Abstract
Consumption of tea is inversely associated with cardiovascular diseases. However, the active compound(s) responsible for the protective effects of tea are unknown. Although many favorable cardiovascular effects in vitro are mediated by epigallocatechin gallate (EGCG), its contribution to the beneficial effects of tea in vivo remains unresolved. In a randomised crossover study, a single dose of 200 mg EGCG was applied in three different formulas (as green tea beverage, green tea extract (GTE), and isolated EGCG) to 50 healthy men. Flow-mediated dilation (FMD) and endothelial-independent nitro-mediated dilation (NMD) was measured before and two hours after ingestion. Plasma levels of tea compounds were determined after each intervention and correlated with FMD. FMD significantly improved after consumption of green tea containing 200 mg EGCG (p < 0.01). However, GTE and EGCG had no significant effect on FMD. NMD did not significantly differ between interventions. EGCG plasma levels were highest after administration of EGCG and lowest after consumption of green tea. Plasma levels of caffeine increased after green tea consumption. The results show that EGCG is most likely not involved in improvement of flow-mediated dilation by green tea. Instead, other tea compounds, metabolites or combinations thereof may play a role.
Similar content being viewed by others
Introduction
Consumption of tea is associated with reduced progression of atherosclerosis and lower cardiovascular mortality1, 2. However, the substance(s) mediating the favorable cardiovascular effects of tea in vivo have not yet been identified. Tea contains high amounts of polyphenols with significant biological activities. The green tea catechin epigallocatechin gallate (EGCG) is the physiologically most active compound in vitro 3. A large number of in vitro studies and animal experiments provide evidence of the beneficial effects of EGCG on cardiovascular parameters4, 5. Stimulation of nitric oxide (NO) production by endothelial cells and subsequent NO-dependent vasodilation in isolated vessels are two of the most prominent cardiovascular actions of EGCG in vitro 6,7,8. Owing to promising results at the cellular level, EGCG has become extensively applied in human intervention studies. More than 80 studies with EGCG, which encompass a wide range of clinical applications, were registered on clinicaltrials.gov as of October 2016. It is largely unknown, however, whether the beneficial cardiovascular effects of EGCG in vitro can be reproduced in humans.
To elucidate this matter, we investigated the contribution of EGCG to tea-induced changes in flow-mediated dilation (FMD). Impaired endothelial function represents an early marker for later cardiovascular events9. It had been previously shown that consumption of green tea10 as well as of black tea11 improved FMD in humans. A recent meta-analysis demonstrated that short- and long-term tea consumption increases endothelial-dependent vasodilation12. The role of single tea compounds, and especially of EGCG, to the described cardiovascular effects, however, remains unclear. We studied the impact of a single dose of EGCG in three different application forms (green tea beverage, GTE, and isolated EGCG) on FMD in healthy volunteers. The amount of ingested EGCG (200 mg) corresponds to approx. 0.5 l of green tea. The results provide insights into the potential role of EGCG and other tea compounds in the beneficial cardiovascular effects of green tea in vivo.
Results
Interventions had no influence on plasma levels of cardiovascular risk markers
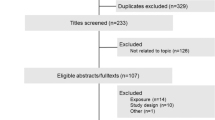
A total of 207 men responded, and interviews were conducted with these volunteers to assess for eligibility. 56 subjects fulfilled the inclusion criteria, including the time requirements, and were enrolled in the study. Four participants were excluded during the study for having blood lipid levels outside the normal range. Two subjects dropped out for personal reasons. 50 subjects finished the study. A flow chart of the study is shown in Fig. 1. Table 1 summarizes the baseline characteristics of the study population. Subjects consumed a single dose of 200 mg EGCG as green tea beverage, as GTE, or as isolated EGCG. FMD was measured before and two hours after ingestion. To prevent food intake from interfering with EGCG absorption and FMD, the interventions were done in a fasting state. The composition of the interventions is shown in Table 2. Levels of LDL, HDL, total cholesterol, TG and CrP did not significantly change two hours after the interventions (Wilcoxon test, data not shown).
