Abstract
There is no consensus on the involvement of zinc (Zn) dysfunctions in Parkinson’s Disease (PD). We performed a meta-analysis to evaluate whether circulating Zn levels in the serum, plasma, and cerebrospinal fluid (CSF) are altered in PD. Twenty-three published studies were selected by searching the databases of PubMed and China National Knowledge Infrastructure (CNKI). A total of 803 PD patients and 796 controls, 342 PD patients and 392 controls, and 135 PD patients and 93 controls were included to study Zn levels in the serum, plasma, and CSF, respectively. Our meta-analysis showed that the serum Zn levels were significantly lower in PD patients compared with health controls (SMD = −0.59; 95% CI [−1.06, −0.12]; P = 0.014). A reduced Zn levels in PD patients were found when serum and plasma studies were analyzed together (SMD = −0.60, 95% CI [−0.98; −0.22]; p = 0.002). PD patients had a tendency toward reduced CSF Zn levels compared with health controls (SMD = −0.50; 95% CI [−1.76, 0.76]; P = 0.439), but no statistical significance was obtained and this data did not allow conclusions due to a small sample size of CSF studies. This study suggests that reduced Zn levels in the serum and plasma are associated with an increased risk for PD.
Similar content being viewed by others
Introduction
Parkinson disease (PD) is a progressive neurodegenerative disorder, and the incidence of the disease rises steeply with age. The lifetime risk of developing the disease is approximately 1.5%1, 2. PD is most commonly associated with motor symptoms, including tremor, rigidity, slowness of movement, and postural instability. The pathological changes of the disease are characterized by a significant loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of intraneuronal proteinaceous cytoplasmic inclusions termed Lewy bodies3,4,5. Although the etiology of PD is largely unknown, PD is associated with many etiological factors including ageing, genetic susceptibility, and disturbances in trace element homeostasis6,7,8,9.
Zn is an essential element for human health, and plays crucial and multiple roles in central nervous system (CNS). Zn are rich in the hippocampus and cerebral cortex10, 11. Zn deprivation influences Zn homeostasis in the brain, leading to changes in behavior, learning, memory, and emotional stability12. Disturbance of Zn homeostasis has been found to be associated with the pathogenesis of many neurodegenerative disorders in CNS, such as PD, Alzheimer’s disease and amyotrophic lateral sclerosis13,14,15,16,17,18. Recently, it has been found that Zn ions directly binds to the peptide fragments from the Parkinson’s disease gene Park919,20,21. Removal of Zn from Park2 causes nearly complete unfolding of the protein and loss of its activity22. However, ambiguous and contradictory results exist in the literature regarding circulating Zn levels in PD patients. For example, some studies reported a decrease of circulating Zn in patients with PD compared with health controls23,24,25,26,27,28,29,30,31,32,33, and other studies found no significant difference or even increased Zn levels in PD patients34,35,36,37,38,39,40,41,42,43,44,45.
To evaluate whether Zn levels is altered in PD, we performed a meta-analysis to compare the zinc levels in the serum, plasma, and CSF in PD patients versus health controls. In this article, we applied this statistical method combining the results of different studies on the levels of zinc in PD, which strengthens the power of the conclusions.
Methods
Search strategy and study selection
The databases of PubMed and China National Knowledge Infrastructure (CNKI) were searched for published studies from inception to 2016 reporting the relationships between circulating zinc levels and PD. We entered the keywords “Parkinson’s disease”, “zinc”, “serum”, “plasma”, “CSF”, “metals” and their combinations in English or Chinese language. No language limits were applied. Eligible articles were retrieved from the above data. In addition, we reviewed references of relevant articles. Eligible studies were selected according to the following inclusion criteria: (1) human study, (2) case-control study, and (3) sample size and Zn levels in serum, plasma or CSF were provided for both PD subjects and health controls. Exclusion criteria included: (1) repeated or overlapped publications, (2) animal study, (3) review, abstracts, letters, and case reports, and (4) studies not providing Zn levels for health controls.
Data extraction
Two authors independently (D.K. and Z.X.) assessed each study and extracted the relevant information: the first author last name, year of publication, sample size, mean age of the participants, the percentage of women included in PD and control sample, and the technique used. The mean Zn levels and the corresponding standard deviations were also recorded, or, were estimated from median, range, and the sample size, if they were not directly reported46.
