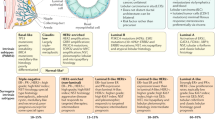
Abstract
Resistance to cancer treatment remains a major clinical hurdle. Here, we demonstrate that the CoREST complex is a key determinant of endocrine resistance and ER+ breast cancer plasticity. In endocrine-sensitive cells, CoREST is recruited to regulatory regions co-bound to ERα and FOXA1 to regulate the estrogen pathway. In contrast, during temporal reprogramming towards a resistant state, CoREST is recruited to AP-1 sites. In reprogrammed cells, CoREST favors chromatin opening, cJUN binding to chromatin, and gene activation by controlling SWI/SNF recruitment independently of the demethylase activity of the CoREST subunit LSD1. Genetic and pharmacological CoREST inhibition reduces tumorigenesis and metastasis of endocrine-sensitive and endocrine-resistant xenograft models. Consistently, CoREST controls a gene signature involved in invasiveness in clinical breast tumors resistant to endocrine therapies. Our studies reveal CoREST functions that are co-opted to drive cellular plasticity and resistance to endocrine therapies and tumorigenesis, thus establishing CoREST as a potential therapeutic target for the treatment of advanced breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All raw and processed NSG data have been deposited in the NCBI Gene Expression Omnibus under accession number GSE168644. Mass spectrophotometry raw files have been deposited on the public repository Chorus (chorusproject.org) with the project number 1763. Source data are provided with this paper.
Code availability
All software and bioinformatic tools used in this study are publicly available.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA Cancer J. Clin. 69, 438–451 (2019).
Hanker, A. B., Sudhan, D. R. & Arteaga, C. L. Overcoming endocrine resistance in breast cancer. Cancer Cell 37, 496–513 (2020).
Patten, D. K. et al. Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat. Med. 24, 1469–1480 (2018).
Marine, J. C., Dawson, S. J. & Dawson, M. A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer 20, 743–756 (2020).
Zhu, C. et al. A non-canonical role of YAP/TEAD is required for activation of estrogen-regulated enhancers in breast cancer. Mol. Cell 75, 791–806 (2019).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Garcia-Martinez, L., Zhang, Y., Nakata, Y., Chan, H. L. & Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 12, 1786 (2021).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 19, 39–56 (2020).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438 (2018).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353–360 (2012).
Berns, E. M. et al. Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res. 60, 2155–2162 (2000).
Abubakar, M. et al. Clinicopathological and epidemiological significance of breast cancer subtype reclassification based on p53 immunohistochemical expression. NPJ Breast Cancer 5, 20 (2019).
Yamashita, H. et al. p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res. 8, R48 (2006).
Yamamoto, M. et al. p53 accumulation is a strong predictor of recurrence in estrogen receptor-positive breast cancer patients treated with aromatase inhibitors. Cancer Sci. 105, 81–88 (2014).
Bertucci, F. et al. Genomic characterization of metastatic breast cancers. Nature 569, 560–564 (2019).
Silwal-Pandit, L., Langerod, A. & Borresen-Dale, A. L. TP53 mutations in breast and ovarian cancer. Cold Spring Harb. Perspect. Med. 7, a026252 (2017).
Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32, 169–184 (2017).
Lee, M. G., Wynder, C., Cooch, N. & Shiekhattar, R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437, 432–435 (2005).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Perillo, B., Tramontano, A., Pezone, A. & Migliaccio, A. LSD1: more than demethylation of histone lysine residues. Exp. Mol. Med 52, 1936–1947 (2020).
Magliulo, D., Bernardi, R. & Messina, S. Lysine-specific demethylase 1A as a promising target in acute myeloid leukemia. Front Oncol. 8, 255 (2018).
Bennani-Baiti, I. M., Machado, I., Llombart-Bosch, A. & Kovar, H. Lysine-specific demethylase 1 (LSD1/KDM1A/AOF2/BHC110) is expressed and is an epigenetic drug target in chondrosarcoma, Ewing’s sarcoma, osteosarcoma, and rhabdomyosarcoma. Hum. Pathol. 43, 1300–1307 (2012).
Wang, Y. et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 138, 660–672 (2009).
