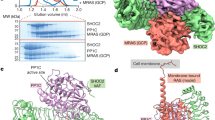
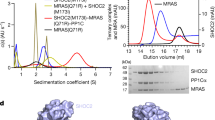
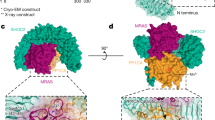
Abstract
SHOC2 acts as a strong synthetic lethal interactor with MEK inhibitors in multiple KRAS cancer cell lines. SHOC2 forms a heterotrimeric complex with MRAS and PP1C that is essential for regulating RAF and MAPK-pathway activation by dephosphorylating a specific phosphoserine on RAF kinases. Here we present the high-resolution crystal structure of the SHOC2–MRAS–PP1C (SMP) complex and apo-SHOC2. Our structures reveal that SHOC2, MRAS, and PP1C form a stable ternary complex in which all three proteins synergistically interact with each other. Our results show that dephosphorylation of RAF substrates by PP1C is enhanced upon interacting with SHOC2 and MRAS. The SMP complex forms only when MRAS is in an active state and is dependent on SHOC2 functioning as a scaffolding protein in the complex by bringing PP1C and MRAS together. Our results provide structural insights into the role of the SMP complex in RAF activation and how mutations found in Noonan syndrome enhance complex formation, and reveal new avenues for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The atomic coordinates and structure factors of the SMP complex and SHOC2 have been deposited in the Protein Data Bank and are available under accession numbers 7TVF and 7TVG, respectively. Structures under PDB accession codes 6DNO and 1X1S were used as initial models for molecular replacement. Structures used for superpositions are available in the PDB, including 4MOV, 5IOH, 4G9J, 5ZQV, 5ZT0, 6ZEF, 3V4Y, 3N5U,4MOY, 4XPN, 3EGG, 2O8G, and 1S70. The individual apparent KD values measured by SPR, mass spectrometry data, and full gel scans are accessible as source data published along this manuscript. Source data are provided with this paper.
References
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Yaeger, R. & Corcoran, R. B. Targeting alterations in the RAF–MEK pathway. Cancer Discov. 9, 329–341 (2019).
Longo, J. F. & Carroll, S. L. The RASopathies: biology, genetics and therapeutic options. Adv. Cancer Res 153, 305–341 (2022).
Lavoie, H. & Therrien, M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 16, 281–298 (2015).
Park, E. et al. Architecture of autoinhibited and active BRAF–MEK1–14-3-3 complexes. Nature 575, 545–550 (2019).
Simanshu, D. K. & Morrison, D. K. A structure is worth a thousand words: new insights for RAS and RAF regulation. Cancer Discov. 12, 899–912 (2022).
Tran, T. H. et al. KRAS interaction with RAF1 RAS-binding domain and cysteine-rich domain provides insights into RAS-mediated RAF activation. Nat. Commun. 12, 1176 (2021).
Cookis, T. & Mattos, C. Crystal structure reveals the full Ras–Raf interface and advances mechanistic understanding of Raf activation. Biomolecules 11, 996 (2021).
Molzan, M. et al. Impaired binding of 14-3-3 to C-RAF in Noonan syndrome suggests new approaches in diseases with increased Ras signaling. Mol. Cell. Biol. 30, 4698–4711 (2010).
Rodriguez-Viciana, P., Oses-Prieto, J., Burlingame, A., Fried, M. & McCormick, F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol. Cell 22, 217–230 (2006).
Young, L. C. et al. SHOC2–MRAS–PP1 complex positively regulates RAF activity and contributes to Noonan syndrome pathogenesis. Proc. Natl Acad. Sci. USA 115, E10576–E10585 (2018).
Jeoung, M., Abdelmoti, L., Jang, E. R., Vander Kooi, C. W. & Galperin, E. Functional integration of the conserved domains of Shoc2 scaffold. PLoS ONE 8, e66067 (2013).