Statistical analysis
We used STATA 12.0 (Stata, College Station, TX, USA) to perform all the statistical analyses. To obtain the pooled estimate of the difference in the Zn level between PD and control group, a random effects model was applied, when significant heterogeneity was observed. Otherwise, the fixed effects mode was adopted. Random-effects models were used to combine study-specific Standardized Mean Difference (SMD), which standardizes the outcome for each individual study to the effect size found in terms of the standard deviation observed in the study. Heterogeneity was evaluated using Chi-square and the I-square test.
To study the causes of heterogeneity, a subgroup analysis was conducted to assess the impact of the method used for measuring Zn levels and the geographic location of studied population. Meta-regression analysis was also performed to analyze the potentially important covariates of patient’s mean age and gender distribution (proportion of women), and to analyze whether the continuous variables had moderating effects on the outcomes of the meta-analysis. Sensitivity analysis was used to investigate the influence of a single study on the overall effect estimate by omitting one study at a time during repeated analyses. Potential publication bias was evaluated by funnel plot with the Egger test. Temporal effect was also evaluated with a cumulative meta-analysis. P-values less than 0.05 were considered statistically significant.
Results
Study Selection and characteristics of eligible studies
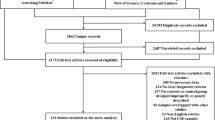
Thirty-six potential studies were identified after an initial search from the Pubmed, CNKI and the references of relevant articles. After further screening, twenty-three studies were finally selected to be related to our meta-analysis (a total of 1306 cases and 1294 controls). The flow diagram of the search process is shown in Fig. 1. Patient samples ranged from 17 to 238 subjects. The mean age of PD patients ranged from 55.4 to 71.1 years and the percentage of female patients ranged from 8% to 62%. The data of mean age was missing in three of these studies, and the data of percentage of female was missing in two of these studies. The analyzed population was European or American in 8 studies, Asian in 14, and African in 1. The detailed characteristics are presented in Table 1.
Meta-analysis of Zn levels in the serum
Data from 18 studies was analyzed in a random-effects model to compare the serum Zn levels in PD patients and health controls (Table 1). The pooled sample size consisted of 1599 subjects including 803 PD and 796 controls. PD patients had significantly lower levels of serum Zn levels than health controls (SMD = −0.59; 95% CI [−1.06, −0.12]; P = 0.014) (Fig. 2). Significant heterogeneity was found among these studies (I2 = 94.7%, P = 0.000). Subgroup analysis by the method used for measuring Zn levels showed a high heterogeneity in each subgroup. Additionally, the subgroup analysis by geographic locations, showed that the heterogeneity was high in European populations and Asian populations. Neither the method for measuring Zn levels nor the geographic location was a main source of heterogeneity (Table 2). In meta-regression analyses, age and gender had no moderating effects (mean age: P = 0.076; gender: P = 0.121). Sensitivity analyses showed that no single study significantly changed the results. The results of cumulative analysis excluded a temporal effect. Furthermore, Egger’s regression asymmetry test did not suggest publication bias (P = 0.163) in the present meta-analysis.
Forest plot for random-effects meta-analysis on differences in serum Zn between PD patients and health controls. 18 studies encompassing a sample of 1599 subjects. The horizontal lines represent 95% CI. The sizes of the shaded squares are proportional to study weight. SMD, Standardized mean difference; CI, confidence interval.
Meta-analysis of Zn levels in the plasma
Three studies were selected with a pooled sample size of 734 subjects including 342 PD patients and 392 health controls (Table 1). Random-effects meta-analysis demonstrated that patients with PD had significantly lower plasma Zn levels compared with health controls (SMD = −0.73; 95% CI [−1.14, −0.32]; P = 0.000; Fig. 3) with significant heterogeneity among these studies (I2 = 77.6%, P = 0.011). We did not perform any further analysis due to the limited number of studies.
Forest plot for random-effects meta-analysis on differences in plasma Zn between PD patients and health controls. 3 studies encompassing a sample of 734 subjects. The horizontal lines represent 95% CI. The sizes of the shaded squares are proportional to study weight. SMD, Standardized mean difference; CI, confidence interval.
Meta-analysis of Zn levels in the serum and plasma
We also performed a joint analysis on 21 studies by combining studies investigating zinc levels in the serum and plasma (Table 1). The pooled sample size consisted of 2333 subjects including 1145 PD patients and 1188 heath controls. The Zn levels were significantly decreased in PD patients compared with health controls (SMD = −0.60, 95% CI [−0.98; −0.22]; p = 0.002; Fig. 4) with high heterogeneity (I2 = 94.3%, P = 0.000). Subgroup analysis by the method used for measuring Zn levels or by geographic locations of the studied population showed high heterogeneity in each subgroup (Table 3). Meta-regression analyses revealed that mean age and gender had no moderating effects on the outcomes of the meta-analysis (mean age: P = 0.076; gender: P = 0.139). Sensitivity analyses indicated that the results were not unduly changed by a particular study. Temporal effect was not observed by the cumulative meta-analysis. According to the Egger’s test, no evidence of publication bias (P = 0.852) was found.