Wu, Y. et al. The deubiquitinase USP28 stabilizes LSD1 and confers stem-cell-like traits to breast cancer cells. Cell Rep. 5, 224–236 (2013).
Shahbandi, A., Nguyen, H. D. & Jackson, J. G. TP53 mutations and outcomes in breast cancer: reading beyond the headlines. Trends Cancer 6, 98–110 (2020).
Fu, X. et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl Acad. Sci. USA 113, E6600–E6609 (2016).
Jeselsohn, R. et al. Embryonic transcription factor SOX9 drives breast cancer endocrine resistance. Proc. Natl Acad. Sci. USA 114, E4482–E4491 (2017).
Morrison, G. et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res. Treat. 144, 263–272 (2014).
Murphy, C. S., Pink, J. J. & Jordan, V. C. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 50, 7285–7292 (1990).
Murphy, C. S., Meisner, L. F., Wu, S. Q. & Jordan, V. C. Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur. J. Cancer Clin. Oncol. 25, 1777–1788 (1989).
Inman, J. L., Robertson, C., Mott, J. D. & Bissell, M. J. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142, 1028–1042 (2015).
Idowu, M. O. et al. CD44+/CD24–/low cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum. Pathol. 43, 364–373 (2012).
Honeth, G. et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 10, R53 (2008).
Fang, Y., Liao, G. & Yu, B. LSD1/KDM1A inhibitors in clinical trials: advances and prospects. J. Hematol. Oncol. 12, 129 (2019).
Ravasio, R. et al. Targeting the scaffolding role of LSD1 (KDM1A) poises acute myeloid leukemia cells for retinoic acid-induced differentiation. Sci. Adv. 6, eaax2746 (2020).
Anastas, J. N. et al. Re-programing chromatin with a bifunctional LSD1/HDAC inhibitor induces therapeutic differentiation in DIPG. Cancer Cell 36, 528–544 (2019).
Kalin, J. H. et al. Targeting the CoREST complex with dual histone deacetylase and demethylase inhibitors. Nat. Commun. 9, 53 (2018).
Foster, C. T. et al. Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol. Cell. Biol. 30, 4851–4863 (2010).
Luo, H. et al. MOF acetylates the histone demethylase LSD1 to suppress epithelial-to-mesenchymal transition. Cell Rep. 15, 2665–2678 (2016).
Zhang, J. et al. SFMBT1 functions with LSD1 to regulate expression of canonical histone genes and chromatin-related factors. Genes Dev. 27, 749–766 (2013).
Liu, J. et al. Arginine methylation-dependent LSD1 stability promotes invasion and metastasis of breast cancer. EMBO Rep. 21, e48597 (2020).
Bahreini, A. et al. Mutation site and context dependent effects of ESR1 mutation in genome-edited breast cancer cell models. Breast Cancer Res. 19, 60 (2017).
Fang, R. et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol. Cell 39, 222–233 (2010).
Karytinos, A. et al. A novel mammalian flavin-dependent histone demethylase. J. Biol. Chem. 284, 17775–17782 (2009).
Hatzi, K. et al. Histone demethylase LSD1 is required for germinal center formation and BCL6-driven lymphomagenesis. Nat. Immunol. 20, 86–96 (2019).
Grose, R. Epithelial migration: open your eyes to c-Jun. Curr. Biol. 13, R678–R680 (2003).
Sioletic, S. et al. c-Jun promotes cell migration and drives expression of the motility factor ENPP2 in soft tissue sarcomas. J. Pathol. 234, 190–202 (2014).
Zhang, Y. et al. Critical role of c-Jun overexpression in liver metastasis of human breast cancer xenograft model. BMC Cancer 7, 145 (2007).
Kappelmann-Fenzl, M. et al. c-Jun drives melanoma progression in PTEN wild type melanoma cells. Cell Death Dis. 10, 584 (2019).
Malorni, L. et al. Blockade of AP-1 potentiates endocrine therapy and overcomes resistance. Mol. Cancer Res. 14, 470–481 (2016).
Bi, M. et al. Enhancer reprogramming driven by high-order assemblies of transcription factors promotes phenotypic plasticity and breast cancer endocrine resistance. Nat. Cell Biol. 22, 701–715 (2020).
