Abstract
Autophagy is a lysosome-dependent degradation pathway essential for cellular homeostasis, which decreases with age. However, it is unclear how aging induces autophagy decline. Here we show the role of protein S-palmitoylation in autophagy. We identify the palmitoyl acyltransferase DHHC5 as a regulator of autophagy by mediating the palmitoylation of beclin 1, which in turn promotes the formation of ATG14L-containing class III phosphatidylinositol-3-kinase complex I and its lipid kinase activity by promoting the hydrophobic interactions between beclin 1 and adapter proteins ATG14L and VPS15. In aging brains of human and nonhuman primate, the levels of DHHC5 exhibit a marked decrease in expression. We show that DHHC5 deficiency in neurons leads to reduced cellular protein homeostasis in two established murine models of Alzheimer’s disease, which exaggerates neurodegeneration in an autophagy-dependent manner. These findings identify reduction of DHHC5-mediated beclin 1 S-palmitoylation as an underlying mechanism by which aging induces autophagy decline.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The atomic structural model file for PI3KC3-C1 was from Dr. Junjie Liu and Dr. Hongwei Wang (Tsinghua University) and has been deposited in figshare (https://doi.org/10.6084/m9.figshare.24356617). The atomic structural model file for PI3KC3-C2 was from Protein Data Bank (PDB; https://www.rcsb.org) with PDB ID 7BL1. The PDB models of molecular docking experiments have been deposited in figshare (https://doi.org/10.6084/m9.figshare.24319816). The initial slope of the fEPSPs and FV amplitude was measured using customized MATLAB code (https://github.com/KaiWen-Helab/Matlab-code.git). The uncropped gel blots have been provided as source data. Raw data for mass spectrometry analysis are provided in Supplementary Tables 1–4. All data generated and/or analyzed in the current study are included in this published article. Source data are provided with this paper.
References
Leidal, A. M., Levine, B. & Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20, 1338–1348 (2018).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Kenyon, C. J. The genetics of ageing. Nature 464, 504–512 (2010).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Kihara, A., Kabeya, Y., Ohsumi, Y. & Yoshimori, T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2, 330–335 (2001).
Itakura, E., Kishi, C., Inoue, K. & Mizushima, N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360–5372 (2008).
Matsunaga, K. et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat. Cell Biol. 11, 385–396 (2009).
Zhong, Y. et al. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 11, 468–476 (2009).
Yu, Y. et al. The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav. Brain Res. 334, 155–162 (2017).
Ott, C., Konig, J., Hohn, A., Jung, T. & Grune, T. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 10, 266–273 (2016).
Kaushik, S. et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 13, 258–265 (2012).
Lipinski, M. M. et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 107, 14164–14169 (2010).
Son, J. H., Shim, J. H., Kim, K. H., Ha, J. Y. & Han, J. Y. Neuronal autophagy and neurodegenerative diseases. Exp. Mol. Med. 44, 89–98 (2012).
Pickford, F. et al. The autophagy-related protein Beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Investig. 118, 2190–2199 (2008).
Linder, M. E. & Deschenes, R. J. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 8, 74–84 (2007).
Yang, X., Chatterjee, V., Ma, Y., Zheng, E. & Yuan, S. Y. Protein palmitoylation in leukocyte signaling and function. Front. Cell Dev. Biol. 8, 600368 (2020).
Chen, B., Sun, Y., Niu, J., Jarugumilli, G. K. & Wu, X. Protein lipidation in cell signaling and diseases: function, regulation, and therapeutic opportunities. Cell Chem. Biol. 25, 817–831 (2018).
Greaves, J. & Chamberlain, L. H. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem. Sci. 36, 245–253 (2011).
Buszka, A., Pytys, A., Colvin, D., Wlodarczyk, J. & Wojtowicz, T. S-palmitoylation of synaptic proteins in neuronal plasticity in normal and pathological brains. Cells https://doi.org/10.3390/cells12030387 (2023).
Fukata, Y. & Fukata, M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 11, 161–175 (2010).
Globa, A. K. & Bamji, S. X. Protein palmitoylation in the development and plasticity of neuronal connections. Curr. Opin. Neurobiol. 45, 210–220 (2017).
Kang, R. et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456, 904–909 (2008).
Andrew, R. J. et al. Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 114, E9665–E9674 (2017).
Bhattacharyya, R., Barren, C. & Kovacs, D. M. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J. Neurosci. 33, 11169–11183 (2013).
Singaraja, R. R. et al. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet. 20, 3899–3909 (2011).
Luebben, A. V. et al. Cln5 represents a new type of cysteine-based S-depalmitoylase linked to neurodegeneration. Sci. Adv. 8, eabj8633 (2022).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Rubinsztein, D. C., Codogno, P. & Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 11, 709–730 (2012).
Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N. & Rubinsztein, D. C. Alpha-synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 278, 25009–25013 (2003).
Ravikumar, B., Duden, R. & Rubinsztein, D. C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11, 1107–1117 (2002).
Berger, Z. et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum. Mol. Genet. 15, 433–442 (2006).
Tian, Y., Bustos, V., Flajolet, M. & Greengard, P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 25, 1934–1942 (2011).
Luhr, M., Saetre, F. & Engedal, N. The long-lived protein degradation assay: an efficient method for quantitative determination of the autophagic flux of endogenous proteins in adherent cell lines. Bio-Protoc. 8, e2836 (2018).
Cao, Y. & Klionsky, D. J. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 17, 839–849 (2007).
Hill, S. M., Wrobel, L. & Rubinsztein, D. C. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 26, 617–629 (2019).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature 402, 672–676 (1999).
Chen, J. J., Marsden, A. N., Scott, C. A., Akimzhanov, A. M. & Boehning, D. DHHC5 mediates beta-adrenergic signaling in cardiomyocytes by targeting Galpha proteins. Biophys. J. 118, 826–835 (2020).
Lu, Y. et al. Palmitoylation of NOD1 and NOD2 is required for bacterial sensing. Science 366, 460–467 (2019).
Sun, Q. et al. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA 105, 19211–19216 (2008).
Shoji-Kawata, S. et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206 (2013).
Martin, B. R. & Cravatt, B. F. Large-scale profiling of protein palmitoylation in mammalian cells. Nat. Methods 6, 135–138 (2009).
Martin, B. R. Nonradioactive analysis of dynamic protein palmitoylation. Curr. Protoc. Protein Sci. 73, 14 15 11–14 15 19 (2013).
Drisdel, R. C. & Green, W. N. Labeling and quantifying sites of protein palmitoylation. BioTechniques 36, 276–285 (2004).
Roth, A. F. et al. Global analysis of protein palmitoylation in yeast. Cell 125, 1003–1013 (2006).
Webb, Y., Hermida-Matsumoto, L. & Resh, M. D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 275, 261–270 (2000).
Hannoush, R. N. & Sun, J. The chemical toolbox for monitoring protein fatty acylation and prenylation. Nat. Chem. Biol. 6, 498–506 (2010).
Antinone, S. E. et al. Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants. J. Biol. Chem. 288, 21606–21617 (2013).
Lu, J. et al. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nat. Commun. 5, 3920 (2014).
Cao, Y. et al. NRBF2 regulates macroautophagy as a component of Vps34 complex I. Biochem. J. 461, 315–322 (2014).
Sun, Q., Westphal, W., Wong, K. N., Tan, I. & Zhong, Q. Rubicon controls endosome maturation as a Rab7 effector. PNAS 107, 19338–19343 (2010).
Liang, C. et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat. Cell Biol. 10, 776–787 (2008).
Liang, C. et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 8, 688–699 (2006).
Tremel, S. et al. Structural basis for VPS34 kinase activation by Rab1 and Rab5 on membranes. Nat. Commun. 12, 1564 (2021).
Ma, M. et al. Cryo-EM structure and biochemical analysis reveal the basis of the functional difference between human PI3KC3-C1 and -C2. Cell Res. 27, 989–1001 (2017).
Lipinski, M. M. et al. A genome-wide siRNA screen reveals multiple mTORC1 independent signaling pathways regulating autophagy under normal nutritional conditions. Dev. Cell 18, 1041–1052 (2010).
Li, Y. et al. DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory. J. Biol. Chem. 285, 13022–13031 (2010).
Yang, L. et al. Ubiquitylome study identifies increased histone 2A ubiquitylation as an evolutionarily conserved aging biomarker. Nat. Commun. 10, 2191 (2019).
Dragatsis, I. & Zeitlin, S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis 26, 133–135 (2000).
Li, L., Wang, Z. V., Hill, J. A. & Lin, F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol. 25, 305–315 (2014).
Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007).
Katayama, H., Yamamoto, A., Mizushima, N., Yoshimori, T. & Miyawaki, A. GFP-like proteins stably accumulate in lysosomes. Cell Struct. Funct. 33, 1–12 (2008).
Boland, B. et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. 28, 6926–6937 (2008).
Castillo, K. et al. Measurement of autophagy flux in the nervous system in vivo. Cell Death Dis. 4, e917 (2013).
Lee, J. H. et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat. Neurosci. 25, 688–701 (2022).
Iba, M. et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci. 33, 1024–1037 (2013).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Goedert, M., Jakes, R. & Vanmechelen, E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 189, 167–169 (1995).
Jicha, G. A., Bowser, R., Kazam, I. G. & Davies, P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 48, 128–132 (1997).
Oakley, H. et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006).
Kugler, S., Kilic, E. & Bahr, M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 10, 337–347 (2003).