Forest plot for random-effects meta-analysis on differences in serum Zn together with plasma Zn between PD patients and health controls. 21 studies encompassing a sample of 2333 subjects. The horizontal lines represent 95% CI. The sizes of the shaded squares are proportional to study weight. SMD, Standardized mean difference; CI, confidence interval.
Meta-analysis of Zn levels in the CSF
Four studies were in the meta-analysis of Zn levels in the CSF (Table 1). The subject sample consisted of 228 subjects including 135 PD patients and 93 health controls. Random-effects meta-analysis showed that PD patients had a tendency toward reduced Zn levels in the CSF compared with health controls (SMD = −0.50; 95% CI [−1.76, 0.76]; P = 0.439; Fig. 5), but no statistical significance was obtained due to a small sample size. Significant heterogeneity was found among these studies (I2 = 94.4%, P = 0.000). As depicted in the forest plot, compared wtih controls, zinc levels in the CSF in PD patients was not significantly different in the Alimonti’s41 study, significantly increased in the Hozumi’s42 study, and was lower in the Jiménez’s23 and Qureshi’s35 studies. No further analysis was performed due to the limited number of studies.
Forest plot for random-effects meta-analysis on differences in CSF Zn between PD patients and health controls. 3 studies encompassing a sample of 229 subjects. The horizontal lines represent 95% CI. The sizes of the shaded squares are proportional to study weight. SMD, Standardized mean difference; CI, confidence interval.
Discussion
It remains unclear whether circulating Zn levels are associated with PD. In this meta-analysis, we found that the Zn levels in the serum and plasma were reduced in PD patients compared with healthy controls. However, strong heterogeneity was found among the studies. Subgroup analyses showed that methods for measuring Zn levels and geographic locations were not the source of the heterogeneity. Furthermore, meta-regression analysis showed that mean age and gender of the patients had no significant effect on the analysis. Despite the high heterogeneity among studies, our study suggests that a reduction in the serum Zn levels is associated with an increased risk of PD. The statistical power was increased when the samples size was increased by pooling of the studies with serum Zn levels and plasma Zn levels. In this meta-analysis, we found that the Zn level in the plasma was significantly lower in PD patients than health controls. However, the small number of studies and high heterogeneity among studies cautions should be taken to interpret the conclusion. In addition, PD patients had a tendency toward reduced CSF Zn levels compared with the health controls. Since the number of studies and the sample size of these studies with CSF Zn levels (4 studies, 135 PD patients and 93 health controls) were relatively small, the association between CSF Zn levels and PD is inconclusive. Further studies with a large sample size are required to investigate the CSF Zn levels in PD.
It is still unclear how Zn reduction affects the progression of PD. It has been reported that Zn can protect against oxidative stress by increasing the superoxide dismutase activities, and inhibiting the nitric oxide synthetase39, 47. Thus, Zn deficiency may result in increased oxidative stress, which leads to nigral cell death in PD26. This idea is supported by the findings that the levels of nitric oxide and lipid peroxidation products are high and the Zn levels are low in the blood plasma of PD patients17, 26. In addition, Zn is required for maintaining the conformation of Parkin, and Parkin dysfunction can cause PD. Since removal of Zn from Parkin can cause unfolding of the protein, Zn deficiency may contribute to the progression of PD by inducing Parkin dysfunction22. Interestingly, in the Drosophila disease model with a defective Parkin protein, Zn-supplemented food intake resulted in an extended lifespan and an improvement of motoric abilities17, 48. Although no clinical reports have shown the effectiveness of Zn therapy in patients with PD, Zn therapy has been proven to favor improvement and even resolution of neurological damage in patients with Wilson’s disease49. Our findings that PD patients had a lower circulating Zn level support the notion that Zn deficiency is a potential risk factor for PD, and Zn-supplemented intervention may be a potential therapy for PD.
