Abstract
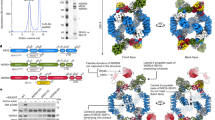
The Rag GTPases (Rags) recruit mTORC1 to the lysosomal membrane in response to nutrients, where it is then activated in response to energy and growth factor availability. The lysosomal folliculin (FLCN) complex (LFC) consists of the inactive Rag dimer, the pentameric scaffold Ragulator, and the FLCN:FNIP2 (FLCN-interacting protein 2) GTPase activating protein (GAP) complex, and prevents Rag dimer activation during amino acid starvation. How the LFC is disassembled upon amino acid refeeding is an outstanding question. Here we show that the cytoplasmic tail of the human lysosomal solute carrier family 38 member 9 (SLC38A9) destabilizes the LFC and thereby triggers GAP activity of FLCN:FNIP2 toward RagC. We present the cryo-EM structures of Rags in complex with their lysosomal anchor complex Ragulator and the cytoplasmic tail of SLC38A9 in the pre- and post-GTP hydrolysis state of RagC, which explain how SLC38A9 destabilizes the LFC and so promotes Rag dimer activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
EM density maps have been deposited in the EMDB with accession codes EMD-21686 (pre-GAP complex) and EMD-21687 (post-GAP complex). Atomic coordinates have been deposited in the PDB with accession codes 6WJ2 (pre-GAP complex) and 6WJ3 (post-GAP complex). Source data are provided with this paper.
References
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-019-0199-y (2020).
Kim, J. & Guan, K. L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 21, 63–71 (2019).
Lawrence, R. E. & Zoncu, R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019).
Sancak, Y. et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 (2008).
Kim, E., Goraksha-Hicks, P., Li, L., Neufeld, T. P. & Guan, K. L. Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–945 (2008).
Sancak, Y. et al. Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Teis, D., Wunderlich, W. & Huber, L. A. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3, 803–814 (2002).
Bar-Peled, L., Schweitzer, L. D., Zoncu, R. & Sabatini, D. M. Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 (2012).
Rogala, K. B. et al. Structural basis for the docking of mTORC1 on the lysosomal surface. Science https://doi.org/10.1126/science.aay0166 (2019).
Anandapadamanaban, M. et al. Architecture of human Rag GTPase heterodimers and their complex with mTORC1. Science 366, 203–210 (2019).
Rebsamen, M. et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481 (2015).
Wang, S. et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347, 188–194 (2015).
Jung, J., Genau, H. M. & Behrends, C. Amino acid-dependent mTORC1 regulation by the lysosomal membrane protein SLC38A9. Mol. Cell. Biol. 35, 2479–2494 (2015).
Wyant, G. A. et al. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 171, 642–654 (2017).
Scalise, M. et al. Insights into the transport side of the human SLC38A9 transceptor. Biochim. Biophys. Acta Biomembr. 1861, 1558–1567 (2019).
Lei, H. T., Ma, J., Sanchez Martinez, S. & Gonen, T. Crystal structure of arginine-bound lysosomal transporter SLC38A9 in the cytosol-open state. Nat. Struct. Mol. Biol. 25, 522–527 (2018).
Ma, J., Lei, H.-T. & Gonen, T. A conformational change in the N terminus of SLC38A9 signals mTORC1 activation. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/339937v1 (2018).
Shen, K. & Sabatini, D. M. Ragulator and SLC38A9 activate the Rag GTPases through noncanonical GEF mechanisms. Proc. Natl Acad. Sci. USA 115, 9545–9550 (2018).
Lawrence, R. E. et al. Structural mechanism of a Rag GTPase activation checkpoint by the lysosomal folliculin complex. Science 366, 971–977 (2019).
Tsun, Z. Y. et al. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell 52, 495–505 (2013).
Shen, K. et al. Cryo-EM structure of the human FLCN–FNIP2–Rag–Ragulator complex. Cell 179, 1319–1329 (2019).
Hong, S. B. et al. Inactivation of the FLCN tumor suppressor gene induces TFE3 transcriptional activity by increasing its nuclear localization. PLoS ONE 5, e15793 (2010).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Wada, S. et al. The tumor suppressor FLCN mediates an alternate mTOR pathway to regulate browning of adipose tissue. Genes Dev. 30, 2551–2564 (2016).
Betschinger, J. et al. Exit from pluripotency is gated by intracellular redistribution of the bHLH transcription factor Tfe3. Cell 153, 335–347 (2013).