Kuma, A. et al. The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 (2004).
Lamb, C. A., Yoshimori, T. & Tooze, S. A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 (2013).
Ito, D. et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 57, 1–9 (1998).
Cotman, C. W. & Su, J. H. Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol. 6, 493–506 (1996).
Eimer, W. A. & Vassar, R. Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol. Neurodegener. 8, 2 (2013).
Forner, S. et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 8, 270 (2021).
Kim, S. W. et al. Palmitoylation controls trafficking of the intracellular Ca(2+) channel MCOLN3/TRPML3 to regulate autophagy. Autophagy 15, 327–340 (2019).
Ra, E. A. et al. TRIM31 promotes Atg5/Atg7-independent autophagy in intestinal cells. Nat. Commun. 7, 11726 (2016).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Rubinsztein, D. C., Marino, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Glatigny, M. et al. Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29, 435–448 e438 (2019).
Boland, B. et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 17, 660–688 (2018).
Qiu, W. et al. Standardized operational protocol for human brain banking in China. Neurosci. Bull. 35, 270–276 (2019).
He, Z. et al. Amyloid-beta plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 24, 29–38 (2018).
Braakman, I., Lamriben, L., van Zadelhoff, G. & Hebert, D. N. Analysis of disulfide bond formation. Curr. Protoc. Protein Sci. 90, 14.1.1–14.1.21 (2017).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Atomic structural model file for PI3KC3-C1. figshare https://doi.org/10.6084/m9.figshare.24356617 (2023).
PDB models of molecular docking between PI3KC3-C1 and palmitic acid. figshare https://doi.org/10.6084/m9.figshare.24319816 (2023).
MATLAB code for measuring initial slope of the fEPSPs and FV amplitude. GitHub https://github.com/KaiWen-Helab/Matlab-code.git (2023).
Acknowledgements
We thank T. Zhao (Fudan University) for Dhhc5f/f mice, J. Liu and H. Wang (Tsinghua University) for providing the atomic structural model files for PI3KC3-C1, W.Wei (Harvard Medical School) for DHHC constructs, H. Jiang (IRCBC) for Alk14-CoA, H. Liu (Chinese Academy of Medical Sciences) for providing modeling software, and the staff members of the Animal Facility at the National Facility for Protein Science in Shanghai for providing technical support. This work was supported in part by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB39030600), the STI2030-Major Projects (2022ZD0213200), the National Natural Science Foundation of China (32070737 and 92049303), the Shanghai Science and Technology Development Funds (20JC1411600, 20QA1411500 and 22JC1410400), the CAS Youth Interdisciplinary Team (JCTD-2022-10), the Shanghai Key Laboratory of Aging Studies (19DZ2260400), and the Shanghai Municipal Science and Technology Major Project (2019SHZDZX02).
Author information
Authors and Affiliations
Contributions
This project was conceived, designed and directed by D.X. R.G. designed and conducted majority of the experiments. J.L. assisted with the structure modeling of PI3KC3 complexes. X.M. and K.H. conducted the LTP experiments and data analysis. W.Z. and Z.H. assisted with tau pffs injection experiments and data analysis. B.S. and M.Z. conducted the mass spectrometry analysis. Y.Z. assisted with the proteomic analysis of DHHC PATs in aged monkey brains. The manuscript was written by D.X. and edited by J.Y., K.H. and J.L.
Corresponding author
Ethics declarations
Competing interests
J.Y. is a consultant of Denali Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Hening Lin, Thomas Wollert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
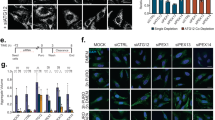
Extended Data Fig. 1 Identification of DHHC5 as a regulator of autophagy.