Munne, P. M. et al. Compressive stress-mediated p38 activation required for ERα + phenotype in breast cancer. Nat. Commun. 12, 6967 (2021).
Gross, K., Wronski, A., Skibinski, A., Phillips, S. & Kuperwasser, C. Cell fate decisions during breast cancer development. J. Dev. Biol. 4, 4 (2016).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Gao, S. et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat. Genet. 52, 1011–1017 (2020).
Smith, L. M. et al. cJun overexpression in MCF-7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype. Oncogene 18, 6063–6070 (1999).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Mariani, O. et al. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell 11, 361–374 (2007).
Shao, J. et al. COP1 and GSK3β cooperate to promote c-Jun degradation and inhibit breast cancer cell tumorigenesis. Neoplasia 15, 1075–1085 (2013).
Musgrove, E. A. & Sutherland, R. L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 9, 631–643 (2009).
Zhang, X., Jin, B. & Huang, C. The PI3K/Akt pathway and its downstream transcriptional factors as targets for chemoprevention. Curr. Cancer Drug Targets 7, 305–316 (2007).
Wang, L. et al. CARM1 methylates chromatin remodeling factor BAF155 to enhance tumor progression and metastasis. Cancer Cell 25, 21–36 (2014).
Mohammad, H. P., Barbash, O. & Creasy, C. L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat. Med. 25, 403–418 (2019).
Sehrawat, A. et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc. Natl Acad. Sci. USA 115, E4179–E4188 (2018).
Zhang, Y. et al. The Polycomb protein RING1B enables estrogen-mediated gene expression by promoting enhancer-promoter interaction and R-loop formation. Nucleic Acids Res. 49, 9768–9782 (2021).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Aguilan, J. T., Kulej, K. & Sidoli, S. Guide for protein fold change and P value calculation for non-experts in proteomics. Mol. Omics 16, 573–582 (2020).
Yuan, Z. F. et al. EpiProfile 2.0: a computational platform for processing epi-proteomics mass spectrometry data. J. Proteome Res. 17, 2533–2541 (2018).
Acknowledgements
We are indebted to members of the Morey laboratory for discussions and to the Oncogenomics Core Facility, Cancer Modeling Shared Resource, and Flow Cytometry Core Facility at the Sylvester Comprehensive Cancer Center (SCCC). T47D-ERαY537S cells were kindly provided by S. Oesterreich (University of Pittsburgh). This work was supported by SCCC funds to L. M. and R. E. V., the Florida Health Bankhead-Coley Cancer Research Program (20B15), the V Foundation (DEC2020-009), the Lampert Breast Cancer Research Fund, and R01GM141349 from the National Institute of General Medical Sciences to L. M., and R01GM121595 from the National Institute of General Medical Sciences and 1R01CA233945 from the National Cancer Institute to R. E. V. Research in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA240139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
L. M., R. E. V., and L. G.-M. designed the study and analyzed the experiments. L. G.-M. conducted most of the experiments and bioinformatic analysis. Extended Data Figure 5a–g was analyzed by N. W. LC–MS/MS experiments were performed and analyzed by S. S. and S. D. Breast cancer datasets were analyzed by M. A. and B. M., and D. B. performed in vivo experiments. A. M. A. performed FOXA1 ChIP–seq experiments in reprogrammed and LSD1 KO cells as well as depletion and characterization of RCOR1 and SMARCC1 knockdown. Y. N. performed CUT&RUN experiments. L. S provided tehcnical support. Z. Z. and Z. L. analyzed the RNA-seq data. S. M. provided the plasmids to generate LSD1 KO cells and the rescue experiments, as well as the MCC2580 and DPP38003 compounds. S. B. K. provided intellectual support. L. M., R. E. V., and T. C.-T. supervised the experiments and provided intellectual support in interpretation of results. L. M., R. E. V., and L. G.-M. wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks Salvador Benitah, Jason Carroll, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Editor recognition statement (if applicable to your journal): Primary Handling Editor: Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Further characterization of LSD1/CoREST in breast cancer and long-term estrogen deprivation (LTED) model.