Only green tea improved FMD
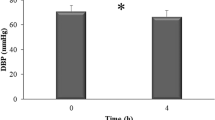
Before interventions, there were no statistical differences in baseline vessel diameter, in FMD, and in NMD between the four study arms by repeated measures ANOVA (Table 3). Hot water decreased FMD non-significantly by −0.84% (5.05 ± 2.65% at baseline and 4.21 ± 2.65% after two hours, p = 0.076, paired t-test). Green tea significantly improved FMD by 1.36% (4.49 ± 2.77% at baseline and 5.85 ± 3.61% after two hours, p = 0.004, paired t-test). GTE and EGCG, although containing the same amount of EGCG as the tea beverage, had no significant effect on FMD (GTE: 4.72 ± 3.16% at baseline and 4.90 ± 3.04% after two hours, p = 0.679, paired t-test; EGCG: 5.00 ± 3.07% at baseline and 4.77 ± 3.99% after two hours, p = 0.648, paired t-test). Between treatments, there were significant overall differences in changes of FMD (p = 0.006, repeated measures ANOVA). After post hoc Bonferroni analysis, only green tea significantly improved FMD compared to water (Fig. 2). No significant changes in FMD were observed between the other treatments. The overall effects on FMD increased with increasing complexity of the interventions – i.e., with an increasing number of compounds contained in the interventions (Fig. 2). There were significant overall differences in absolute FMD by repeated measures ANOVA two hours after ingestion between treatments (p = 0.032). After Bonferroni post hoc analysis, the absolute FMD differed significantly only between green tea and water (5.85 ± 3.61% after tea and 4.21 ± 2.65% after water, p = 0.017), whereas no significant differences were observed between the other interventions. The net response in FMD between green tea and water (mean FMD after green tea minus mean FMD after hot water as control) was 1.64%.
Only green tea increased flow-mediated dilation (FMD). Subjects consumed 200 mg of EGCG as isolated EGCG, GTE, or green tea after fasting overnight. An equal volume of hot water served as control. Green tea significantly increased FMD compared to GTE, EGCG, and water as control. Water slightly decreased FMD, whereas EGCG and GTE had little effects. Data are means ± SEM from n = 50 subjects. All p-values by repeated measures ANOVA followed by post hoc Bonferroni.
NMD was not affected by the interventions
Changes in endothelial-independent NMD did not significantly differ between interventions (water: −0.32 ± 5.42%, EGCG: −0.15 ± 4.78%, GTE: −0.36 ± 4.71%, green tea: −0.79 ± 5.00%; p = 0.92, repeated measures ANOVA), and did not coincide with FMD.
EGCG plasma levels did not correlate with changes in FMD
Plasma levels of EGCG and of the other tea catechins were below detection limits before interventions and after consumption of hot water (data not shown). The highest EGCG plasma levels were observed after intake of isolated EGCG and the lowest after consumption of green tea. Administration of GTE resulted in intermediate EGCG plasma levels (Fig. 3a). EGCG was present in the free (unconjugated) form in human plasma, in agreement with previous studies13. No significant correlations between EGCG plasma levels and changes in FMD were observed for all interventions (Fig. 3b).
EGCG plasma levels did not correlate with changes in FMD. Shown are the sum of free and conjugated EGCG plasma levels two hours after consumption (a). Data are means ± SEM from n = 50 subjects for water, EGCG, and green tea and n = 49 for GTE. *p < 0.05 versus GTE and green tea, #p < 0.05 versus EGCG and green tea (Wilcoxon test). Correlations of EGCG plasma levels with changes in FMD after green tea, GTE, and EGCG (b). There were no significant correlations between EGCG plasma levels and FMD for all treatments (Spearman’s). Data are from n = 50 subjects for EGCG and green tea and n = 49 for GTE.
No correlations of catechin plasma levels with FMD
Beneficial effects on endothelial function have also been described for epicatechin (EC)14. EC plasma levels were higher after GTE and lower after green tea (Fig. 4a), and no correlation with changes in FMD was observed after green tea consumption (Fig. 4b). Similar results were obtained for epigallocatechin (EGC) (Fig. 4a,b). Mean plasma concentrations for epicatechin gallate (ECG) were very low, and a negative correlation with changes in FMD after green tea was observed (Fig. 4a,b). The sum of all catechins (total catechin plasma levels) showed a non-significant negative correlation with changes in FMD after tea (r = −0.239, p = 0.095, Spearman’s). An overview of catechin plasma levels after the interventions before and after deconjugation is given in Table 4.