Cordeddu, V. et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat. Genet. 41, 1022–1026 (2009).
Hannig, V., Jeoung, M., Jang, E. R., Phillips, J. A. 3rd & Galperin, E. A novel SHOC2 variant in rasopathy. Hum. Mutat. 35, 1290–1294 (2014).
Motta, M. et al. Clinical and functional characterization of a novel RASopathy-causing SHOC2 mutation associated with prenatal-onset hypertrophic cardiomyopathy. Hum. Mutat. 40, 1046–1056 (2019).
Young, L. C. et al. An MRAS, SHOC2, and SCRIB complex coordinates ERK pathway activation with polarity and tumorigenic growth. Mol. Cell 52, 679–692 (2013).
Sulahian, R. et al. Synthetic lethal interaction of SHOC2 sepletion with MEK inhibition in RAS-driven cancers. Cell Rep. 29, 118–134 (2019).
Jones, G. G. et al. SHOC2 phosphatase-dependent RAF dimerization mediates resistance to MEK inhibition in RAS-mutant cancers. Nat. Commun. 10, 2532 (2019).
Kaplan, F. M. et al. SHOC2 and CRAF mediate ERK1/2 reactivation in mutant NRAS-mediated resistance to RAF inhibitor. J. Biol. Chem. 287, 41797–41807 (2012).
Terai, H. et al. SHOC2 is a critical modulator of sensitivity to EGFR-TKIs in non-small cell lung cancer cells. Mol. Cancer Res. 19, 317–328 (2021).
Boned Del Rio, I. et al. SHOC2 complex-driven RAF dimerization selectively contributes to ERK pathway dynamics. Proc. Natl Acad. Sci. USA 116, 13330–13339 (2019).
Han, K. et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 580, 136–141 (2020).
Young, L. C. & Rodriguez-Viciana, P. MRAS: a close but understudied member of the RAS family. Cold Spring Harb. Perspect. Med. 8, a033621 (2018).
Kota, P. et al. M-Ras/Shoc2 signaling modulates E-cadherin turnover and cell-cell adhesion during collective cell migration. Proc. Natl Acad. Sci. USA 116, 3536–3545 (2019).
Higgins, E. M. et al. Elucidation of MRAS-mediated Noonan syndrome with cardiac hypertrophy. JCI Insight 2, e91225 (2017).
Suzuki, H. et al. Severe Noonan syndrome phenotype associated with a germline Q71R MRAS variant: a recurrent substitution in RAS homologs in various cancers. Am. J. Med. Genet. A 179, 1628–1630 (2019).
Verbinnen, I., Ferreira, M. & Bollen, M. Biogenesis and activity regulation of protein phosphatase 1. Biochem. Soc. Trans. 45, 89–99 (2017).
Korrodi-Gregorio, L., Esteves, S. L. & Fardilha, M. Protein phosphatase 1 catalytic isoforms: specificity toward interacting proteins. Transl. Res. 164, 366–391 (2014).
Peti, W., Nairn, A. C. & Page, R. Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280, 596–611 (2013).
Bertola, D. et al. The recurrent PPP1CB mutation p.Pro49Arg in an additional Noonan-like syndrome individual: broadening the clinical phenotype. Am. J. Med. Genet. A 173, 824–828 (2017).
Gripp, K. W. et al. A novel rasopathy caused by recurrent de novo missense mutations in PPP1CB closely resembles Noonan syndrome with loose anagen hair. Am. J. Med. Genet. A 170, 2237–2247 (2016).
Huckstadt, V., Chinton, J., Gomez, A., Obregon, M. G. & Gravina, L. P. Noonan syndrome with loose anagen hair with variants in the PPP1CB gene: First familial case reported. Am. J. Med. Genet A 185, 1256–1260 (2021).
Zambrano, R. M. et al. Further evidence that variants in PPP1CB cause a rasopathy similar to Noonan syndrome with loose anagen hair. Am. J. Med. Genet A 173, 565–567 (2017).