a, Kaplan–Meier survival curves segregated by ERα expression and TP53 mutation status (METABRIC dataset of 1,423 samples). Overall survival of patients with ER−/TP53 mutations is significantly diminished. P value was calculated using a log-rank (Mantel–Cox) test. b, CD24 and CD44 expression after 12 months (M) in LTED. c, FACS of T47D-LTED biological replicate (FM, full media). d, e, CD24 and CD44 expression in FM, TamR, FulR, and LTED T47D (d) and MCF7 (e). f, Growth curves of 2 × 105 FM and LTED T47D (3–9 M) cultured with DMSO (vehicle) or 1 µM tamoxifen or fulvestrant for 5 days, n = 3 biological independent replicates, data are presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). g, Heatmap of 3,206 significantly downregulated genes (FC > 2, q-value < 0.05) during acquisition of resistance in T47D. Major transcriptomic changes occurred after 6 M in LTED. h, GSEA of 4 M and 6 M T47D-LTED cells. Basal breast cancer, EMT transition, and ductal invasive signatures were upregulated while response to estrogen and luminal breast cancer signatures were downregulated after 6 months in LTED conditions. NES, normalized enrichment score. i, Single-cell SNV (single nucleotide variant) analysis from 6 × 103 FM and 6 M T47D-LTED. No FM cells harboured BRAF or KRAS mutations while ~60% of 6M cells acquired mutations in both genes. j, RNA-seq signal at ESR1 and PGR in FM and 9M T47D-LTED.
Extended Data Fig. 2 ESR1 loss is not mediated by epigenetic repressive mechanisms, and corin treatments in parental and endocrine resistant MCF7 cells.
a, b, WB of PRC2 subunits (EZH2 and SUZ12) and ERα from whole cell extracts of FM and reprogrammed (Repro.) cells cultured for 3 and 5 days in the presence of 1 µM or 5 µM of PRC2 inhibitors EPZ6438 (a) and GSK343 (b). Total H3K27me3, the primary substrate of EZH2, decreased after PRC2 inhibition. c, ERα WB from whole cell extracts of FM and reprogrammed cells cultured for 3 and 6 days in the presence of vehicle (DMSO), 1 µM, 5 µM, or 10 µM of the G9A inhibitor, UNC0638. d, ERα WB from whole cell extracts of FM and reprogrammed cells cultured for 6 days in vehicle (DMSO), 5 µM, or 10 µM of the DNMT inhibitor, decitabine (Deci.), 0.5 µM of the HDAC inhibitor, SAHA, or in combination. e, Proliferation of reprogrammed cells treated with 5 µM DMSO (vehicle) or two LSD1 enzymatic inhibitors (MCC2580, DPP38003) for 6 days, n = 3 biological independent replicates, data are presented as mean values + SEM, p-value < 0.05 for treatment with MCC2580 on day 6 (two-way ANOVA). f, Growth curves of 2 × 105 FM, LTED, TamR, and FulR MCF7 cultured with DMSO (vehicle) or 500 nM corin for 7 days, n = 3 independent experiments (except FulR cells, n = 2 independent experiments), Data are presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). Uncropped images are available as source data.
Extended Data Fig. 3 Genetic abrogation of LSD1 impaired proliferation and survival of endocrine sensitive and resistant cells, and the LSD1 interactome.
a, Proliferation of siLSD1 parental T47D 72 h after siRNA transfection, n = 3 biological independent replicates. Data are presented as mean values + SEM. p-value < 0.005 (one-way ANOVA). b-e, WB of proteins indicated, with VINCULIN as the loading control, in siCTR and siLSD1 T47D 6 days after transfection (b), primed siLSD1 T47D 3- and 6-days post siRNA transfection (c) and in WT and KO reprogrammed T47D (d), and reprogrammed shCTR and shLSD1 T47D (e). f, Clonogenic assay of reprogrammed shLSD1 T47D, n = 3 biological independent replicates. g, Proliferation of shCTR and shRCOR1 parental and reprogrammed T47D for 7 days, n = 2 biological independent replicates. h, DEG overlap between parental and reprogrammed LSD1 KO T47D. i, Endogenous LSD1 immunoprecipitation (IP) with CoREST subunits in whole cell lysates using two antibodies in parental or primed T47D. IgG and MBD3 were used as negative controls. j, Interaction network of LSD1 interactome in parental and reprogrammed T47D. k, Relative peptide abundance of selected SWI/SNF subunits identified by LC-MS/MS in parental and reprogrammed T47D. IP = LSD1 IP, IgG = IgG IP. n = 3 biological independent replicates. Number of peptides are represented as shades of blue and orange. Uncropped images are available as source data.