There are some limitations in this meta-analysis. First, although we collected the most comprehensive and updated publications, especially those for CSF or plasma Zn levels, were still limited. Further studies with larger sample sizes of PD patients are required to confirm our conclusion. Second, the dietary intake of metal elements has been reported to be related to risk for PD6, 50. However, we could not analyze possible associations between dietary intake of Zn and the risk for PD due to limited data of the included studies. In addition to Zn, Fe and Cu are also essential nutrients in food for human health. Mariani et al. found that plasma/serum Fe and Cu in PD patients did not differ from that in healthy controls51, whereas Jiao et al. reported a higher serum Fe levels in PD patients compared to the healthy controls52. Therefore, it seems that we could not explain the reasons for the differences in plasma/serum essential metals only based on differences in dietary intake. The mechanisms underlying disbalance of circulating Zn levels in PD patients remain unknown. Dysfunction of Zn transporters, such as divalent metal transporter 1, ZnT3 and LC30A10, has been found in PD53,54,55,56,57. Therefore, the low Zn level in PD patients may be the result of dysfunction of Zn transporters. Third, the mean Zn levels varied greatly among studies. The variability suggested problems with the technique for sampling or maybe the methods used. In addition, we repeated the analysis after excluding the studies with the maximum and the minimum Zn concentrations (Pan 1991; Zhang 2010), and further performed the analysis of the difference in serum Zn levels again. However, the results also showed lower levels of Zn in PD patients compared with health controls (SMD = −0.65; 95% CI [−1.17, −0.13]; P = 0.015), suggesting good stability of our meta-analysis. Forth, we have reviewed the English or Chinese studies, but the studies published in other languages were excluded, though these studies might meet the inclusion criteria.
In conclusion, to the best of our knowledge, this meta-analysis is the first study that investigated the association of circulating Zn levels in PD patients compared with health controls. Our meta-analysis indicated that the Zn level in the serum and plasma was significantly lower in PD patients compared with health controls. However, the results need to be interpreted with caution because there is a high level of heterogeneity among studies.
References
Lees, A. J., Hardy, J. & Revesz, T. Parkinson’s disease. Lancet 373, 2055–2066, doi:10.1016/S0140-6736(09)60492-X (2009).
de Rijk, M. C. et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology 45, 2143–2146 (1995).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Jankovic, J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79, 368–376, doi:10.1136/jnnp.2007.131045 (2008).
Ziemssen, T. & Reichmann, H. Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 13, 323–332, doi:10.1016/j.parkreldis.2006.12.014 (2007).
Powers, K. M. et al. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 60, 1761–1766 (2003).
Gao, X. et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr 86, 1486–1494 (2007).
Liu, R. et al. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol 175, 1200–1207, doi:10.1093/aje/kwr451 (2012).
Nandipati, S. & Litvan, I. Environmental Exposures and Parkinson’s Disease. Int J Environ Res Public Health 13, doi:10.3390/ijerph13090881 (2016).
Frederickson, C. J. Neurobiology of zinc and zinc-containing neurons. International review of neurobiology 31, 145–238 (1989).
Vallee, B. L. & Falchuk, K. H. The biochemical basis of zinc physiology. Physiol Rev 73, 79–118 (1993).
Takeda, A. Movement of zinc and its functional significance in the brain. Brain Res Brain Res Rev 34, 137–148 (2000).
Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Frontiers in aging neuroscience 5, 33, doi:10.3389/fnagi.2013.00033 (2013).
Prakash, A., Bharti, K. & Majeed, A. B. Zinc: indications in brain disorders. Fundam Clin Pharmacol 29, 131–149, doi:10.1111/fcp.12110 (2015).
Dexter, D. T. et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. Journal of neurochemistry 52, 1830–1836 (1989).
Ventriglia, M. et al. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. Journal of Alzheimer’s disease: JAD 46, 75–87, doi:10.3233/JAD-141296 (2015).
Stelmashook, E. V. et al. Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochemistry. Biokhimiia 79, 391–396, doi:10.1134/S0006297914050022 (2014).
Yasui, M., Ota, K. & Garruto, R. M. Concentrations of zinc and iron in the brains of Guamanian patients with amyotrophic lateral sclerosis and parkinsonism-dementia. Neurotoxicology 14, 445–450 (1993).
Remelli, M., Peana, M., Medici, S., Delogu, L. G. & Zoroddu, M. A. Interaction of divalent cations with peptide fragments from Parkinson’s disease genes. Dalton transactions (Cambridge, England: 2003) 42, 5964–5974, doi:10.1039/c2dt32222f (2013).