No correlations of catechin plasma levels with changes in FMD. Plasma levels of EC (epicatechin), EGC (epigallocatechin), and ECG (epicatechin gallate) after hydrolysis (sum of free and conjugated catechins) two hours after consumption (a). The scale of the y axes are equal to Fig. 3 to facilitate comparisons with EGCG levels. Data are means ± SEM from n = 50 subjects for water, EGCG, and green tea and n = 49 for GTE. Correlations of catechin plasma levels with FMD changes after tea consumption (b). Non-significant negative correlations were observed between catechin plasma levels and FMD changes (Spearman’s). Data are from n = 50 subjects.
Caffeine plasma levels increased after green tea consumption
Since catechin plasma levels were not sufficient to account for differential effects on FMD between interventions, we measured blood levels of additional tea compounds. Plasma levels of caffeine markedly increased two hours after consumption of green tea, whereas a slight decrease was observed in the other interventions (Fig. 5a). Plasma levels of theobromine slightly decreased after all interventions (Fig. 5a). Caffeine and theobromine were also present at baseline (before intervention) in all treatments. We observed a slight, but significant positive correlation between plasma concentrations of caffeine and changes in FMD after green tea consumption, whereas plasma levels of theobromine did not correlate with FMD (Fig. 5b).
Caffeine but not theobromine plasma levels correlate with FMD changes after green tea consumption. Plasma levels of caffeine and theobromine before and two hours after interventions (a). For comparison, the scale is identical for both compounds. Data are means ± SEM from n = 50 subjects for water, EGCG, and green tea and n = 49 for GTE. Significant positive correlations between plasma levels and changes in FMD after green tea consumption were obtained for caffeine, but not for theobromine (Spearman’s) (b). Data are from n = 50 subjects.
Discussion
This study shows for the first time that EGCG is most likely not involved in tea-induced improvement of endothelial function in humans. Although EGCG plasma levels were highest after intake of EGCG and lowest after consumption of green tea, only green tea improved FMD. It has been consistently demonstrated that green tea improves endothelial function in humans10, 15, 16. The results of our study confirmed these findings. Although not statistically significant, a minor effect on FMD was also observed for GTE in our study.
Green tea and EGCG stimulated NO production in endothelial cells by activating endothelial nitric oxide synthase (eNOS) and induced vasodilation in aortic rings10, 17. However, the contribution of EGCG to the effects of green tea in vivo remains elusive. In our study, we observed no effect of 200 mg isolated EGCG on endothelial function in humans. Widlansky et al. found an acute (but not long-term) improvement in FMD with 300 mg EGCG in patients with coronary artery disease18. However, apart from different EGCG doses used, in contrast to our study Widlansky et al. observed no differences in the absolute FMD between EGCG and placebo after supplementation. A numerical lower baseline FMD before EGCG as compared to before placebo could have contributed to their results. In vitro, significant effects of EGCG on NO production and vasodilation were obtained only at concentrations of 1 µM or higher6,7,8, concentrations that were not achieved in plasma in our study and in Widlansky et al.18. Surprisingly, mean plasma levels of EGCG in our study were inversely associated with changes in FMD between interventions (Fig. 6). All of the above results make a substantial contribution of EGCG to the vasodilating effects of green tea in vivo very unlikely.
Stimulation of endothelial NO production and vasodilation in isolated vessels have also been described for EC8, 19. In a small study by Schroeter et al., the administration of 1–2 mg/kg body weight of EC resulted in improved FMD in three subjects14. However, EC supplementation for 4 weeks (100 mg/d) had no significant effects on FMD in 35 subjects20. Consumption of 442 ml green tea in our study resulted in the intake of 23 mg EC, which is much less than the amount of EC ingested in the study by Schroeter et al. EC plasma levels in our study were very low and did not parallel changes in FMD between interventions. In line with previous reports13, EC was fully conjugated and not present in the free form in plasma. Therefore, the involvement of EC in the effects of green tea on FMD is rather unlikely. Mean plasma levels of the other catechins ECG and EGC were also very low and showed no correlations with FMD, indicating that both catechins are in all likelihood not involved in the improvement of FMD by green tea. Also, the sum of all catechins showed no correlation with FMD.