Snead, K., Wall, V., Ambrose, H., Esposito, D. & Drew, M. Polycistronic baculovirus expression of SUGT1 enables high-yield production of recombinant leucine-rich repeat proteins and protein complexes. Protein Expr. Purif. 193, 106061 (2022).
Selfors, L. M., Schutzman, J. L., Borland, C. Z. & Stern, M. J. soc-2 encodes a leucine-rich repeat protein implicated in fibroblast growth factor receptor signaling. Proc. Natl Acad. Sci. USA 95, 6903–6908 (1998).
Ye, M. et al. Crystal structure of M-Ras reveals a GTP-bound “off” state conformation of Ras family small GTPases. J. Biol. Chem. 280, 31267–31275 (2005).
Choy, M. S. et al. Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proc. Natl Acad. Sci. USA 111, 4097–4102 (2014).
Salvi, F. et al. Towards dissecting the mechanism of protein phosphatase-1 inhibition by its C-terminal phosphorylation. ChemBioChem 22, 834–838 (2021).
Wakula, P., Beullens, M., Ceulemans, H., Stalmans, W. & Bollen, M. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J. Biol. Chem. 278, 18817–18823 (2003).
Hendrickx, A. et al. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem. Biol. 16, 365–371 (2009).
Lee, K. H. et al. Stabilization of Sur8 via PKCα/∆ degradation promotes transformation and migration of colorectal cancer cells. Oncotarget 8, 115596–115608 (2017).
Meehan, T. F. et al. Disease model discovery from 3,328 gene knockouts by The International Mouse Phenotyping Consortium. Nat. Genet. 49, 1231–1238 (2017).
Ferreira, M., Beullens, M., Bollen, M. & Van Eynde, A. Functions and therapeutic potential of protein phosphatase 1: insights from mouse genetics. Biochim. Biophys. Acta Mol. Cell. Res. 1866, 16–30 (2019).
Burd, C. E. et al. Mutation-specific RAS oncogenicity explains NRAS codon 61 selection in melanoma. Cancer Discov. 4, 1418–1429 (2014).
Endo, T. M-Ras is muscle-Ras, moderate-Ras, mineral-Ras, migration-Ras, and many more-Ras. Exp. Cell. Res. 397, 112342 (2020).
Terrak, M., Kerff, F., Langsetmo, K., Tao, T. & Dominguez, R. Structural basis of protein phosphatase 1 regulation. Nature 429, 780–784 (2004).
Goldberg, J. et al. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 376, 745–753 (1995).
Kurcinski, M., Badaczewska-Dawid, A., Kolinski, M., Kolinski, A. & Kmiecik, S. Flexible docking of peptides to proteins using CABS-dock. Protein Sci. 29, 211–222 (2020).
Hoermann, B. et al. Dissecting the sequence determinants for dephosphorylation by the catalytic subunits of phosphatases PP1 and PP2A. Nat. Commun. 11, 3583 (2020).
Yi, J. et al. Endothelial SUR-8 acts in an ERK-independent pathway during atrioventricular cushion development. Dev. Dyn. 239, 2005–2013 (2010).
Nunez Rodriguez, N. et al. Characterization of R-ras3/m-ras null mice reveals a potential role in trophic factor signaling. Mol. Cell. Biol. 26, 7145–7154 (2006).
Lai, L. P. et al. Classical RAS proteins are not essential for paradoxical ERK activation induced by RAF inhibitors. Proc. Natl Acad. Sci. USA 119, e2113491119 (2022).
Kumar, G. S. et al. Identification of the substrate recruitment mechanism of the muscle glycogen protein phosphatase 1 holoenzyme. Sci. Adv. 4, eaau6044 (2018).
Ehrhardt, A., Ehrhardt, G. R., Guo, X. & Schrader, J. W. Ras and relatives–job sharing and networking keep an old family together. Exp. Hematol. 30, 1089–1106 (2002).
