binding proteins and phospholipases A2 from the venom of the coral snake Micrurus nigrocinctus. FEBS Lett 380, 29–32 (1996).
Fernandez, J. et al. Venomic and antivenomic analyses of the Central American coral snake, Micrurus nigrocinctus (Elapidae). J Proteome Res 10, 1816–1827 (2011).
Klett, R. P. et al. The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J Biol Chem 248, 6841–6853 (1973).
Schiettecatte, J., Anckaert, E. & Smitz, J. in Advances in Immunoassay Technology (eds Norman H., L. Chiu, & Theodore K. Christopoulos) (2012).
Gillet, P., Mori, M., Van Esbroeck, M., Van den Ende, J. & Jacobs, J. Assessment of the prozone effect in malaria rapid diagnostic tests. Malar J 8, 271 (2009).
Abe, T., Alema, S. & Miledi, R. Isolation and characterization of presynaptically acting neurotoxins from the venom of Bungarus snakes. Eur J Biochem 80, 1–12 (1977).
Pungercar, J. & Krizaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon 50, 871–892 (2007).
Harrison, R. A., Hargreaves, A., Wagstaff, S. C., Faragher, B. & Lalloo, D. G. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 3, e569 (2009).
Karlsson, E., Arnberg, H. & Eaker, D. Isolation of the principal neurotoxins of two Naja naja subspecies. Eur J Biochem 21, 1–16 (1971).
Tan, K. Y., Tan, C. H., Fung, S. Y. & Tan, N. H. Neutralization of the Principal Toxins from the Venoms of Thai Naja kaouthia and Malaysian Hydrophis schistosus: Insights into Toxin-Specific Neutralization by Two Different Antivenoms. Toxins (Basel) 8, (86 (2016).
Lindstrom, J. et al. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem 255, 8340–8350 (1980).
Tan, K. Y., Tan, C. H., Fung, S. Y. & Tan, N. H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J Proteomics 120, 105–125 (2015).
Ramos-Cerrillo, B. et al. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon 52, 881–888 (2008).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275 (1951).
Acknowledgements
This study was funded by a research grant (no. IM 2011-01 to KR) from the Chulabhorn Research Institute and a research grant from the University of Malaya (grant no. UM.C/625/1/HIR/MOE/E00040-20001 to NHT). The authors are deeply grateful to Prof Ram Sasekaram for the kind gift of benzoquinolinium dibromide, and for the valuable suggestions and assistance of Assoc. Prof. Dr. Withawat Wiriyarat of the Faculty of Veterinary Science, Mahidol University, Dr. Lawan Chanhome of the Queen Soavabha Memorial Institute and Ms. Prapada Chaisuriya of the Laboratory of Immunology, Chulabhorn Research Institute and Mr. Sutat Lapanan A.N.H. Scientific Marketing Co.,Ltd. Bangkok.
Author information
Authors and Affiliations
Contributions
K.R. and C.H.T. designed the study; P.S., K.P., K.Y.T., S.E., B.C. and W.W. undertook the laboratory studies; K.Y.T., C.H.T., K.R., P.S. and S.Y. undertook the analysis; K.R., P.S., C.H.T. and N.H.T. drafted the manuscript and all authors contributed to the final version; K.R. and C.H.T. are responsible for the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ratanabanangkoon, K., Simsiriwong, P., Pruksaphon, K. et al. A novel in vitro potency assay of antisera against Thai Naja kaouthia based on nicotinic acetylcholine receptor binding. Sci Rep 7, 8545 (2017). https://doi.org/10.1038/s41598-017-08962-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08962-3
This article is cited by
-
A pan-specific antiserum produced by a novel immunization strategy shows a high spectrum of neutralization against neurotoxic snake venoms
Scientific Reports (2020)
-
An in vitro potency assay using nicotinic acetylcholine receptor binding works well with antivenoms against Bungarus candidus and Naja naja
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.