What other compounds could potentially contribute to the effects of green tea? Caffeine is present in high amounts in green and black tea. Consequently, plasma levels of caffeine substantially increased after consumption of green tea in our study. However, the published data on the role of caffeine on flow-mediated dilation are conflicting. A cup of decaffeinated coffee had no effect on FMD in healthy adults, whereas caffeinated coffee (with 80 mg of caffeine) even declined FMD21. In a similar study, decaffeinated coffee resulted in a slight increase in FMD, whereas caffeinated coffee significantly decreased FMD in healthy subjects22. Consumption of green tea significantly improved FMD, however, the corresponding amount of caffeine contained in this tea (125 mg) did only slightly increase FMD16. Likewise, a significant improvement of FMD was observed two hours after consumption of black tea, but an equivalent dose of 200 mg caffeine had no effect11. But there is also evidence that caffeine exerts beneficial effects on endothelial function. A single dose of caffeine acutely improved FMD in patients with stable CAD, and increased forearm blood flow23, 24. Chronic coffee consumption was associated with higher FMD25, and moderate coffee consumption resulted in a decreased risk of cardiovascular diseases26. An improved FMD was also observed after administration of a green tea extract containing caffeine27. In addition, an inverse association with risk of CVD and stroke was shown for higher coffee and green tea consumption28. Tea (mainly black) as well as moderate coffee consumption have been shown to decrease the risk for coronary heart disease2. Taken together, these studies point to a cardiovascular risk reduction for both tea and coffee.
In black tea, the amount of EGCG (and of other catechins) is much lower compared to green tea, depending on the degree of fermentation. Theaflavins and thearubigins, the major polyphenolic compounds in black tea, are too large for substantial absorption into the circulation29. Green and black tea are, however, equally effective in the improvement of FMD10, 12, and both teas contain a similar amount of caffeine. However, other compounds including plasma metabolites of tea polyphenols, such as methylated compounds/phase I-breakdown or gut microbial metabolites (in addition to glucuronidated and sulphated substances), could also be involved. Although EGCG can be methylated30, very low31, or no methylated (or otherwise metabolised) EGCG32 was found in human plasma after consumption of a green tea extract or green tea. An inverse association between the degree of urinary flavonoid O-methylation (4OMGA) and FMD after consumption of black tea indicates that methylation of tea flavonoids could rather affect endothelial function33. However, the influence of other (as yet unknown) phase I-breakdown or gut microbial metabolites on tea-induced FMD cannot be fully excluded.
Some study limitations should be acknowledged. The observed correlation between caffeine plasma levels and FMD is only weak and does not prove a role of caffeine in tea-induced endothelial function. A study with caffeine and hot water, EGCG and green tea or with caffeinated and decaffeinated green tea would be required to verify the role of caffeine in tea-induced FMD. Although EGCG did not mediate the effects of green tea on FMD in our study with healthy subjects, a contribution of EGCG to tea-mediated improvements of endothelial dysfunction cannot be fully excluded. Even though the amount of EGCG used in our study (200 mg) corresponds to a nutritionally relevant dose that can be consumed by drinking tea, it does not necessarily reflect habitual tea drinking of several cups per day. Also, it should be noted that the contributions of single tea compounds to tea-induced FMD cannot be generalised for other cardiovascular, neurological, or anti-tumor effects of green tea.
In summary, our data indicate that EGCG is not involved in changes of FMD by green tea. The lack of an impact of EGCG on tea-induced improvements in endothelial function clearly points out that caution is indicated when translating promising in vitro experiments using a single isolated compound from a food matrix into the human situation. In light of many ongoing human studies with EGCG it will be of considerable interest to study the contribution of this promising in vitro candidate to other putative beneficial health effects of green tea.
Methods
Study population
Healthy men between 20 and 50 years were recruited by press advertisements. Subjects with chronic diseases and known cardiovascular risk factors such as high blood cholesterol (≥240 mg/dl), diabetes, arterial hypertension, and body mass index >27 kg/m2 were excluded. Plasma lipid profiles and blood pressure were required to be within the normal range for study inclusion. Regular tea consumption, current smoking, chronic drug use, and alcohol abuse were additional exclusion criteria. The study was approved by the Charité University Hospital Ethics Committee and participants provided their written informed consent. The study protocol was in accordance to local university guidelines and with the principles outlined in the Declaration of Helsinki.