Extended Data Fig. 4 LSD1-BAF interaction in endocrine sensitive and resistant cells.
a, Superose 6 gel filtration in parental cells showing co-elution of LSD1, RCOR1, and members of the SWI/SNF complex (SMARCC1 and ARID2). b, LSD1 IP with SWI/SNF subunits in reprogrammed T47D. Note that the DPF2-LSD1 interaction was also not detected by LC-MS/MS (Fig. 2e). c, SMARCC1 IP with LSD1 in reprogrammed T47D. d, The LSD1-SMARCC1 interaction is DNA-independent. EtBr, ethidium bromide1. e, f, Proliferation of T47D treated with 1 µM corin for 5 days (e), and TSA or SAHA for 7 days (f), n = 3 biological independent replicates. Data are presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). g, Proliferation of WT and LSD1 KO reprogrammed T47D treated with TSA or SAHA for 7 days, n = 2 independent experiments. h, ERα WB in parental and endocrine resistant T47D lines with VINCULIN as a loading control. i, Synergy maps of parental and TamR T47D treated with tamoxifen and corin, or fulvestrant and corin. The 3D synergy matrix was generated with SynergyFinder 2.0, n = 3 biological independent replicates. Uncropped images are available as source data.
Extended Data Fig. 5 Mechanisms of action following CoREST chemical inhibition.
a, b, Cell cycle analysis (a) and representative γH2AX staining (b) 4 days after treatment with 500 nM DMSO or corin. PI, propidium iodide (inset zoom = 4X, scale bar = 5 μm). c, Quantification of γH2AX staining intensity from three biologically independent experiments; 200 cells per sample/experiment were analyzed. d, Quantification of the cell cycle distribution of γH2AX defined as the sum of the intensities of γH2AX foci per nucleus post-exposure to DMSO or 500 nM corin and stained with DAPI. Data from 3 biologically independent experiments; 200 cells per sample/experiment were analyzed. e, f, Representative images (left) and quantification (right) of ß-galactosidase (e) or PI/Annexin V (f) staining following exposure to DMSO or 500 nM corin for 4 days, n = 3 biological independent replicates (two-way ANOVA, scalebar = 10 μm), or LSD1 depletion, n = 2 biological independent replicates. g, CDKN2A (encoding p16) log2 TPM values in parental and reprogrammed T47D. (p-value = 0.0007, Two-tailed unpaired t-test), n = 2 biological independent replicates. h, The LSD1-RCOR1 interaction in reprogrammed whole cell lysate is destabilized in the presence of 1 µM corin. i, Cellular fractionation of reprogrammed cells treated with 500 nM corin for 24 h. Two different exposures are shown for LSD1 and RCOR1. j, WB of proteins indicated on the left from parental and reprogrammed cells treated with 1 µM of corin for 5 days. Uncropped images are available as source data.
Extended Data Fig. 6 LSD1/CoREST genomic occupancy during reprogramming.
LSD1 ChIP-qPCR. Validation of LSD1 ChIP-seq with two different LSD1 antibodies and concentrations in parental T47D, n = 2 biological independent replicates. NANOG was used as a negative control. Validation of LSD1 ChIP-seq in reprogrammed cells. NANOG was used as a negative control. b, LSD1 ChIP-seq signal at selected genomic regions. c, LSD1 ChIP-seq peak distribution. e-f, ChIP-seq signal of biological replicates in parental and reprogrammed cells.