a, Quantification of GFP-LC3 puncta in DHHC5-WT/KD H4-GFP-LC3 cells that were treated with BafA1 (100 nM) for 8 h. b, Fluorescent micrographs and quantification of mean GFP-LC3 puncta in Dhhc5-WT/KD MEFs-GFP-LC3 cells that were treated with BafA1 (100 nM) for 8 h. DHHC5 blotting is shown on the right. c, Western blots detection and quantification of LC3 II in Dhhc5-WT/KD HT22 cells with BafA1 (100 nM) for indicated time. d-g, A schematic of the Cas9/sgRNA/oligo targeting site in the exon2 of DHHC5 (d). The deleted sequences are presented (e). Sequencing analysis with the arrow indicating the missing sequences (f). Western blot of DHHC5 (g). Similar results were observed in n = 3 independent experiments (g). h, Western blots detection and quantification of LC3 II in DHHC5-WT and DHHC5-KO clonal cell line with BafA1 (100 nM) for 4 h. i, Quantification of GFP-LC3 puncta in DHHC5-WT/KD H4-GFP-LC3 cells that were treated with Torin-1 (200 nM) for 8 h. j, k, Western blots detection and quantification of LC3 II in Dhhc5-WT/KD MEFs (j) or DHHC5-WT/KD H4 cells (k) with the presence of Torin-1 (200 nM) for indicated time (j) or with the presence of Torin-1 (200 nM) alone or together with BafA1 (100 nM) for 8 h (k). l, m, Fluorescent micrographs and quantification of GFP-LC3 puncta in each cell (l) or mean GFP-LC3 puncta per cell (m) in DHHC5-WT/KD H4-GFP-LC3 cells that were starved in EBSS for 1 h. Quantification of LC3 II/Tubulin ratios is relative to WT untreated control for each independent experiment (c, h, j, k). The violin plot represents 1000–2000 cells analysed for one sample per condition. The centre line shows the median, the height represents the interquartile range and the width represents the frequency distribution of the data (a, i, l). Bars represent mean ± s.d. of 9 samples of n = 3 independent experiments (b, m). Bars represent mean ± s.e.m. of n = 3 independent experiments (c, h, j, k). Each dot represents average quantification for ~1000 cells from one sample (b, m). Two-way ANOVA, post hoc Bonferroni’s test (a, b, i, l, m).
Extended Data Fig. 2 DHHC5 interacts with Beclin 1.
a, Schematic diagram of mass spectrometry analysis for identification of binding proteins of DHHC5. b, Chemical structure of palmitic acid and its alkyne-tagged analogue 17-octadecynoic acid (17-ODYA). c, Schematic diagram of mass spectrometry analysis for identification of palmitoylation substrates of DHHC5. d, HEK293T cells were co-transfected with Flag-Beclin 1 and HA-DHHC5 as indicated for 24 h. The interaction between Beclin 1 and DHHC5 was revealed by immunoprecipitation-immunoblotting (IP-IB) assay. e, Western blots showing co-immunoprecipitation of Flag-Beclin 1 with HA-DHHC5 in HEK293T cells that were untreated or treated with 200 nM Torin-1 for 8 h or starved in EBSS for 1 h or serum starved for 8 h. SD: serum deprivation. f-h, Western blots showing co-immunoprecipitation of Beclin 1 with DHHC5 in DHHC5-WT HEK293T cells (f), Dhhc5-WT MEFs (g), and Dhhc5-WT HT22 cells (h). i, HEK293T cells were co-transfected with HA-DHHC5 and Flag-tagged full-length Beclin 1, or truncation mutants of Beclin 1 as indicated for 24 h. The interaction of DHHC5 and Beclin 1 truncation mutants was revealed by IP-IB assay. FL: full length; CCD: coiled coil domain; ECD: evolutionarily conserved domain. j, HEK293T cells were co-transfected with Flag-Beclin 1 and HA-tagged full-length DHHC5 or truncation mutants of DHHC5 as indicated for 24 h. The interaction of Beclin 1 and DHHC5 truncation mutants was revealed by IP-IB assay. k, Localization of Beclin 1, mCherry-DHHC5 and TGN46 under basal and induced autophagy (treated with 200 nM Torin-1 for 8 h) conditions was analysed using confocal imaging in H4 cells. Quantification of the colocalization of Beclin 1 and mcherry-DHHC5 using Pearson’s correlation coefficients was shown on the right as box plot with whiskers from minimum to maximum. The box plot represents 20 cells analysed for one of n = 3 independent samples per condition. Boxes represent quartiles, center lines denote medians, and whiskers extend to most extreme values within 1.5 × interquartile range. Unpaired two-tailed t-tests. Similar results were observed in n = 3 independent experiments (d-j).
Extended Data Fig. 3 Beclin 1 is palmitoylated by DHHC5.
a, HEK293T cells expressing Flag-Beclin 1 were incubated with 17-ODYA or palmitic acid (PA) overnight followed by immunoprecipitation and Cu(I)-assisted cross linking to TAMRA-azide and the immunoprecipitated samples were then resolved by SDS-PAGE and scanned to record the in-gel fluorescence signal. After scanning, the gel was stained with Coomassie Brilliant Blue (CBB) for protein loading. b, Schematic of the immunoprecipitation and acyl-biotin exchange (IP-ABE) assay followed by streptavidin pulldown to purify and detect Beclin 1 palmitoylation. c, HEK293T cells expressing Flag-Beclin 1 were subjected to IP-ABE assay to detect Palm-Beclin 1. d, e, HEK293T cells expressing Flag-Beclin 1 were treated with 200 nM Torin-1 (d) or starved in EBSS (e) for indicated time. The cell lysates were then subjected to IP-ABE assay to detect Palm-Beclin 1. Quantification of Palm-Beclin 1 normalized to total Beclin 1 is relative to untreated control for each independent experiment. f, HEK293T cells expressing Flag-Beclin 1 and indicated DHHCs were subjected to IP-ABE assay to detect Palm-Beclin 1. Quantification of Palm-Beclin 1 normalized to total Beclin 1 is relative to no DHHC control. g, WT and indicated DHHC-KD H4 cells expressing Flag-Beclin 1 were subjected to IP-ABE assay to detect Palm-Beclin 1. The knockdown efficiency of DHHCs was confirmed by quantitative PCR. h, DHHC5-WT/KD HEK293T cells were transfected with Flag-Beclin 1 for 24 h. The cell lysates were then subjected to IP-ABE assay with or without HAM treatment. Palm-Beclin 1 was detected by streptavidin pulldown followed by western blots. i, j, DHHC5-WT and DHHC5-KD (i) or clonal KO (j) H4 cells were subjected to IP-ABE assay with or without HAM treatment. Palm-Beclin 1 was detected by streptavidin pulldown followed by western blots. Similar results were observed in n = 3 independent experiments (c-e, g-j). Bars represent mean ± s.e.m. of n = 3 independent experiments (d-f). Bars represent mean ± s.d. of 3 independent samples (g). One-way ANOVA, post hoc Dunnett’s test (f).