Study design
This pilot study was a prospective, exploratory, randomised, crossover study. A single dose of 200 mg of EGCG was orally ingested in different application forms. After fasting overnight, each subject consumed 200 mg of EGCG on different days, either as green tea beverage, green tea extract (GTE), or purified EGCG. The same amount of hot water was applied with each of the EGCG forms ingested. The corresponding amount of hot water served as control. Randomization was performed using computer-generated random numbers for the order of treatments. The interventions were at least 3 days apart. Before and two hours after ingestion, flow-mediated and nitro-mediated dilation of the brachial artery was assessed by vascular ultrasound. Blood was taken before and two hours after intervention, and plasma levels of tea compounds were measured. The study was performed at the Department of Cardiology and Angiology (Campus Mitte) at Charité – University Medicine Berlin, Germany. The study has been registered under ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01662232, registration No. NCT01662232; date of registration: August 7, 2012.
Interventions
Darjeeling green tea was obtained from King’s Teagarden, Berlin, Germany. For determination of the EGCG content of the beverage before the start of the study, 6.75 grams of ground tea solids were brewed with 550 ml of boiling water for 3 min. The infusion had an EGCG content of 452.5 ± 8.7 mg/l (mean ± SD, n = 6). For administration of 200 mg of EGCG, the participants consumed 442 ml of the beverage. The GTE was supplied by Nutri-Fit GmbH & Co. KG. The EGCG content of the extract was 492.3 ± 1.4 mg/g (n = 3). For ingestion of 200 mg of EGCG, the subjects took a capsule containing 406.3 mg of GTE. The purity of the EGCG (kindly provided by Nutri-Fit GmbH & Co. KG) as determined by HPLC prior to the study was 93.6 ± 1.3% (n = 3). To correct for EGCG content, the subjects took a capsule containing 214 mg of EGCG for ingestion of 200 mg EGCG. An equal amount of hot water (442 ml, according to the volume of the beverage) was applied together with all capsules. 442 ml of hot water served as control.
Assessment of flow-mediated (FMD) and nitro-mediated dilation (NMD)
Endothelium-dependent FMD was assessed by measuring the change in brachial artery diameter during reactive hyperemia after cuff occlusion of the forearm, according to established guidelines34. Endothelial function was measured by high-resolution vascular ultrasound using Vivid 7 (General Electric Medical Systems) with a 12-MHz linear array transducer. We performed all measurements in the morning at the same time of the day. Data were analysed using the automatic edge detection software FMD Studio Cardiovascular Suite (CVS 2.0, QUIPU, Pisa, Italy), which analyses the data in real time35. Baseline diameter was assessed for 1 min, followed by 5 min of cuff occlusion and determination of diameter changes after reactive hyperemia for 4 min after cuff release. FMD was defined as the maximum percentage change in diameter compared with baseline measurements. Percent changes in FMD of the brachial artery were determined before and two hours after each intervention. Endothelium-independent vasodilation (NMD) was measured 10 min after FMD by sublingual application of nitroglycerin spray (0.4 mg) before and after each intervention. Baseline diameter was assessed for 1 min, and the increase in diameter of the brachial artery after application of nitroglycerin was measured for 9 min.
Blood parameters
For cardiovascular risk assessment, the following parameters were measured by conventional laboratory methods: total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides (TG), C-reactive protein (CrP), lipoprotein(a), HbA1c, and homocysteine. Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and CrP were determined before and two hours after each intervention.
Composition of the interventions and determination of plasma levels of tea compounds
Concentrations of the individual tea substances in the interventions were measured on a Waters Acquity UPLC (Waters, Milford, MA, USA). The equipment consists of a binary pump (BSM), an autosampler (SM) cooled at 10 °C, a column oven (CM) set at 40 °C, a diode array detector (PDA) scanning from 190 to 500 nm, and an Acquity TQD triple-quadrupole mass spectrometer with an electrospray interface. A Waters BEH phenyl column (50 mm x 2.1 mm, 1.7 µm) with a VanGuard precolumn was employed at a flow rate of 0.6 ml/min. The eluents acetonitrile/0.1% formic acid (A) and water/0.1% formic acid (B) were run with the following gradient: 0 min: 6% A; 1.5 min 13% A; 2.3–3.3 min: 100% A; 4.3–5.3 min: 6% A. Peak identity was confirmed by MS/MS. For quantification, authentic reference compounds were used (detection wavelength 278 nm) for external calibration. Liquid samples were adequately diluted, solid samples were dissolved with methanol/water (80/20), and filtered through 0.2 µm Chromafil RC-20/15 MS filters (Macherey-Nagel, Düren, Germany).