Remelli, M. et al. Manganism and Parkinson’s disease: Mn(II) and Zn(II) interaction with a 30-amino acid fragment. Dalton transactions (Cambridge, England: 2003) 45, 5151–5161, doi:10.1039/c6dt00184j (2016).
Medici, S., Peana, M., Delogu, L. G. & Zoroddu, M. A. Mn(II) and Zn(II) interactions with peptide fragments from Parkinson’s disease genes. Dalton transactions (Cambridge, England: 2003) 41, 4378–4388, doi:10.1039/c2dt12168a (2012).
Hristova, V. A., Beasley, S. A., Rylett, R. J. & Shaw, G. S. Identification of a novel Zn2+ -binding domain in the autosomal recessive juvenile Parkinson-related E3 ligase parkin. The Journal of biological chemistry 284, 14978–14986, doi:10.1074/jbc.M808700200 (2009).
Jimenez-Jimenez, F. J. et al. Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. Journal of neural transmission (Vienna, Austria: 1996) 105, 497–505, doi:10.1007/s007020050073 (1998).
Hegde, M. L. et al. Serum trace element levels and the complexity of inter-element relations in patients with Parkinson’s disease. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 18, 163–171 (2004).
Alimonti, A. et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 28, 450–456, doi:10.1016/j.neuro.2006.12.001 (2007).
Nikam, S., Nikam, P., Ahaley, S. K. & Sontakke, A. V. Oxidative stress in Parkinson’s disease. Indian J Clin Biochem 24, 98–101, doi:10.1007/s12291-009-0017-y (2009).
Brewer, G. J. et al. Subclinical zinc deficiency in Alzheimer’s disease and Parkinson’s disease. American journal of Alzheimer’s disease and other dementias 25, 572–575, doi:10.1177/1533317510382283 (2010).
Ahmed, S. S. & Santosh, W. Metallomic profiling and linkage map analysis of early Parkinson’s disease: a new insight to aluminum marker for the possible diagnosis. PloS one 5, e11252, doi:10.1371/journal.pone.0011252 (2010).
Zhao, H. W. et al. Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PloS one 8, e83060, doi:10.1371/journal.pone.0083060 (2013).
Luo, P. Y., zhu, X., Zhang, Y. P., Zheng, Z. Z. & Duan, Z. G. Determination of serum trace elements levels and CT scans of brain in Parkinson’s disease. Acta Universitatis Medicinalis Secondae Shanghai 7, 332–334 (1987).
Hou, H. & Wang, X. The relationship between Parkinson’ disease and trace elements and oxidative stress. Chin J Neuroimmunol & Neurol 21, 123–125 (2014).
Pan, B. Y. et al. Research of the relationship between the serum content of microelents and Parkinson’s disease. Guangdong Medical Journal 12, 3–5 (1991).
Fang, S. Y. et al. Study on the changes of trace elements and antioxidant enzymes in blood of patients with Parkinson’s disease. Chinese Journal of Nervous and Mental Diseases 20, 45–47 (1994).
Forte, G. et al. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson’s disease. Journal of neural transmission (Vienna, Austria: 1996) 111, 1031–1040, doi:10.1007/s00702-004-0124-0 (2004).
Qureshi, G. A., Qureshi, A. A., Memon, S. A. & Parvez, S. H. Impact of selenium, iron, copper and zinc in on/off Parkinson’s patients on L-dopa therapy. Journal of neural transmission. Supplementum, 229–236 (2006).
Gellein, K. et al. Trace elements in serum from patients with Parkinson’s disease-a prospective case-control study: the Nord-Trondelag Health Study (HUNT). Brain research 1219, 111–115, doi:10.1016/j.brainres.2008.05.002 (2008).
Squitti, R. et al. Implications of metal exposure and liver function in Parkinsonian patients resident in the vicinities of ferroalloy plants. Journal of neural transmission (Vienna, Austria: 1996) 116, 1281–1287, doi:10.1007/s00702-009-0283-0 (2009).
Fukushima, T., Tan, X., Luo, Y. & Kanda, H. Serum vitamins and heavy metals in blood and urine, and the correlations among them in Parkinson’s disease patients in China. Neuroepidemiology 36, 240–244, doi:10.1159/000328253 (2011).
Younes-Mhenni, S. et al. Serum copper, zinc and selenium levels in Tunisian patients with Parkinson’s disease. La Tunisie medicale 91, 402–405 (2013).
Kocaturk, P. A., Akbostanci, M. C., Tan, F. & Kavas, G. O. Superoxide dismutase activity and zinc and copper concentrations in Parkinson’s disease. Pathophysiology 7, 63–67 (2000).