Extended Data Fig. 4 Beclin 1 is S-palmitoylated at evolutionarily conserved cysteine residue.
a, Schematic of the immunoprecipitation and acyl-NMM exchange assay coupled to mass spectrometry to identify palmitoylation sites. b-d, HEK293T cells expressing either WT Beclin 1 or mutants in the absence (b, c) or presence (d) of DHHC5 were subjected to IP-ABE assay to detect Palm-Beclin 1. e, HEK293T cells were transfected with Flag-tagged WT Beclin 1 or Beclin 1-C137S/C159S mutant (2CS) for 24 h. Flag-Beclin 1 was then enriched from cell lysates and free thiols of protein were blocked by NEM. Disulfide bonds were reduced by DTT. Biotin-BMCC was applied to attach at the reduced thiols. Samples were analysed by western blots using streptavidin-HRP. f, HEK293T cells were transfected with either Flag-tagged WT Beclin 1 or mutants as indicated for 48 h. Flag-Beclin 1 was then purified from cell lysates. The eluted proteins (0.2 μg/μl) were then subjected to thermal shift assay. The apparent melting temperature (Tm) is the peak in the derivative melting curve (dF/dT), which is used as an indicator of thermal stability. g, Quantification of mean GFP-LC3 puncta per cell (~1000 cells analysed per sample) in BECN1-WT/KD H4-GFP-LC3 cells. Western blot of Beclin 1 was shown on the right. h, Confirmation of the expression of WT Beclin 1 and different mutants in BECN1-KD H4-GFP-LC3 cells. i, Confirmation of Beclin 1 knockdown in BECN1-KD H4 cells by western blots. j, Western blots detection and quantification of LC3 II in BECN1-KD H4 cells that reconstituted with WT Beclin 1 or C137S mutant with the presence of Torin-1 (TORi, 200 nM) alone or together with BafA1 (100 nM) for 8 h. Quantification of LC3 II/Tubulin ratios is relative to Beclin 1-WT untreated control for each independent experiment. Similar results were observed in n = 3 independent experiments (b-f, h, i). Bars represent mean ± s.d. of 5 independent samples of one representative experiment (g). Bars represent mean ± s.e.m. of n = 3 independent experiments (j). Unpaired two-tailed t-tests (g).
Extended Data Fig. 5 Beclin 1 C137 palmitoylation regulates PI3KC3-C1 assembly.
a, BECN1-KD H4 cells expressing mCherry-tagged WT Beclin 1 or C137S mutant were analysed for the localization of mCherry-Beclin 1 and TGN46. Pearson’s coefficient was calculated from 20 cells that expressing mCherry-Beclin 1 of one representative experiment out of n = 3 independent experiments and was shown on the right as box plot with whiskers from minimum to maximum. b, c, BECN1-KD (b) or DHHC5-WT/KD (c) H4-GFP-LC3 cells expressing mCherry-tagged WT Beclin 1 or C137S mutant were analysed for the localization of mCherry-Beclin 1 and GFP-LC3 without or with Torin-1 (200 nM, 8 h) using Operetta CLS High Content Analysis System. mCherry+GFP+ puncta and GFP+ puncta were identified and quantified for each cell automatically. Ratio of mCherry+GFP+/GFP+ was calculated for the determination of Beclin 1 recruitment to autophagosomes. d, HEK293T cells were co-transfected with HA-Beclin 1 and Flag-tagged WT or mutant Beclin 1 as indicated for 24 h. The interaction of HA-Beclin 1 and Flag-Beclin 1 was detected by IP-IB assay. e, HEK293T cells were co-transfected with expression vectors of HA-DHHC5 and Flag-tagged WT or mutant Beclin 1 as indicated for 24 h. The interaction of HA-DHHC5 and Flag-Beclin 1 was detected by IP-IB assay. f, Quantification of proteins in Flag-IPs in Fig. 4b. g, BECN1-KD H4 cells expressing WT murine Beclin 1 (mBeclin 1) or C135S mutant mBeclin 1 were subjected to IP-IB of mBeclin 1/VPS34 complex. Quantification of indicated proteins in Flag-IPs is shown below. h, Quantification of proteins in ATG14L and UVRAG-IPs in Fig. 4c. Similar results were observed in n = 3 independent experiments (d, e). Quantification of indicated proteins is relative to Beclin 1-WT control for each independent experiment (f-h). Bars represent mean ± s.e.m. of n = 3 independent experiments (f-h). The box plot with whiskers from minimum to maximum represents 100–200 cells analysed for one of n = 3 independent samples per condition (b, c). Boxes represent quartiles, center lines denote medians, and whiskers extend to most extreme values within 1.5 × interquartile range (a-c). Unpaired two-tailed t-tests (a-c).