Plasma levels of green tea catechins were measured as previously described13. To determine the levels of free (unconjugated) catechins, all samples were analysed in parallel, with omission of enzymatic hydrolysis. Plasma levels of caffeine and theobromine were determined as described36, with slight modifications: 40 µl of 20% aqueous perchloric acid were added to 100 µl of thawed plasma samples and centrifuged. The supernatant was injected into the UHPLC system (as described above). For external calibration, authentic reference compounds were used (detection wavelength 278 nm).
Statistical analysis
For this exploratory pilot study, comparisons of parameters between the four interventions water, EGCG, GTE, and green tea were performed by repeated measures ANOVA. After overall statistical differences between treatments (p < 0.05), this was followed by post hoc Bonferroni correction to adjust for multiple testing. Pairwise comparisons of parameters before and after interventions within treatments were performed using paired t-tests or Wilcoxon tests (for non-Gaussian distributions). Correlations between plasma levels of individual tea substances and FMD were calculated by using Spearman’s rho coefficient. All statistical tests were two-sided, with the level of significance accepted at p < 0.05. Statistical analysis was performed using SPSS, release 22.0 (SPSS, Inc., Chicago, IL, USA). Values are given as means±SD.
References
Kuriyama, S. et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296, 1255–1265, doi:10.1001/jama.296.10.1255 (2006).
de Koning Gans, J. M. et al. Tea and coffee consumption and cardiovascular morbidity and mortality. Arterioscler. Thromb. Vasc. Biol. 30, 1665–1671, doi:10.1161/ATVBAHA.109.201939 (2010).
Lorenz, M. Cellular targets for the beneficial actions of tea polyphenols. Am. J. Clin. Nutr. 98(6 Suppl), 1642S–1650S, doi:10.3945/ajcn.113.058230 (2013).
Chyu, K. Y. et al. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation 109, 2448–2453, doi:10.1161/01.CIR.0000128034.70732.C2 (2004).
Stangl, V., Dreger, H., Stangl, K. & Lorenz, M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 73, 348–358, doi:10.1016/j.cardiores.2006.08.022 (2007).
Lorenz, M. et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 279, 6190–6195, doi:10.1074/jbc.M309114200 (2004).
Kim, J. A. et al. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J. Biol. Chem. 282, 13736–13745, doi:10.1074/jbc.M609725200 (2007).
Aggio, A. et al. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 52, 263–272, doi:10.1007/s00394-012-0320-x (2013).
Ras, R. T., Streppel, M. T., Draijer, R. & Zock, P. L. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int. J. Cardiol. 168, 344–351, doi:10.1016/j.ijcard.2012.09.047 (2013).
Jochmann, N. et al. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br. J. Nutr. 99, 863–868, doi:10.1017/S0007114507838992 (2008).
Duffy, S. J. et al. Short- and long term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 104, 151–156, doi:10.1161/01.CIR.104.2.151 (2001).
Ras, R. T., Zock, P. L. & Draijer, R. Tea consumption enhances endothelial-dependent vasodilation; a Meta-analysis. PLoS ONE 6(3), e16974, doi:10.1371/journal.pone.0016974 (2011).
Zimmermann, B. F. et al. A shortcut from plasma to chromatographic analysis: straight-forward and fast sample preparation for analysis of green tea catechins in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 823–826, doi:10.1016/j.jchromb.2009.01.013 (2009).
Schroeter, H. et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 103, 1024–1029, doi:10.1073/pnas.0510168103 (2006).
Nagaya, N. et al. Green tea reverses endothelial dysfunction in healthy smokers. Heart 90, 1485–1486, doi:10.1136/hrt.2003.026740 (2004).
Alexopoulos, N. et al. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur. J. Cardiovasc. Prev. Rehabil. 15, 300–305, doi:10.1097/HJR.0b013e3282f4832f (2008).
Lorenz, M. et al. Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic Res. Cardiol. 104, 100–110, doi:10.1007/s00395-008-0759-3 (2009).
Widlansky, M. E. et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 26, 95–102, doi:10.1080/07315724.2007.10719590 (2007).
Ramirez-Sanchez, I., Maya, L., Ceballos, G. & Villarreal, F. (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 55, 1398–1405, doi:10.1161/HYPERTENSIONAHA.109.147892 (2010).