Alimonti, A. et al. Elemental profile of cerebrospinal fluid in patients with Parkinson’s disease. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 21, 234–241, doi:10.1016/j.jtemb.2007.05.001 (2007).
Hozumi, I. et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. Journal of the neurological sciences 303, 95–99, doi:10.1016/j.jns.2011.01.003 (2011).
Yang, Z. Z. et al. The concentration of five kinds of trace elements in patients with MD and PD at Plateau area and its significance. Journal of Qinghai medical college 22, 5–9 (2001).
Zhou, P. Y., Cao, Z. H., Song, J. H., Gong, S. H. & Lou, Y. W. Determination of somvitamins and trace elements in serum of patients with Parkinson’s disease. Chin J Clinicians 5, 5213–5215 (2011).
Zhang, W., Lei, Z. L. & Chen, H. B. Investigation on the level of trace elements in blood for the patients with vascular Parkinsonism and Parkinson’s disease. Chinese Journal of Stroke 5, 37–41 (2010).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5, 13, doi:10.1186/1471-2288-5-13 (2005).
Miao, L. & St Clair, D. K. Regulation of superoxide dismutase genes: implications in disease. Free radical biology & medicine 47, 344–356, doi:10.1016/j.freeradbiomed.2009.05.018 (2009).
Saini, N. & Schaffner, W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biological chemistry 391, 513–518, doi:10.1515/BC.2010.052 (2010).
Rodriguez-Castro, K. I., Hevia-Urrutia, F. J. & Sturniolo, G. C. Wilson’s disease: A review of what we have learned. World J Hepatol 7, 2859–2870, doi:10.4254/wjh.v7.i29.2859 (2015).
Cheng, P. et al. Dietary intake of iron, zinc, copper, and risk of Parkinson’s disease: a meta-analysis. Neurol Sci 36, 2269–2275, doi:10.1007/s10072-015-2349-0 (2015).
Mariani, S. et al. Fe and Cu do not differ in Parkinson’s disease: a replication study plus meta-analysis. Neurobiology of aging 34, 632–633, doi:10.1016/j.neurobiolaging.2012.05.015 (2013).
Jiao, J. et al. Meta-analysis of the association between serum iron levels and Parkinson’s disease: Evidence from 11 publications. Brain research 1646, 490–493, doi:10.1016/j.brainres.2016.06.044 (2016).
Kong, S. M. et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Human molecular genetics 23, 2816–2833, doi:10.1093/hmg/ddu099 (2014).
Tsunemi, T. & Krainc, D. Zn(2)(+) dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Human molecular genetics 23, 2791–2801, doi:10.1093/hmg/ddt572 (2014).
Peng, J., Peng, L., Stevenson, F. F., Doctrow, S. R. & Andersen, J. K. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience 27, 6914–6922, doi:10.1523/JNEUROSCI.1569-07.2007 (2007).
Whitfield, D. R. et al. Assessment of ZnT3 and PSD95 protein levels in Lewy body dementias and Alzheimer’s disease: association with cognitive impairment. Neurobiology of aging 35, 2836–2844, doi:10.1016/j.neurobiolaging.2014.06.015 (2014).
Chen, P. et al. Manganese homeostasis in the nervous system. Journal of neurochemistry 134, 601–610, doi:10.1111/jnc.13170 (2015).
Acknowledgements
This study was supported by grants from Program for Liaoning Innovation Research Team in University (NO.LT2014016); Liaoning Province Scientific Research Foundation (2014226033); Program for Ministry of national science and technology create significant new drugs (No. 2014ZX09201002-004); Key Laboratory Foundation from Shenyang S&T Projects (F16-094-1-00). This study was also supported in part by National Natural Science Foundation of China (No. 81501098) and National Natural Science Foundation of China (No. 81603112) and National Science and Technology. The funders have no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
K.D. and M.-J.W. conceived and designed the experiments; K.D. and X.Z. searched databases and collected full-text papers; K.D. and M.-Y.L. analyzed data; K.D. wrote the manuscript; M.-J.W. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, K., Liu, MY., Zhong, X. et al. Decreased circulating Zinc levels in Parkinson’s disease: a meta-analysis study. Sci Rep 7, 3902 (2017). https://doi.org/10.1038/s41598-017-04252-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04252-0
This article is cited by
-
The Changes of Blood and CSF Ion Levels in Depressed Patients: a Systematic Review and Meta-analysis
Molecular Neurobiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.