Extended Data Fig. 6 Beclin 1 C137 palmitoylation promotes its interaction with ATG14L and VPS15 in PI3KC3-C1.
a, A cartoon representation showing the environment of Beclin 1 C137 residue in the cryo-EM structure model for PI3KC3-C2 (PDB ID: 7BL1). C137 residue is far from VPS15 and completely exposed to solvent. b, A cartoon representation showing the binding model of palmitic acid on molecular surface of PI3KC3-C1 (left) and 3D binding mode of PI3KC3-C1 and palmitic acid (right). Palmitic acid is colored in blue, the surrounding residues in the binding pockets are depicted as sticks. c-e, Quantification of proteins in Flag-IPs in Fig. 4e (c), Fig. 4f (d), and Fig. 4g (e). Quantification of indicated proteins is relative to Beclin 1-WT and ATG14L-WT control (c), Beclin 1-WT and VPS15-WT control (d), and DHHC5-WT control (e) for each independent experiment. f, DHHC5-WT and clonal KO H4 cells were subjected to IP-IB of Beclin 1/VPS34 complex. Quantification of indicated proteins in Beclin 1-IPs is relative to DHHC5-WT control for each independent experiment and shown on the right. g, Quantification of proteins in Flag-IPs in Fig. 4h. Quantification of indicated proteins is relative to DHHC5-WT and Beclin 1-WT control for each independent experiment. h, Confirmation of Beclin 1 knockdown in BECN1-KD H4-DsRed-FYVE cells by western blots. i, Fluorescent micrographs and quantification of mean DsRed-FYVE puncta per cell in BECN1-WT and BECN1-KD H4-DsRed-FYVE cells. j, Confirmation of the expression of WT Beclin 1 and different mutants in BECN1-KD H4-DsRed-FYVE cells by western blots. k, Quantification of mean DsRed-FYVE puncta per cell in BECN1-KD H4-DsRed-FYVE cells that were reconstituted with either WT Beclin 1 or indicated mutant. Similar results were observed in n = 3 independent experiments (h, j). Bars represent mean ± s.e.m. of n = 3 independent experiments (c-g). Bars represent mean ± s.d. of 8 (i) and 7 (k) samples of n = 3 independent experiments. Each dot represents average quantification for ~1000 cells from one sample (i, k). Unpaired two-tailed t-tests (i). One-way ANOVA, post hoc Dunnett’s test (k).
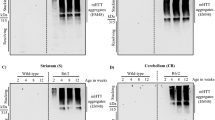
Extended Data Fig. 7 DHHC5 is highly expressed by neurons and aging induces the reduction of DHHC5.
a, Tissue distribution of DHHC5 in mice is shown. Immunoblotting of whole tissue extracts (25 μg of protein/lane) was performed. Ponceau S staining was used as a loading control. b, Quantitative RT-PCR analysis of the relative mRNA levels of DHHC5 in frontal cortex of monkey (left, n = 4 young and n = 5 older monkeys) and human (right, n = 4 young and n = 6 older individuals). Gene expression was normalized to ACTB mRNA levels. c, Immunoblotting analysis of hippocampus from mice (n = 6 young and n = 6 older mice) and the quantification of DHHC5 and Beclin 1 levels normalized to Tubulin were shown on the right. d, Representative images showing co-localization of DHHC5 and the neuronal marker NeuN in the hippocampus of 4-month-old WT mice. e, Representative images showing DHHC5 immunostaining in cortex and hippocampus of 4-month-old Dhhc5f/f and Dhhc5f/f;Camk2a-Cre mice. f, Tissue lysates of cortex from mice were incubated with anti-Beclin 1 antibody and then the immunoprecipitate was subjected to ABE assay followed by streptavidin pulldown. Palm-Beclin 1 was detected by western blots using anti-Beclin 1 antibody. n = 4 mice for each genotype were analysed. g, Confocal image (left) and quantification (right) of RFP-EGFP-LC3 in cortical neurons of mice with indicated genotype. Quantification of LC3-positive dots presented in individual neurons were expressed as a percentage of cell with LC3 dots (minimum three dots per cell) (right, top). RFP puncta per cell were measured to calculate LC3 flux (right, bottom). Similar results were observed in n = 3 independent experiments (a, d, e). The box plot shows whiskers from minimum to maximum, each dot represents one individual. Boxes represent quartiles, center lines denote medians, and whiskers extend to most extreme values within 1.5 × interquartile range (b, c). Bars represent mean ± s.e.m., each dot represents average quantification from one of n = 6 mice (g). Unpaired two-tailed t-tests (b, c, g).