Fedoryshchak, R. O. et al. Molecular basis for substrate specificity of the Phactr1/PP1 phosphatase holoenzyme. eLife 9, e61509 (2020).
O’Connell, N. et al. The molecular basis for substrate specificity of the nuclear NIPP1:PP1 holoenzyme. Structure 20, 1746–1756 (2012).
Choy, M. S. et al. SDS22 selectively recognizes and traps metal-deficient inactive PP1. Proc. Natl Acad. Sci. USA 116, 20472–20481 (2019).
Martinez Fiesco, J. A., Durrant, D. E., Morrison, D. K. & Zhang, P. Structural insights into the BRAF monomer-to-dimer transition mediated by RAS binding. Nat. Commun. 13, 486 (2022).
Kwon, J. J. & Hahn, W. C. A leucine-rich repeat protein provides a SHOC2 the RAS circuit: a structure–function perspective. Mol. Cell Biol. 41, e00627-20 (2021).
Jang, H., Stevens, P., Gao, T. & Galperin, E. The leucine-rich repeat signaling scaffolds Shoc2 and Erbin: cellular mechanism and role in disease. FEBS J. 288, 721–739 (2021).
Sieburth, D. S., Sun, Q. & Han, M. SUR-8, a conserved Ras-binding protein with leucine-rich repeats, positively regulates Ras-mediated signaling in C. elegans. Cell 94, 119–130 (1998).
Talsania, K. et al. Genome assembly and annotation of the Trichoplusia ni Tni-FNL insect cell line enabled by long-read technologies. Genes (Basel) 10, 79 (2019).
Taylor, T., Denson, J. P. & Esposito, D. Optimizing expression and solubility of proteins in E. coli using modified media and induction parameters. Methods Mol. Biol. 1586, 65–82 (2017).
Kopra, K. et al. Homogeneous dual-parametric-coupled assay for simultaneous nucleotide exchange and KRAS/RAF-RBd interaction monitoring. Anal. Chem. 92, 4971–4979 (2020).
D’Arcy, A., Bergfors, T., Cowan-Jacob, S. W. & Marsh, M. Microseed matrix screening for optimization in protein crystallization: what have we learned? Acta Crystallogr. F. Struct. Biol. Commun. 70, 1117–1126 (2014).
Kabsch, W. XDS. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D. Biol. Crystallogr. 69, 1204–1214 (2013).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D. Biol. Crystallogr. 67, 235–242 (2011).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D. Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D. Biol. Crystallogr. 67, 355–367 (2011).
Schrodinger, L. L. C. The PyMOL Molecular Graphics System version 1.8 (2015).
Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Siddiqui, F. A. et al. PDE6D inhibitors with a new design principle selectively block K-Ras activity. ACS Omega 5, 832–842 (2020).
Kumar, G. S. et al. The Ki-67 and RepoMan mitotic phosphatases assemble via an identical, yet novel mechanism. eLife 5, e16539 (2016).
Chatterjee, J. et al. Development of a peptide that selectively activates protein phosphatase-1 in living cells. Angew. Chem. Int. Ed. Engl. 51, 10054–10059 (2012).
Yu, J., Deng, T. & Xiang, S. Structural basis for protein phosphatase 1 recruitment by glycogen-targeting subunits. FEBS J. 285, 4646–4659 (2018).
Hirschi, A. et al. An overlapping kinase and phosphatase docking site regulates activity of the retinoblastoma protein. Nat. Struct. Mol. Biol. 17, 1051–1057 (2010).
Choy, M. S. et al. Structural and functional analysis of the GADD34:PP1 eIF2α phosphatase. Cell Rep. 11, 1885–1891 (2015).
Ragusa, M. J. et al. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat. Struct. Mol. Biol. 17, 459–464 (2010).
Hurley, T. D. et al. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J. Biol. Chem. 282, 28874–28883 (2007).