Extended Data Fig. 7 Analysis of T47D-ERαY537S and role of the LSD1 paralog, LSD2.
a, Growth curves of 2 × 105 T47D-ERαY537S cells cultured with 1 µM of tamoxifen, fulvestrant, or palbociclib for 5 days, n = 3 biological independent replicates. Data are presented as mean values + SEM, **p-value < 0.01, *p-value < 0.05 (two-way ANOVA). b, Parental and T47D-ERαY537S expressing luciferase were transplanted into the mammary fat pad of NSG mice (n = 8 biological replicates). Data are presented as mean values + SEM. Metastasis was analyzed by IVIS 45 days after orthotopic injection. ***p-value < 0.001, **p-value < 0.01 (two-way ANOVA). c, Endogenous LSD1 immunoprecipitation (IP) in whole cell lysates with CoREST subunits using two antibodies in parental or primed T47D. IgG and MBD3 were used as negative controls. d, Growth curves of 2 × 105 T47D-ERαY537S cells cultured with 1 µM of GSK-LSD1 or corin for 7 days, n = 3 biological independent replicates. Data are presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). e, Synergy maps for T47D-ERαY537S cells treated with tamoxifen and corin, or fulvestrant and corin. The 3D synergy matrix was generated with SynergyFinder 2.0. n = 3. f, LSD1, ERα, FOXA1, and RNA Pol II ChIP-seq signal at selected genomic regions in parental and T47D-ERαY537S cells. g-h, RNA-seq heat maps (g) and TPM values (h) of differentially expressed LSD1 target genes in parental and reprogrammed T47D treated with corin (500 nM, 72 h), n = 2 biologically independent samples. The box plots span from the 25th to 75th percentiles, the center line shows the median and whiskers show maximum and minimum values. i, H3K4me2 ChIP-seq signal at LSD1 target genes in parental and reprogrammed cells treated with corin (500 nM, 72 h). j, H3K4me2 WB of acid-extracted histones from parental and reprogrammed siCTR and siLSD2 cells. k, WB of proteins indicated from parental and reprogrammed WT and LSD1 KO T47D transfected with siCTR or siLSD2. Uncropped images are available as source data.
Extended Data Fig. 8 CoREST genomic occupancy and role in cJUN and SMARCC1 chromatin recruitment during reprogramming.
a, cJUN and SMARCC1 WB in reprogrammed control and LSD1 KO T47D. b, c, ChIP-seq signal of factors indicated in reprogrammed WT, LSD1 KO, shCTR and shcJUN T47D at selected regions. d, Second biological replicate of cJUN ChIP-seq in reprogrammed T47D treated with 500 nM corin for 72 h. e, LSD1 WB in reprogrammed shCTR and shcJUN T47D. f, cJUN ChIP-seq in shCTR and shcJUN reprogrammed cells. g, TPM values of genes identified in each co-occupancy profile (significance determined by the Mann-Whitney test, two-sided), n = 2 biologically independent samples. The box plots span from the 25th to 75th percentiles, the center line shows the median and whiskers show maximum and minimum values and statistical significance determined by the Mann-Whitney test). h, Second biological replicate of SMARRC1 ChIP-seq signal in reprogrammed WT and LSD1 T47D. i, j, Proliferation (i) and survival (j) of shCTR and shcJUN reprogrammed T47D. n = 3 independent transductions. Data are presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). k, Proliferation of shCTR and shSMARCC1 parental and reprogrammed T47D, n = 2 biologically independent experiments. l, SMARCC1, LSD1, RCOR1, and cJUN WB in shCTR and shSMARCC1 parental and reprogrammed T47D. Uncropped images are available as source data.
Extended Data Fig. 9 Extended characterization of the CoREST role in chromatin accessibility.
a, Percentage of common and specific ATAC-seq peaks in parental and reprogrammed WT and LSD1 KO T47D. b, ATAC-seq signal in WT and LSD1-KO reprogrammed T47D (top) and cells treated with 500 nM corin for 72 h (bottom). c, Second biological replicate of SMARCC1 ChIP-seq signal in WT and LSD1 KO from analysis in Fig. 6d. d, ATAC-seq signal in reprogrammed T47D treated with 500 nM corin for 72 h at accessible sites in LSD1 KO cells that are inaccessible in WT cells. e, log2 TPM values of FOXA1 expression in WT and KO LSD1 parental and reprogrammed cells, n = 2 from biological independent experiments. Data are presented as mean values + SD, unpaired t-test, two-sided, p values (parental vs reprogrammed = 0.0008, reprogrammed WT vs KO = 0.0299). f, log2 TPM values of ESR1 in parental and reprogrammed WT and LSD1 KO T47D, n = 2 biologically independent samples Data are presented as mean values + SD, unpaired t-test, two-sided, p values (parental vs reprogrammed <0.0001, parental WT vs KO < 0.0001, reprogrammed WT vs KO = 0.0041. g, h, WB of LSD1, RCOR1, and ERα from whole cell extracts of reprogrammed WT and LSD1 KO T47D cultured for 7 days in the presence of 1 µM PRC2i EPZ6438, GSK343, and DNMTi 5-azacitidine (g) or anisomycin (h). i, Proliferation of WT and LSD1 KO reprogrammed T47D cells treated with 1 µM of tamoxifen or fulvestrant for 5 days. j, Second biological replicate of SMARCC1 ChIP-seq signal in WT and LSD1 KO from analysis in Fig. 6j. Uncropped images are available as source data.