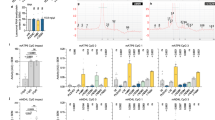
Extended Data Fig. 8 DHHC5 regulates cellular protein homeostasis in vivo.
a, Fluorescent microscopy images of hippocampus two months after local stereotactic injections with AAV-shRNA-Dhhc5 and AAV-shRNA-NC. The construct expresses mCherry, which allows for verification of the site and efficacy of infection. Similar results were observed in n = 3 independent experiments. b, Effects of DHHC5 on the pathological tau tangles-like aggregates in contralateral hippocampus of PS19 mice intrahippocampally injected with tau pffs. Mice were sacrificed and the hippocampi were isolated for immunohistochemistry (IHC) for phospho-Tau (AT8). A focus was made on the CA1 and CA3 regions of the hippocampus. n = 5 mice per condition. Quantification of AT8-positive cell body was presented as mean ± s.e.m., each dot represents average quantification from one individual mouse. Unpaired two-tailed t-tests. c, Fluorescent microscopy images of hippocampus two months after local stereotactic injections with AAV-hSyn-3×Flag (AAV-Ctrl, driven by human synapsin 1 gene promoter) and AAV-hSyn-mDHHC5-3×Flag (AAV-DHHC5). The construct expresses mCherry, which allows for verification of the site and efficacy of infection. Similar results were observed in n = 3 independent experiments. d, Confirmation of DHHC5 expression by western blots in isolated hippocampal neurons of 5×FAD;Dhhc5f/f;Camk2a-Cre mice two months after local stereotactic injections with AAV-Ctrl and AAV-DHHC5. Similar results were observed in n = 3 independent experiments. e, Representative images for Aβ (green) deposition and quantification of Aβ plaque number in the hippocampus of 4-month-old 5×FAD;Dhhc5f/f;Camk2a-Cre mice 2 months after stereotactic injections with either AAV-DHHC5 or their respective AAV-Ctrl. n = 5 mice per condition. Bars represent mean ± s.e.m., each dot represents average quantification from one individual mouse. Unpaired two-tailed t-tests. f, IHC for Aβ in hippocampus from 8-month-old Dhhc5f/f and Dhhc5f/f;Camk2a-Cre mice. No positive signal was detected. Similar results were observed in n = 3 independent experiments.
Extended Data Fig. 9 DHHC5 regulates Aβ deposition in 5×FAD mice through autophagy.
a, b, Confocal image (a) and quantification (b) of RFP-EGFP-LC3 in hippocampal neurons of tfLC3;Dhhc5f/f;Camk2a-Cre mice three days after intraperitoneally treated with 5.3 μmol/kg Tat-scramble or Tat-Beclin 1 peptide. A focus was made on the CA1, CA2 and CA3 regions of the hippocampus. Quantification of LC3-positive dots presented in individual neurons were expressed as a percentage of cell with LC3 dots (minimum three dots per cell) (b, left). RFP puncta per cell were measured to calculate LC3 flux (b, right). Bars represent mean ± s.e.m., each dot represents average quantification from one individual mouse (n = 6 mice for each group). Unpaired two-tailed t-tests. c, Fluorescent microscopy images of hippocampus two months after local stereotactic injections with AAV-hSyn-shmiR-Atg5 and AAV-hSyn-shmiR-NC. The construct expresses EGFP, which allows for verification of the site and efficacy of infection. Similar results were observed in n = 3 independent experiments. d, Confirmation of ATG5 expression by western blots in isolated hippocampal neurons of Dhhc5f/f mice two months after local stereotactic injections with AAV-hSyn-shmiR-Atg5 and AAV-hSyn-shmiR-NC. Similar results were observed in n = 3 independent experiments. e, f, Confocal image (e) and quantification (f) of RFP-EGFP-LC3 in neurons of CA1, CA2 and CA3 regions of mice with indicated genotypes two months after local stereotactic injections with AAV-hSyn-shmiR-Atg5 or AAV-hSyn-shmiR-NC. Quantification of LC3-positive dots presented in individual neurons were expressed as a percentage of cell with LC3 dots (minimum three dots per cell) (f, left). RFP puncta per cell were measured to calculate LC3 flux (f, right). Bars represent mean ± s.e.m., each dot represents average quantification from one individual mouse (n = 6 mice for each group). Two-way ANOVA, post hoc Bonferroni’s test.