Acknowledgements
We thank B. Gillette, H. Ambrose, J. Cregger, P. Frank, B. Higgins, M. Hong, J. Mehalko, A. Mitchell, S. Perkins, N. Ramakrishnan, M. Sherekar, M. Smith, T. Taylor, V. Wall, and S. Widmeyer of the Protein Expression Laboratory (Frederick National Laboratory for Cancer Research) for their help in preparing recombinant proteins. We are grateful to S. Dharmaiah and D. Czyzyk for their help with crystallization, and to T. Waybright for nucleotide analysis (Frederick National Laboratory for Cancer Research). We thank M. Murphy (Cytiva Life Sciences) for his advice on fitting the SPR kinetic data. We thank L. Young for critical feedback on the manuscript (University of California San Francisco). We acknowledge W. Peti (University of Connecticut Health Center) for advice on the expression and purification of PP1CA. X-ray diffraction data were collected at the Northeastern Collaborative Access Team beamlines (24-ID-C/E), funded by the US National Institutes of Health (NIGMS P30 GM124165). The Pilatus 6M detector on the 24-ID-C beamline is funded by an NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. This project was funded in part with federal funds from the National Cancer Institute, National Institutes of Health Contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, and the mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
Author information
Authors and Affiliations
Contributions
D.A.B. and D.K.S. carried out crystallography work, structural analysis, and ITC experiments; P.A. and A.G.S. performed S.P.R. measurements; N.H., M.D., D.E., and P.R.-V. carried out enzymatic assays and western blot analysis. K.S., M.D., S.M., and D.E. prepared recombinant proteins. L.I.F., D.V.N., P.R.-V., and F.M. contributed to the structural and functional analysis. D.A.B. and D.K.S. wrote the manuscript with inputs from all co-authors.
Corresponding author
Ethics declarations
Competing interests
F.M. is a consultant for Amgen, Daiichi, Frontiers Med, Exuma Biotech, Ideaya Biosciences, Kura Oncology, Leidos Biomedical Research, PellePharm, Pfizer, P.M.V. Pharma, and Quanta Therapeutics. F.M. is a consultant and co-founder for (with ownership interest including stock options) BridgeBio, Olema Pharmaceuticals, and Quartz. F.M. has received research grants from Daiichi Sankyo and Gilead Sciences and has a current grant from Boehringer-Ingelheim. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editors: Beth Moorefield and Carolina Perdigoto, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Assembly and selectivity of the SMP complex.
a Nucleotide analysis of the SMP complex by HPLC. Nucleotide standards and their retention time are shown above. b ITC experiment to measure the dissociation constant between SHOC2, MRAS1-178 and PP1CA7-300. c A steady-state plot of measured RU values from the formation of the SHOC2-HRAS-PP1CA complex against concentrations of HRASGMPPNP. d A steady-state plot of measured RU values from the formation of the SHOC2-NRAS-PP1CA complex against concentrations of NRASGMPPNP.
Extended Data Fig. 2 Comparison of the individual components of the SMP complex with their apo-forms.
a Superposition of the two SMP complexes found in the asymmetric subunit in cartoon form. Both chains of MRAS and PP1CA are in the same color, while the two SHOC2 chains are colored pink and cyan. The overall, SHOC2, MRAS and PP1CA RMSDs are 0.62 Å, 1.74 Å, 0.19 Å and 0.15 Å, respectively. b Top view as shown in panel a without MRAS and PP1CA present. LRR10 is marked, highlighting the hinge. c A cartoon of SHOC2 with a color gradient from blue to red showing the RMSD between the two SHOC2 molecules in the SMP complex, with blue and red representing low and high RMSD, respectively. LRR10 is marked highlighting the hinge. d Superposition of apo-SHOC2 (yellow) with SHOC2 from the SMP complex (pink) which was used for all subsequent analysis. All LRRs are labeled. e Superposition of mouse MRAS bound to GMPPNP (yellow; PDB ID 1X1S36) with human MRAS from the SMP complex (blue). Switch I, switch II, nucleotide and Mg2+ ions are shown in dark blue, purple, sticks and green spheres, respectively. The overall RMSD is 0.32 Å. f Superposition of apo-PP1CA (olive, PDB ID 4MOV37) with PP1CA from the SMP complex (green). Mn2+ ions from SMP and apo-PP1CA are shown in green and gray, respectively. The overall RMSD is 0.28 Å.
