Abstract
Neurotransmitter release is mediated by proteins that drive synaptic vesicle fusion with the presynaptic plasma membrane. While soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs) form the core of the fusion apparatus, additional proteins play key roles in the fusion pathway. Here, we report that the C-terminal amphipathic helix of the mammalian accessory protein, complexin (Cpx), exerts profound effects on membranes, including the formation of pores and the efficient budding and fission of vesicles. Using nanodisc-black lipid membrane electrophysiology, we demonstrate that the membrane remodeling activity of Cpx modulates the structure and stability of recombinant exocytic fusion pores. Cpx had particularly strong effects on pores formed by small numbers of SNAREs. Under these conditions, Cpx increased the current through individual pores 3.5-fold, and increased the open time fraction from roughly 0.1 to 1.0. We propose that the membrane sculpting activity of Cpx contributes to the phospholipid rearrangements that underlie fusion by stabilizing highly curved membrane fusion intermediates.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All graphed raw data are provided as source data. With the exception of RCSB Protein Data Bank to access PDB 1KIL, no specific databases or third party data were used in this study. Source data are provided with this paper.
Code availability
No unique code was used in this study.
References
Reim, K. et al. Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 169, 669–680 (2005).
Mcmahon, H. T., Missler, M., Li, C. & Sudhof, T. C. Complexins—cytosolic proteins that regulate snap receptor function. Cell 83, 111–119 (1995).
Reim, K. et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71–81 (2001).
Ishizuka, T., Saisu, H., Odani, S. & Abe, T. Synaphin—a protein associated with the docking/fusion complex in presynaptic terminals. Biochem. Biophys. Res. Commun. 213, 1107–1114 (1995).
Trimbuch, T. & Rosenmund, C. Should I stop or should I go? The role of complexin in neurotransmitter release. Nat. Rev. Neurosci. 17, 118–125 (2016).
Lopez-Murcia, F. J., Reim, K., Jahn, O., Taschenberger, H. & Brose, N. Acute complexin knockout abates spontaneous and evoked transmitter release. Cell Rep. 26, 2521–2530 e2525 (2019).
Courtney, N. A., Bao, H., Briguglio, J. S. & Chapman, E. R. Synaptotagmin 1 clamps synaptic vesicle fusion in mammalian neurons independent of complexin. Nat. Commun. 10, 14 (2019).
Wragg, R. T. et al. Evolutionary divergence of the C-terminal domain of complexin accounts for functional disparities between vertebrate and invertebrate complexins. Front. Mol. Neurosci. 10, 24 (2017).
Cai, H. et al. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc. Natl Acad. Sci. USA 105, 19538–19543 (2008).
Xue, M. et al. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc. Natl Acad. Sci. USA 105, 7875–7880 (2008).
Xue, M. et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila complexins orchestrates synaptic vesicle exocytosis. Neuron 64, 367–380 (2009).
Lin, M. Y. et al. Complexin facilitates exocytosis and synchronizes vesicle release in two secretory model systems. J. Physiol. 591, 2463–2473 (2013).
Zhou, Q. J. et al. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 548, 420 (2017).
Chicka, M. C. & Chapman, E. R. Concurrent binding of complexin and synaptotagmin to liposome-embedded SNARE complexes. Biochemistry 48, 657–659 (2009).
Martin, J. A., Hu, Z., Fenz, K. M., Fernandez, J. & Dittman, J. S. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol. 21, 97–105 (2011).
Diao, J. et al. Complexin-1 enhances the on-rate of vesicle docking via simultaneous SNARE and membrane interactions. J. Am. Chem. Soc. 135, 15274–15277 (2013).
Snead, D., Wragg, R. T., Dittman, J. S. & Eliezer, D. Membrane curvature sensing by the C-terminal domain of complexin. Nat. Commun. 5, 4955 (2014).
Snead, D. et al. Unique structural features of membrane-bound C-terminal domain motifs modulate complexin inhibitory function. Front. Mol. Neurosci. 10, 154 (2017).
Jorquera, R. A., Huntwork-Rodriguez, S., Akbergenova, Y., Cho, R. W. & Littleton, J. T. Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J. Neurosci. 32, 18234–18245 (2012).
Bao, H. et al. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature 554, 260–263 (2018).