Extended Data Fig. 10 DHHC5 deficiency exacerbates neurodegeneration in murine Alzheimer’s disease.
a, Representative images showing microglia activation and expansion (green, IBA1 positive) in the hippocampus of 4-month-old 5×FAD;Dhhc5f/f;Camk2a-Cre mice two months after local stereotactic injections with AAV-Ctrl or AAV-DHHC5. n = 5 mice per group. Quantification of activated microglia was shown on the right. Data shown are the MFI of IBA1 staining for each group relative to AAV-Ctrl. b, Representative images showing microglia activation and expansion (green, IBA1 positive) in the hippocampus of 4-month-old 5×FAD;Dhhc5f/f;Camk2a-Cre mice that were intraperitoneally treated with 5.3 μmol/kg Tat-scramble or Tat-Beclin 1 peptide every three days for 2 months. n = 5 mice per treatment. Quantification of activated microglia was shown on the right. Data shown are the MFI of IBA1 staining for each group relative to Tat-scramble controls. c, Representative images showing microglia activation and expansion (green, IBA1 positive) in the hippocampus of 4-month-old 5×FAD;Dhhc5f/f;Camk2a-Cre mice two months after local stereotactic injections with AAV-hSyn-shmiR-Atg5 or AAV-hSyn-shmiR-NC. n = 5 mice per group. Quantification of activated microglia was shown on the right. Data shown are the MFI of IBA1 staining for each group relative to AAV-hSyn-shmiR-NC controls. d, Representative images showing neurons and quantification of neuronal content (#) in the hippocampus of 8-month-old Dhhc5f/f and Dhhc5f/f;Camk2a-Cre mice. n = 5 mice per genotype. e, Representative images identifying neuronal apoptosis (red, CC3 positive) within the CA1-region of the hippocampus of the indicated 6-month-old 5×FAD genotypes. n = 5 mice per genotype. f, Analysis of hippocampal synaptic transmission in 4-month-old 5×FAD DHHC5-deficient mice and their littermate controls. n = 3 mice per genotype, with a minimum of 6 slices per mouse. g, Y-maze test measuring total distance in 5×FAD;Dhhc5f/f (n = 7) and 5×FAD;Dhhc5f/f;Camk2a-Cre (n = 8) mice at 4 months of age. h, Analysis of novel object recognition measuring total exploration time in 4-month-old 5×FAD;Dhhc5f/f (n = 8) and 5×FAD;Dhhc5f/f;Camk2a-Cre (n = 8) mice. Bars represent mean ± s.e.m. (a-d, f-h). Each dot represents one individual mouse (a-d, g, h). Unpaired two-tailed t-tests (a-c, f-h).
Supplementary information
Supplementary Table
Supplementary Table 1. Proteins pull-down by anti-DHHC5 in DHHC5-WT versus DHHC5-KD H4 cells. Related to Fig.2a. An Excel file of the proteomics data showing the quantification of the abundance of each protein interacted with DHHC5 in DHHC5-WT versus DHHC5-KD H4 cells. Unpaired two-tailed t-tests. Supplementary Table 2. Proteomics analysis to identify the targets of DHHC5 by click chemistry-based enrichment of palmitoylated proteins coupled to mass spectrometry in DHHC5-KD versus DHHC5-WT H4 cells. Related to Fig. 2b. The quantitative mass spectroscopy analysis showing the quantification of the abundance of each protein enriched by click chemistry-based enrichment of palmitoylated proteins coupled to mass spectrometry in DHHC5-WT versus DHHC5-KD H4 cells. Unpaired two-tailed t-tests. Supplementary Table 3. Proteomics analysis to identify the palmitoylation sites of beclin 1. Related to Fig. 3a. An Excel file of the proteomics data showing the palmitoylation sites of beclin 1 from ABE-labeled murine beclin 1. Supplementary Table 4. Proteomics analysis of beclin 1–VPS34 complex. Related to Fig. 4a. An Excel file of the proteomics data showing the quantification of the abundance of each protein interacted with WT beclin 1 or its C137S mutant. Unpaired two-tailed t-tests.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western Blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western Blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western Blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western Blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western Blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western Blots and/or gels.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, R., Liu, J., Min, X. et al. Reduction of DHHC5-mediated beclin 1 S-palmitoylation underlies autophagy decline in aging. Nat Struct Mol Biol 31, 232–245 (2024). https://doi.org/10.1038/s41594-023-01163-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01163-9