Extended Data Fig. 3 PP1C isoform specificity of the SMP complex.
ITC experiments to measure the dissociation constant between a SHOC2, MRAS1-178 and PP1CA2-330 and b SHOC2, MRAS1-178 and PP1CB2-327.
Extended Data Fig. 4 Analysis of the SHOC2-PP1CA interface.
a The proposed SILK and RVxF binding motifs mapped onto SHOC2 (pink spheres) of the SMP complex. b Superposition of all RVxF-PP1C complexes present in the PDB onto the SMP complex. Surface of PP1CA (green) with the RVxF motif of SHOC2 (current work, pink), muscle glycogen-targeting subunit (PDB ID 6DNO, cyan53), RepoMan (PDB ID 5IOH, magenta76), cell-permeable peptide (PDB ID 4G9J, salmon77), PP1 regulatory subunit 3 A (PDB ID 5ZQV, light gray78), PP1 regulatory subunit 3B (PDB ID 5ZT0, violet78), Phactr1 (PDB ID 6ZEF, teal55), NIPP1 (PDB ID 3V4Y, orange56), Retinoblastoma-associated protein (PDB ID 3N5U, purple79), PP1 regulatory subunit 10 (PDB ID 4MOY, gray37), GADD34 (PDB ID 4XPN, dark blue80), Spinophilin (PDB ID 3EGG, gold81) and mouse-inhibitor 2 (PDB ID 2O8G, dark olive82). c Sequence alignment of the RVxF motif of SHOC2 across different species. Totally conserved residues are bold and highlighted in black, while similar residues are bold and highlighted in white. The RVxF motif is denoted with black stars. d Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 mutants as denoted in the figure with five injections of MRASGMPPNP and PP1CA (blue). The data were fit to a 1:1 kinetic model (black). e The SILK binding pocket on PP1C (green surface), as shown by the SILK of mouse inhibitor-2 (yellow cartoon) is occluded by SHOC2 (pink). A hydrogen bond forms between E54 of PP1CA at the periphery of the SILK binding pocket to R203 of SHOC2.
Extended Data Fig. 5 Isoforms and Noonan Syndrome mutations of PP1C.
a Sequence alignment of the three human isoforms of PP1C. Totally conserved residues are bold and highlighted in black, while similar residues are bold and highlighted in white. Non-conserved residues are only highlighted in white. The secondary structure of the PP1CA structure is shown above the alignment. α-helices and β-strands are labeled according to the nomenclature of Peti et al.29. Residues of PP1CA which interact with SHOC2 and MRAS are denoted with pink ovals and blue stars, respectively. R188 is the only residue of PP1CA which interacts with both SHOC2 and MRAS. b Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 with five injections of MRASGMPPNP and PP1CA mutants as denoted in the figure (blue). The data were fit to a 1:1 kinetic model (black).
Extended Data Fig. 6 Analysis of SHOC2 binding to the surface of MRAS.
a The N-terminal LRRs of SHOC2 (pink) are shown interacting with the switch I (dark blue) and switch II (purple) of MRAS (blue). b The C-terminal LRRs of SHOC2 (pink) are shown interacting with the C-terminus of MRAS (blue surface). c Residues of MRAS found mutated in NS highlighted as spheres on the structure of MRAS. d Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 mutants as denoted in the figure with five injections of MRASGMPPNP and PP1CA (blue). The data were fit to a 1:1 kinetic model (black).