Das, D., Bao, H., Courtney, K. C., Wu, L. & Chapman, E. R. Resolving kinetic intermediates during the regulated assembly and disassembly of fusion pores. Nat. Commun. 11, 231 (2020).
Malsam, J. et al. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl Acad. Sci. USA 106, 2001–2006 (2009).
Lee, E. Y., Fulan, B. M., Wong, G. C. L. & Ferguson, A. L. Mapping membrane activity in undiscovered peptide sequence space using machine learning. Proc. Natl Acad. Sci. USA 113, 13588–13593 (2016).
Seiler, F., Malsam, J., Krause, J. M. & Sollner, T. H. A role of complexin-lipid interactions in membrane fusion. FEBS Lett. 583, 2343–2348 (2009).
Vogel, H. & Jahnig, F. The structure of melittin in membranes. Biophys. J. 50, 573–582 (1986).
Williams, R. W. et al. Raman spectroscopy of synthetic antimicrobial frog peptides magainin 2a and PGLa. Biochemistry 29, 4490–4496 (1990).
Tuerkova, A. et al. Effect of helical kink in antimicrobial peptides on membrane pore formation. eLife 9, 38 (2020).
Jonker, C. T. H. et al. Accurate measurement of fast endocytic recycling kinetics in real time. J. Cell Sci. 133, jcs231225 (2020).
Cho, R. W. et al. Phosphorylation of Complexin by PKA regulates activity-dependent spontaneous neurotransmitter release and structural synaptic plasticity. Neuron 88, 749–761 (2015).
Kaeser-Woo, Y. J., Yang, X. F. & Sudhof, T. C. C-terminal complexin sequence is selectively required for clamping and priming but not for Ca2+ triggering of synaptic exocytosis. J. Neurosci. 32, 2877–2885 (2012).
Hui, E., Johnson, C. P., Yao, J., Dunning, F. M. & Chapman, E. R. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell 138, 709–721 (2009).
Martens, S., Kozlov, M. M. & McMahon, H. T. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 (2007).
Dubochet, J. et al. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228 (1988).
Malsam, J. et al. Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release. EMBO J. 31, 3270–3281 (2012).
Wu, Z. Y. et al. Dilation of fusion pores by crowding of SNARE proteins. eLife 6, 26 (2017).
Shata, A., Saisu, H., Odani, S. & Abe, T. Phosphorylated synaphin/complexin found in the brain exhibits enhanced SNARE complex binding. Biochem. Biophys. Res. Commun. 354, 808–813 (2007).
Wong, Y. H. et al. KinasePhos 2.0: a web server for identifying protein kinase-specific phosphorylation sites based on sequences and coupling patterns. Nucleic Acids Res. 35, W588–W594 (2007).
Miller, M. L. & Blom, N. Kinase-specific prediction of protein phosphorylation sites. Methods Mol. Biol. 527, 299–310, x (2009).
Chapman, E. R., Alexander, K., Vorherr, T., Carafoli, E. & Storm, D. R. Fluorescence energy transfer analysis of calmodulin-peptide complexes. Biochemistry 31, 12819–12825 (1992).
Lee, M. T., Sun, T. L., Hung, W. C. & Huang, H. W. Process of inducing pores in membranes by melittin. Proc. Natl Acad. Sci. USA 110, 14243–14248 (2013).
Wu, L., Courtney, K. C. & Chapman, E. R. Cholesterol stabilizes recombinant exocytic fusion pores by altering membrane bending rigidity. Biophys. J. 120, 1367–1377 (2021).
Xue, M. et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 14, 949–958 (2007).
Chen, X. C. et al. Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409 (2002).
Buhl, L. K. et al. Differential regulation of evoked and spontaneous neurotransmitter release by C-terminal modifications of complexin. Mol. Cell. Neurosci. 52, 161–172 (2013).
Yang, X. F., Cao, P. & Sudhof, T. C. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc. Natl Acad. Sci. USA 110, 20777–20782 (2013).
Dhara, M. et al. Complexin synchronizes primed vesicle exocytosis and regulates fusion pore dynamics. J. Cell Biol. 204, 1123–1140 (2014).