Napolitano, G. et al. A substrate-specific mTORC1 pathway underlies Birt–Hogg–Dubé syndrome. Nature https://doi.org/10.1038/s41586-020-2444-0 (2020).
Settembre, C. et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108 (2012).
Napolitano, G. et al. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 9, 3312 (2018).
de Araujo, M. E. G. et al. Crystal structure of the human lysosomal mTORC1 scaffold complex and its impact on signaling. Science 358, 377–381 (2017).
Su, M. Y. et al. Hybrid structure of the RagA/C–Ragulator mTORC1 activation complex. Mol. Cell 68, 835–846 (2017).
Antonny, B., Beraud-Dufour, S., Chardin, P. & Chabre, M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36, 4675–4684 (1997).
Ahmadian, M. R., Wittinghofer, A. & Herrmann, C. Fluorescence methods in the study of small GTP-binding proteins. Methods Mol. Biol. 189, 45–63 (2002).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Scheres, S. H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife https://doi.org/10.7554/eLife.42166 (2018).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single particle cryo-EM reconstruction. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2019.12.15.877092v1 (2019).
Asarnow, D., Palovcak, E. & Cheng, Y. UCSF pyem v0.5 (Zenodo, 2019); https://doi.org/10.5281/zenodo.3576630
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods https://doi.org/10.1038/s41592-020-0914-9 (2020).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Jakobi, A. J., Wilmanns, M. & Sachse, C. Model-based local density sharpening of cryo-EM maps. Elife https://doi.org/10.7554/eLife.27131 (2017).
Burnley, T., Palmer, C. M. & Winn, M. Recent developments in the CCP-EM software suite. Acta Crystallogr. D Struct. Biol. 73, 469–477 (2017).
Scheres, S. H. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Acknowledgements
We thank R. Zoncu for comments on the manuscript, C. Hecksel for assistance in pre-GAP complex cryo-EM data acquisition, which was performed at the Stanford-SLAC Cryo-EM Center (S2C2) supported by the NIH Common Fund Transformative High Resolution Cryo-Electron Microscopy program (U24 GM129541), and D. Toso, J. Remis and P. Tobias for assistance in post-GAP complex cryo-EM data acquisition. This work was supported by NIH R01GM111730 (J.H.H.), an EMBO Long-Term Fellowship (S.A.F.) and a University of California Cancer Research Coordinating Committee Predoctoral Fellowship (R.E.L.).
Author information
Authors and Affiliations
Contributions
S.A.F. designed and carried out all experiments and carried out all data analysis. R.E.L. performed initial GEF and LFC disassembly experiments. S.A.F. and J.H.H. conceptualized the project and wrote the first manuscript draft. All authors contributed to editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.H.H. is a scientific founder and receives research funding from Casma Therapeutics.
Additional information
Peer review information Peer reviewer reports are available. Anke Sparmann was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Pre−GAP complex cryo-EM structure determination.
a, Exemplary raw cryo-EM micrograph at −2.2 μm defocus. Scale bar 50 nm. b, Power spectrum of micrograph shown in a with CTF estimation. c, Exemplary 2D class averages. Scale bar 150 Å. d, Cryo-EM data processing workflow. Used software is indicated with italic font. Red asterisk indicates the map used for model building to subsequently generate simulated maps w/o SLC38A9. e, Particle orientation distribution of the final particle set. f, Fourier shell correlation (FSC) of the final 3D reconstruction. g-h, Overlay of the final density map with the masks used during refinement (g, blue, transparent), FSC calculation (h, pink, transparent). i, Both masks from g and h overlaid with the final density map.
Extended Data Fig. 2 Pre−GAP complex atomic coordinate building and refinement.
a, Overlay of half-map (green) and map-model (purple) FSC to assess map to model agreement. b, Overlay of FSC work (blue) and FSC test (yellow) of the cross-validation test to assess overfitting. The refinement target resolution is indicated by a vertical dashed line. c, Final model composition and chain assignment. Parts not resolved by the cryo-EM density are represented by thin black lines. Red lines indicate regions where side chains are truncated to alanine. d, Model fit in the cryo-EM density (mesh) of selected regions. The threshold level used to display the density in UCSF Chimera is given in parentheses.
Extended Data Fig. 3 HDX-MS analysis of SLC38A9NT in isolation and bound to Rags.
a, Deuterium uptake and peptide coverage (grey lines) of SLC38A9NT in complex with inactive Rags (left) or in isolation (right) at 6, 60, 600 and 60,000 s exchange time. b, HDX difference plots of SLC38A9NT in complex with inactive Rags and in isolation at 60 (left), 600 (middle) and 60,000 s (right) exchange time. Plotted are the mean±SD of technical replicates (n = 3). c, Individual SLC38A9NT MS peptide spectra of selected peptides in isolation (black) and in complex with inactive Rags (red). Undeuterated reference spectra are shown at the top. Data for graphs in b are available as source data online.