Extended Data Fig. 7 Analysis of MRAS binding to the surface of SHOC2.
a Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 with five injections of MRASmutant-GMPPNP as denoted in the figure and PP1CA (blue). The data were fit to a 1:1 kinetic model (black). b Sequence alignment of human MRAS, KRAS, HRAS and NRAS sequences. Totally conserved residues are bold and highlighted in black, while highly conserved residues are bold and highlighted in white. Non-conserved residues are only highlighted in white. The secondary structure of the MRAS present in the SMP complex is shown above the alignment. Residues of MRAS which interact with SHOC2 and PP1CA are denoted with pink stars and green ovals, respectively.
Extended Data Fig. 8 Analysis of the MRAS-PP1CA interface.
a The N-terminus of MRAS (blue) occupies the MyPhoNE cleft (dark red) on PP1CA (green). The helical MyPhoNE motif of MYPT1 (PDB ID 1s70 (ref. 46)) is shown in cyan. b ITC experiment to measure the dissociation constant between SHOC2, MRAS11-178 and PP1CA7-300. c Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 with five injections of MRASH53A-GMPPNP and PP1CA (blue). d Single-cycle kinetic analysis was performed on immobilized avi-tagged SHOC2 with five injections of MRASGMPPNP and PP1CAR188A (blue). In each case, the data were fit to a 1:1 kinetic model (black).
Extended Data Fig. 9 Docking of CRAF substrates and Noonan syndrome mutations found in the active site of PP1C.
a The CABS-dock server docked 15-mer peptides of the CR2-pS region of CRAF into the PP1CA structure of the SMP complex. All 167 peptides were placed in the active site, with all peptides placed with the N- and C-termini in the acidic and hydrophobic active site channels (magenta ribbons). PP1CA is shown as an electrostatic surface. b Two NS mutations are found to line the acidic and C-terminal channels of PP1CB. These were mapped onto the PP1CA surface with D253Y and E275K shown in blue (D252Y and E274K in PP1CB). c Fluorescent Western blot (representative of three independent experiments) of different concentrations of PP1CA or SMP incubated with either BRAF or CRAF substrates monitoring loss of CR2-pS signal (top gel, green bands). Total RAF loaded are shown as red bands (2nd from top gel). d Western blot (representative of three independent experiments) of different concentrations of PP1CA or SMP with either BRAF or CRAF substrates. Blots were probed at different phosphorylation sites in the substrates. SMP complex only dephosphorylates CR2-pS.
Extended Data Fig. 10 Dephosphorylation of BRAF CR2-pS phosphopeptides by the SMP complex.
Dephosphorylation of BRAF CR2-pS 15mer wild type peptide (top) and +1-position mutation to glutamic acid (bottom) as monitored by MALDI-TOF over 16 hours by the SMP complex. Sodium adducts of both dephosphorylated and phosphorylated peptides are denoted with *.
Supplementary information
Source data
Source Data Fig. 3
SPR kinetic data
Source Data Fig. 4
SPR kinetic data
Source Data Fig. 5
SPR kinetic data
Source Data Fig. 6
Unprocessed Western Blots
Source Data Extended Data Fig. 9
Unprocessed Western Blots
Source Data Extended Data Fig. 10
Mass spectrometry peak lists
Rights and permissions
About this article
Cite this article
Bonsor, D.A., Alexander, P., Snead, K. et al. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. Nat Struct Mol Biol 29, 966–977 (2022). https://doi.org/10.1038/s41594-022-00841-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00841-4
This article is cited by
-
Combinatorial strategies to target RAS-driven cancers
Nature Reviews Cancer (2024)
-
Cryo-EM structure of a RAS/RAF recruitment complex
Nature Communications (2023)
-
Scribble mis-localization induces adaptive resistance to KRAS G12C inhibitors through feedback activation of MAPK signaling mediated by YAP-induced MRAS
Nature Cancer (2023)
-
SHOCing RAF into action
Nature Structural & Molecular Biology (2022)