Gong, J. H. et al. C-terminal domain of mammalian complexin-1 localizes to highly curved membranes. Proc. Natl Acad. Sci. USA 113, E7590–E7599 (2016).
Makke, M. et al. A mechanism for exocytotic arrest by the complexin C-terminus. eLife 7, 25 (2018).
Wragg, R. T. et al. Synaptic vesicles position complexin to block spontaneous fusion. Neuron 77, 323–334 (2013).
Sharpe, H. J., Stevens, T. J. & Munro, S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169 (2010).
Liu, T. Y. et al. Lipid interaction of the C terminus and association of the transmembrane segments facilitate atlastin-mediated homotypic endoplasmic reticulum fusion. Proc. Natl Acad. Sci. USA 109, E2146–E2154 (2012).
Podbilewicz, B. Virus and cell fusion mechanisms. Annu. Rev. Cell Dev. Biol. 30, 111–139 (2014).
Kozlov, M. M. & Chernomordik, L. V. The protein coat in membrane fusion: lessons from fission. Traffic 3, 256–267 (2002).
Daste, F. et al. The heptad repeat domain 1 of Mitofusin has membrane destabilization function in mitochondrial fusion. Embo Rep. 19, e43637 (2018).
Krawczyk, P. A., Laub, M. & Kozik, P. To kill but not be killed: controlling the activity of mammalian pore-forming proteins. Front Immunol. 11, 601405 (2020).
An, S. J., Grabner, C. P. & Zenisek, D. Real-time visualization of complexin during single exocytic events. Nat. Neurosci. 13, 577–U583 (2010).
Radhakrishnan, A. et al. Symmetrical arrangement of proteins under release-ready vesicles in presynaptic terminals. Proc. Natl Acad. Sci. USA 118, e2024029118 (2021).
Huntwork, S. & Littleton, J. T. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 10, 1235–1237 (2007).
Littleton, J. T., Stern, M., Perin, M. & Bellen, H. J. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc. Natl Acad. Sci. USA 91, 10888–10892 (1994).
Vevea, J. D. & Chapman, E. R. Acute disruption of the synaptic vesicle membrane protein synaptotagmin 1 using knockoff in mouse hippocampal neurons. eLife 9, 24 (2020).
Chapman, E. R. & Davis, A. F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273, 13995–14001 (1998).
Wang, P., Wang, C. T., Bai, J. H., Jackson, M. B. & Chapman, E. R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 278, 47030–47037 (2003).
Bai, J., Tucker, W. C. & Chapman, E. R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004).
Liu, H. S. et al. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat. Neurosci. 17, 670 (2014).
Bai, H. et al. Different states of synaptotagmin regulate evoked versus spontaneous release. Nat. Commun. 7, 9 (2016).
Bradberry, M. M. et al. Molecular basis for Synaptotagmin-1-associated neurodevelopmental disorder. Neuron 107, 52–64 (2020).
Liu, L. et al. Beyond amphiphilic balance: changing subunit stereochemistry alters the pore-forming activity of nylon-3 polymers. J. Am. Chem. Soc. 143, 3219–3230 (2021).
Yang, Z. et al. UCSF Chimera, MODELLER, and IMP: an integrated modeling system. J. Struct. Biol. 179, 269–278 (2012).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Jo, S., Lim, J. B., Klauda, J. B. & Im, W. CHARMM-GUI membrane builder for mixed bilayers and its application to yeast membranes. Biophys. J. 97, 50–58 (2009).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 114, 7830–7843 (2010).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Huang, J. & MacKerell, A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Ohkubo, Y. Z., Pogorelov, T. V., Arcario, M. J., Christensen, G. A. & Tajkhorshid, E. Accelerating membrane insertion of peripheral proteins with a novel membrane mimetic model. Biophys. J. 102, 2130–2139 (2012).
Qi, Y. et al. CHARMM-GUI HMMM builder for membrane simulations with the highly mobile membrane-mimetic model. Biophys. J. 109, 2012–2022 (2015).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 81, 511–519 (1984).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. Software X 1, 19–25 (2015).