Extended Data Fig. 4 Post-GAP complex cryo-EM structure determination.
a, Exemplary raw cryo-EM micrograph at −2.2 μm defocus. Scale bar 50 nm. b, Power spectrum of micrograph shown in a with CTF estimation. c, Exemplary 2D class averages. Scale bar 150 Å. d, Cryo-EM data processing workflow. Used software is indicated with italic font. e, Particle orientation distribution of the final particle set. f, Fourier shell correlation (FSC) of the final 3D reconstruction.
Extended Data Fig. 5 Post-GAP complex atomic coordinate building and refinement.
a, Overlay of half-map (green) and map-model (purple) FSC to assess map to model agreement. b, Overlay of FSC work (blue) and FSC test (yellow) of the cross-validation test to assess overfitting. The refinement target resolution is indicated by a vertical dashed line. c, Model fit in the cryo-EM density (mesh) of selected regions. The threshold level used to display the density in UCSF Chimera is given in parentheses.
Extended Data Fig. 6 Overview of Rag GTPase structures in different states.
a–g, Top (middle) and side view (right) of published Rag GTPase structures in surface representation (cyan, RagA G domain; blue, RagC G domain; pink, GTP or GTP analogue; red, GDP or GDP analogue; yellow, RagA or RagC switch I region; grey, RagA or RagC C-terminal roadblock domain). The dashed line connects the Cα atoms of RagA Trp165 and RagC Tyr221 representing the width of the G domain cleft. The two vertical solid lines represent the RagA Trp165 and RagC Tyr221 Cα position in a. PDB codes, experimental method, de facto nucleotide state and Rag binding partners (if any) are summarized on the left.
Extended Data Fig. 7 Tryptophan fluorescence-based RagC XTPase assay.
a, Intrinsic tryptophan fluorescence based RagC XTPase assay of Ragulator-RagAGDP:RagCXTP in the absence (blue) and presence (orange) of SLC38A9NT. Tryptophan fluorescence of a Ragulator-RagAGTP:RagCXTP substrate used in the same assay serves as a positive control (green). Plotted is the mean ± SD mantGDP fluorescence at each time point of one experiment performed in quadruplicates (n = 4). The experiment has been performed twice with similar results. norm., normalized. b, Top view of the pre-GAP complex structure illustrating the SLC38A9NT-RagC switch I and GDP (RagA, red) interaction. SLC38A9 (yellow) and RagC switch I (blue) are displayed in surface representation. Data for graph in a is available as source data online.
Supplementary information
Source data
Source Data Fig. 1
Source data for SEC and statistical source data for HPLC-based XTPase assay.
Source Data Fig. 1
Unprocessed SDS gels.
Source Data Fig. 3
Statistical source data for HDX-MS experiment.
Source Data Fig. 4
Source data for SEC.
Source Data Fig. 4
Unprocessed SDS gels.
Source Data Fig. 5
Statistical source data for HPLC and mantGDP-based GEF assays.
Source Data Extended Data Fig. 3
Statistical source data for HDX-MS experiment.
Source Data Extended Data Fig. 7
Statistical source data for Trp fluorescence–based GEF assay.
Rights and permissions
About this article
Cite this article
Fromm, S.A., Lawrence, R.E. & Hurley, J.H. Structural mechanism for amino acid-dependent Rag GTPase nucleotide state switching by SLC38A9. Nat Struct Mol Biol 27, 1017–1023 (2020). https://doi.org/10.1038/s41594-020-0490-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-020-0490-9
This article is cited by
-
Sex differences in response to obesity and caloric restriction on cognition and hippocampal measures of autophagic-lysosomal transcripts and signaling pathways
BMC Neuroscience (2024)
-
The eukaryotic-like characteristics of small GTPase, roadblock and TRAPPC3 proteins from Asgard archaea
Communications Biology (2024)
-
The Ragulator complex: delving its multifunctional impact on metabolism and beyond
Inflammation and Regeneration (2023)
-
Structure of the lysosomal mTORC1–TFEB–Rag–Ragulator megacomplex
Nature (2023)
-
The molecular basis of nutrient sensing and signalling by mTORC1 in metabolism regulation and disease
Nature Reviews Molecular Cell Biology (2023)