Extended Data Fig. 10 Characterization of primed, CD24+, and CD44+ primed cells in vivo and proliferation defects of LSD1 depletion in TNBC.
a, Schematic (left) and representative IVIS images of mice (right) injected with serial dilutions of primed T47D and 1 × 105 MDA-MB-231 (positive control, n = 3/group, data presented as mean values + SEM). b, c, FACS (b) and representative IVIS images at day 32 post-intracardial injection (c) of sorted CD24+ and CD44+ primed T47D two weeks after sorting without estrogen supplementation (n = 5/group). d-e, Tumor size quantification (d) (****p-value < 0.0001, data presented as mean values + SEM) and survival of mice (e) (**** p-value < 0.0001, Log-rank [Mantel-Cox test]). f, Metastasis quantification from WT and LSD1 KO reprogrammed T47D. *** (p-value < 0.0001, Two-way RM ANOVA). 50,000 cells were injected in the tail vain (n = 10/group,). g, WB of H3K27ac from liver extracts of mice treated with increasing concentrations of corin. SC, subcutaneous. IP, intraperitoneal. Histone H3 was used as a loading control. h, Representative hematoxylin and eosin (H&E) and Ki67 staining in WT LSD1 or KO (top) and corin treated tumors (bottom). i, LSD1 WB from total extracts of shCTR and shLSD1 MDA-MB-231. j, Effect of LSD1 knockdown on MDA-MB-231 proliferation (p-value =0.0255, unpaired t-test), n = 3 independent infections and experiments, data presented as mean values + SEM. k, Clonogenic assay of shCTR and shLSD1 MDA-MB-231 performed in three biological and three technical replicates. l, Proliferation of MDA-MB-231 treated with 1 µM corin for 7 days, n = 3 biological independent experiments. Data presented as mean values + SEM, p-value < 0.001 (two-way ANOVA). Uncropped images are available as source data.
Supplementary information
Supplementary Table
Supplementary Tables 1–6
Source data
Source Data Fig. 1
Unprocessed Western Blots
Source Data Fig. 3
Unprocessed Western Blots
Source Data Fig. 4
Unprocessed Western Blots
Source Data Fig. 5
Unprocessed Western Blots
Source Data Fig. 6
Unprocessed Western Blots
Source Data Extended Data Fig. 2
Unprocessed Western Blots
Source Data Extended Data Fig. 3
Unprocessed Western Blots
Source Data Extended Data Fig. 4
Unprocessed Western Blots
Source Data Extended Data Fig. 5
Unprocessed Western Blots
Source Data Extended Data Fig. 7
Unprocessed Western Blots
Source Data Extended Data Fig. 8
Unprocessed Western Blots
Source Data Extended Data Fig. 9
Unprocessed Western Blots
Source Data Extended Data Fig. 10
Unprocessed Western Blots
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Martinez, L., Adams, A.M., Chan, H.L. et al. Endocrine resistance and breast cancer plasticity are controlled by CoREST. Nat Struct Mol Biol 29, 1122–1135 (2022). https://doi.org/10.1038/s41594-022-00856-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00856-x
This article is cited by
-
Advancing breast cancer diagnosis with a near-infrared fluorescence imaging smart sensor for estrogen/progesterone receptor detection
Scientific Reports (2023)
-
MAF amplification licenses ERα through epigenetic remodelling to drive breast cancer metastasis
Nature Cell Biology (2023)
-
Switching under selection: how CoREST controls endocrine therapy resistance in ER+ breast cancer
Nature Structural & Molecular Biology (2022)