Zhang, Z. & Chapman, E. R. Programmable nanodisc patterning by DNA origami. Nano Lett. 20, 6032–6037 (2020).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Volkmann, N. & Hanein, D. Quantitative fitting of atomic models into observed densities derived by electron microscopy. J. Struct. Biol. 125, 176–184 (1999).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Agulleiro, J. I. & Fernandez, J. J. Tomo3D 2.0-exploitation of advanced vector extensions (AVX) for 3D reconstruction. J. Struct. Biol. 189, 147–152 (2015).
van der Heide, P., Xu, X. P., Marsh, B. J., Hanein, D. & Volkmann, N. Efficient automatic noise reduction of electron tomographic reconstructions based on iterative median filtering. J. Struct. Biol. 158, 196–204 (2007).
Volkmann, N. A novel three-dimensional variant of the watershed transform for segmentation of electron density maps. J. Struct. Biol. 138, 123–129 (2002).
Goddard, T. D., Huang, C. C. & Ferrin, T. E. Software extensions to UCSF Chimera for interactive visualization of large molecular assemblies. Structure 13, 473–482 (2005).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Tucker, W. C., Weber, T. & Chapman, E. R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Acknowledgements
We thank the members of the Chapman laboratory and M.B. Jackson for helpful discussions. We also thank A. Ferguson (University of Chicago) for providing access to the AMP prediction tool and S. Mishra for preliminary work performing HEK cell patch-clamp recordings. This work was supported by Pew Charitable Trust grant no. 864K625 (E.R.C. and D.H.), National Institutes of Health grant nos. MH061876 and NS097362 (E.R.C.), P01-GM121203 (N.V.) and DP2GM140920 (H.B.). Equipment for the cryo-CLEM and in situ cellular tomography workflow used in this work was funded by National Institutes of Health grant nos. S10-OD012372 (D.H.), S10-OD026926 (D.H.), P01-GM121203 (N.V.) and R01-AI132378 (N.V., D.H.), and Pew Charitable Trust grant no. 864K625. The computational component is supported by the grant no. NSF-DMS1661900 (Q.C.) Computational resources from the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by NSF grant no. OCI-1053575 (Q.C.), are greatly appreciated; computations are also supported in part by the Shared Computing Cluster, which is administered by Boston University’s Research Computing Services. E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
K.C.C. was involved with the conceptualization; methodology; validation; formal analysis; investigation; original draft preparation, review and editing of the paper and visualization. L.W. was involved with the methodology, formal analysis, investigation, review and editing of the paper and visualization. T.M. was involved with the methodology, formal analysis, investigation, review and editing of the paper and visualization. M.S. was involved with the methodology and investigation. Z.Z. was involved with the formal analysis, investigation, review and editing of the paper and visualization. M.A. was involved with the formal analysis and investigation. Z.W. was involved with the formal analysis and investigation. M.M.B. was involved with the investigation and review and editing of the paper. C.D. was involved with the methodology and resources. L.D.L. was involved with the resources, supervision and funding acquisition. N.V. was involved with the formal analysis, investigation, supervision, review and editing of the paper, visualization and funding acquisition. D.H. was involved with the methodology, formal analysis, investigation, supervision, review and editing of the paper, visualization and funding acquisition. Q.C. was involved with the methodology, supervision and funding acquisition. H.B. was involved with the conceptualization; methodology; validation; formal analysis; investigation; original draft preparation, review and editing of the paper and visualization. E.R.C. was involved with the conceptualization; validation; resources; original draft preparation, review and editing of the paper; supervision and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural and Molecular Biology thanks Frederic Pincet and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The C-terminal alpha helix of Cpx creates pores in unilamellar vesicles and lyses spheroplasts.
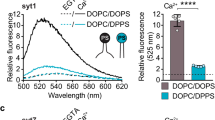
(a) Leakage of glutamate from LUVs composed of PC (black), PC/PS (blue) or total brain lipids (red) after treatment with increasing concentrations of Cpx, Cpx ΔCt21 and Ct21. Glutamate release was monitored via the glutamate sensor, iGluSnFR, in the media (1 µM). (b) Lysis of E. coli spheroplasts with increasing concentrations of Cpx, Cpx ΔCt21 and Ct21 peptide monitored via absorbance at 500 nm. Panels A and B represent the average of three independent experiments. Error bars represent standard error of the mean. (c) Image of the JF635i HaloTag ligand structure. (d) Representative fluorescence emission traces of 5 µM of the JF635i ligand, excited at 635 nm, with and without 5 µM of recombinant HaloTag protein. (e) GUVs labeled with rhodamine-PE (white), and with encapsulated HaloTag protein, before and after treatment with various concentrations of Cpx. JF635i (magenta) is present in the media; this dye strongly fluoresces upon entering GUVs and binding the HaloTag protein. (f) GUV leak assay, described in Fig. 3, performed with 1 µM of multiple Cpx isoforms. (g) GUV leak assay performed with 2 µM of the cytoplasmic domain of synaptotagmin 1 (syt1), bovine serum albumin (BSA), Munc18, α-SNAP, NSF or α-synuclein. Each of the GUV conditions were repeated at least three times.