Dower, J. I. et al. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 101, 914–921, doi:10.3945/ajcn.114.098590 (2015).
Papamichael, C. M. et al. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin. Sci. (Lond). 109, 55–60, doi:10.1042/CS20040358 (2005).
Buscemi, S. et al. Acute effects of coffee on endothelial function in healthy subjects. Eur. J. Clin. Nutr. 64, 483–489, doi:10.1038/ejcn.2010.9 (2010).
Shechter, M. et al. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am. J. Cardiol. 107, 1255–1261, doi:10.1016/j.amjcard.2010.12.035 (2011).
Umemura, T. et al. Effects of acute administration of caffeine on vascular function. Am. J. Cardiol. 98, 1538–1541, doi:10.1016/j.amjcard.2006.06.058 (2006).
Siasos, G. et al. Consumption of a boiled Greek type of coffee is associated with improved endothelial function: the Ikaria study. Vasc. Med. 18, 55–62, doi:10.1177/1358863X13480258 (2013).
Ding, M., Bhupathiraju, S. N., Satija, A., van Dam, R. M. & Hu, F. B. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129, 643–659, doi:10.1161/CIRCULATIONAHA.113.005925 (2014).
Tinahones, F. J. et al. Green tea reduces LDL oxidability and improves vascular function. J. Am. Coll. Nutr. 27, 209–213, doi:10.1080/07315724.2008.10719692 (2008).
Kokubo, Y. et al. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan public health center-based study cohort. Stroke 44, 1369–1374, doi:10.1161/STROKEAHA.111.677500 (2013).
Clifford, M. N., van der Hooft, J. J. & Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 98(6 Suppl), 1619S–1630S, doi:10.3945/ajcn.113.058958 (2013).
Lu, H., Meng, X. & Yang, C. S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos 31, 572–579, doi:10.1124/dmd.31.5.572 (2003).
Miller, R. J. et al. A preliminary investigation of the impact of catechol-O-methyltransferase genotype on the absorption and metabolism of green tea catechins. Eur. J. Nutr. 51, 47–55, doi:10.1007/s00394-011-0189-0 (2012).
Stalmach, A., Troufflard, S., Serafini, M. & Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 53(Suppl 1), S44–S53, doi:10.1002/mnfr.v53.5s (2009).
Hodgson, J. M., Puddey, I. B., Burke, V. & Croft, K. D. Is reversal of endothelial dysfunction by tea related to flavonoid metabolism? Br. J. Nutr. 95, 14–17, doi:10.1079/BJN20051621 (2006).
Thijssen, D. H. et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 300, H2–H12, doi:10.1152/ajpheart.00471.2010 (2011).
Faita, F. et al. Comparison of two automatic methods for the assessment of brachial artery flow-mediated dilation. J. Hypertens. 29, 85–90, doi:10.1097/HJH.0b013e32833fc938 (2011).
Schreiber-Deturmeny, E. & Bruguerolle, B. Simultaneous high-performance liquid chromatographic determination of caffeine and theophylline for routine drug monitoring in human plasma. J. Chromatogr. B Biomed. Appl. 677, 305–312, doi:10.1016/0378-4347(95)00383-5 (1996).
Acknowledgements
The study was supported by the Friede Springer Herz Stiftung. The authors would like to thank Angelika Westphal, Urte Lemma, Thomas Düsterhöft, and Anja Stratmann for excellent technical assistance. We thank all the volunteers for participating in the study.
Author information
Authors and Affiliations
Contributions
M.L. and V.S. conceived and designed the study and wrote the manuscript. F.R., C.H., S.G., E.M., D.P. and B.F.Z. performed the experiments and acquired the data. K.-D.W. did the statistical analysis. G.B. and K.S. made critical revision of the manuscript for key intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenz, M., Rauhut, F., Hofer, C. et al. Tea-induced improvement of endothelial function in humans: No role for epigallocatechin gallate (EGCG). Sci Rep 7, 2279 (2017). https://doi.org/10.1038/s41598-017-02384-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02384-x
This article is cited by
-
Green tea consumption and incidence of cardiovascular disease in type 2 diabetic patients with overweight/obesity: a community-based cohort study
Archives of Public Health (2024)
-
Cardioprotective effect of epigallocatechin gallate in myocardial ischemia/reperfusion injury and myocardial infarction: a meta-analysis in preclinical animal studies
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.