Extended Data Fig. 2 Negative-stain TEM images of miscellaneous structures of Cpx-treated LUVs.
(a) A zoom-out image with four distinct features highlighted by colored boxes that were consistently observed from three independent experiments. Each feature belongs to a category of relevant structures, and more representative cropped images are given in panels b-e: (b) mouth-like opening (pink); (c) ring-like attachment (green); (d) discoidal structures (blue); (e) potential vesicle budding intermediates (yellow). Scale bars: 50 nm.
Extended Data Fig. 3 Electron cryo-tomography of liposomes before and after Cpx treatment.
Electron cryo-tomography of 100 nm LUVs with or without treatment of 10 µM Cpx. Three-dimensional surface representations and the corresponding two-dimensional cross sections are shown. In all cases, the scale bar represents 50 nm. The experiment was repeated twice. (a) Representative electron cryo-tomography of untreated 100 nm LUVs. (b) Representative electron cryo-tomography of 100 nm LUVs showing Cpx treatment often induced vesiculation. (c) Representative electron cryo-tomography of 100 nm LUVs showing Cpx treatment commonly causes LUVs to fragment into membranous networks. (d) Representative electron cryo-tomography of 100 nm LUVs showing Cpx treatment can cause LUVs to twist into highly curved structures.
Extended Data Fig. 4 Recombinant Cpx forms pores in the plasma membrane of mammalian cells.
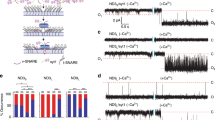
(a) Illustration of the HEK-293T cell patch-clamp setup. A typical cell-attached patch was formed on the surface of the cell, but using a pipette that contained WT full-length or ΔCt21 Cpx. (b) Representative electrophysiological recordings; in 7 out of 21 trials, WT Cpx generated positive currents. In the control or Cpx ΔCt21 conditions, no pores were observed in 5 and 8 trials, respectively. (c) Zoomed-in regions of interest (ROI), from the WT Cpx condition in panel b, showing transient positive currents that precede the large upswing in positive current.
Extended Data Fig. 5 Cpx activity can be regulated by phosphorylation and calmodulin.
(a) Illustration of the GUV leakage assay using Alexa 647 dye. Rhodamine-PE labelled GUVs, pseudo-colored in white, were formed in the presence of 10 µM Alexa 647 dye, followed by iso-osmolar buffer exchange. Upon pore formation, the dye, pseudo-colored in yellow, escapes from the lumen of the GUVs. b) Representative images of Control GUVs and after treatment with 5 µM Cpx (orange), Cpx ΔCt21 (purple), Cpx Ct21 scrambled (beige), Cpx (S115, T119D) (green), 10 µM Calmodulin (CaM) (light gray) or CaM + Cpx in EGTA (blue) and Ca2+ (red). The indicated conditions were independently repeated >3 times with consistent results. The sequence for the Cpx Ct21 scramble is found in Table 1. The white scale bar represents 10 µm. (c) Quantification of GUV lumenal fluorescence after the treatments described in panel B. The data are pooled from three independent experiments and the errors bars represent standard deviation. **** denotes p < 0.0001 and ns denotes not significant, determined by ANOVA analysis and Tukey multiple comparisons test. (d) Illustration of the Cpx-CaM binding assay. A single cysteine variant (L124C) of Cpx was labeled with an environment-sensitive dye, NBD, and the fluorescence emission was monitored by fluorometer. In the presence of Ca2+, CaM binds the Cpx C-terminal helix, causing an increase in fluorescence (e) Representative fluorescence spectrum of Cpx (L124C-NBD). NBD fluorescence in 25 mM HEPES (pH 7.4), 100 mM KCl was monitored without CaM (black) and with CaM in EGTA (blue) or Ca2+ (red). The change in NBD fluorescence in the Ca2+ condition indicates CaM binds the Cpx amphipathic helix.
Extended Data Fig. 6 Five copies of TMD-Cpx and three copies of TMD-Cpx ∆Ct21-melittin do not form SNARE-independent pores in the ND-BLM system.
(a) Illustration for TMD-Cpx (left panel) and TMD- Cpx ∆Ct21-melittin (right panel). The yellow asterisk indicates residue E114, the beginning of Ct21. (b) Sample trace from TMD-Cpx control experiments in the absence of syb2. Five copies of TMD-Cpx alone (no syb2) were reconstituted in the NDs. NDs with TMD-Cpx alone were added to the BLM system along with t-SNARE-liposomes, no pores formed, after 2 hrs. After NDs with syb2 were introduced, multiple pores opened. (c) Sample trace from TMD-Cpx ∆Ct21-melittin experiments in the absence of t-SNAREs. Three copies of syb2 and three copies of TMD-Cpx ∆Ct21-melittin were co-reconstituted in the NDs. NDs were introduced to the BLM system, no pore formed, after 2 hrs. After t-SNARE liposomes were added to the same system, multiple pores opened.
Extended Data Fig. 7 Truncation of the Cpx C-terminus causes fusion pore instability and reduces SUV lipid mixing.
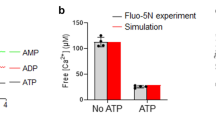
(a) Crystal structure from pdb 1KIL showing Cpx (shown in red) bound to the SNARE complex formed by syb2 (shown in yellow), SNAP25B (shown in orange) and syntaxin 1a (Syx1a) (shown in green). Cpx residues R48 and R59 are shown in cyan. (b) Lipid mixing assay performed with v-SNARE and t-SNARE containing vesicles over time. The t-SNARE SUVs remain constant, while the v-SNARE SUVs were reconstituted as syb2 alone (grey), syb2 + TMD-Cpx (blue), syb2 + TMD-Cpx ΔCt21 (orange) or syb2 + TMD-Cpx ΔCt21-SBM (beige). In addition to the syb2 alone condition, equimolar TMD-anchored Cpx variants were also separately co-reconstituted into the v-SNARE vesicles. The plot shows the average percent of lipid mixing over time from five individual experiments that each contained three technical replicates. The standard error of the mean from each condition is shown as dotted lines. (c) Initial lipid mixing rates for each of the listed conditions, derived from the plots in panel b. (d) Total lipid mixing for each condition after 60 minutes, derived from the plots in panel b. **** denotes p-value < 0.0001; *** denotes p-value < 0.001; ** denotes p-value < 0.01; * denotes p-value < 0.05 and error bars represent standard error of the mean.
Supplementary information
Supplementary Video 1
Cpx creates pores and deforms GUVs. GUVs, labeled with rhodamine-PE (white) were generated with encapsulated recombinant HaloTag protein. The external media contains the fluorogenic HaloTag ligand, JF635i (Magenta). Application of 5 µM Cpx creates pores in the GUV membrane, allowing the JF635i dye to enter and bind the HaloTag protein, causing a fluorescence increase. Membrane remodeling and vesiculation is also observed. Scale bar represents 20 µm.
Source data
Source Data Fig. 1
Figs. 1b,e source data.
Source Data Fig. 2
Figs. 2e,f,g source data.
Source Data Fig. 5
Figs. 5e,g source data.
Source Data Fig. 6
Figs. 6b,d source data.
Source Data Extended Data Fig. 1
Extended Data Fig. 1a,b.
Source Data Extended Data Fig. 5
Extended Data Fig. 5c.
Source Data Extended Data Fig. 7
Extended Data Fig. 7c,d.
Rights and permissions
About this article
Cite this article
Courtney, K.C., Wu, L., Mandal, T. et al. The complexin C-terminal amphipathic helix stabilizes the fusion pore open state by sculpting membranes. Nat Struct Mol Biol 29, 97–107 (2022). https://doi.org/10.1038/s41594-021-00716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00716-0