Abstract
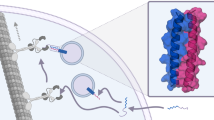
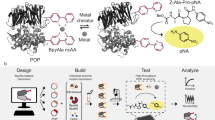
Natural or engineered peptides serve important biological functions. A general approach to achieve chemical-dependent activation of short peptides will be valuable for spatial and temporal control of cellular processes. Here we present a pair of chemically activated protein domains (CAPs) for controlling the accessibility of both the N- and C-terminal portion of a peptide. CAPs were developed through directed evolution of an FK506-binding protein. By fusing a peptide to one or both CAPs, the function of the peptide is blocked until a small molecule displaces them from the FK506-binding protein ligand-binding site. We demonstrate that CAPs are generally applicable to a range of short peptides, including a protease cleavage site, a dimerization-inducing heptapeptide, a nuclear localization signal peptide, and an opioid peptide, with a chemical dependence up to 156-fold. We show that the CAPs system can be utilized in cell cultures and multiple organs in living animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the paper and its supplementary information files. PDB-1FAP, PDB-1NSG, and PDB-6DDF cited in this study are available at Protein Data Bank. All the DNA constructs used in this study are available upon request to the corresponding author. Source data are provided with this paper.
Code availability
The GROMACS software package including source code is freely available at https://www.gromacs.org and https://gitlab.com/gromacs/gromacs and includes implementations of all simulation and analysis algorithms used for this project. The VMD (Visual Molecular Dynamics) package used to visualize the simulated proteins is available from https://www.ks.uiuc.edu/Research/vmd.
References
Song, H. K. & Eck, M. J. Structural basis of degradation signal recognition by SspB, a specificity-enhancing factor for the ClpXP proteolytic machine. Mol. Cell 12, 75–86 (2003).
Yi, J. J., Wang, H., Vilela, M., Danuser, G. & Hahn, K. M. Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides. ACS Synth. Biol. 3, 788–795 (2014).
Matthew, A. N. et al. Hepatitis C virus NS3/4A protease inhibitors incorporating flexible P2 quinoxalines target drug resistant viral variants. J. Med. Chem. 60, 5699–5716 (2017).
Dingwall, C. & Laskey, R. A. Nuclear target sequences a consensus? Tibs 16, 478–481 (1991).
Xu, D., Farmer, A., Collett, G., Grishin, N. V. & Chook, Y. M. Sequence and structural analyses of nuclear export signals in the NESdb database. Mol. Biol. Cell 23, 3677–3693 (2012).
Renicke, C., Schuster, D., Usherenko, S., Essen, L.-O. & Taxis, C. A LOV2 domain-based optogenetic tool to control protein degradation and cellular function. Chem. Biol. 20, 619–626 (2013).
Bonger, K. M., Chen, L. C., Liu, C. W. & Wandless, T. J. Small-molecule displacement of a cryptic degron causes conditional protein degradation. Nat. Chem. Biol. 7, 531–537 (2011).
Wong, S., Mosabbir, A. A. & Truong, K. An engineered split intein for photoactivated protein trans-splicing. PLoS One 10, 1–16 (2015).
So, W. H., Wong, C. T. T. & Xia, J. Peptide photocaging: a brief account of the chemistry and biological applications. Chin. Chem. Lett. 29, 1058–1062 (2018).
Renner, C. & Moroder, L. Azobenzene as conformational switch in model peptides. Chembiochem 7, 868–878 (2006).
Lungu, O. I. et al. Designing photoswitchable peptides using the AsLOV2 domain. Chem. Biol. 19, 507–517 (2012).
Bonger, K. M., Rakhit, R., Payumo, A. Y., Chen, J. K. & Wandless, T. J. General method for regulating protein stability with light. ACS Chem. Biol. 9, 111–115 (2014).
Niopek, D. et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat. Commun. 5, 4404 (2014).
Yumerefendi, H. et al. Control of protein activity and cell fate specification via light-mediated nuclear translocation. PLoS One 10, e0128443 (2015).
Niopek, D., Wehler, P., Roensch, J., Eils, R. & Di Ventura, B. Optogenetic control of nuclear protein export. Nat. Commun. 7, 1–9 (2016).
Guntas, G. et al. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl Acad. Sci. USA 112, 112–117 (2015).
Konold, P. E. et al. Unfolding of the C-terminal Jα helix in the LOV2 photoreceptor domain observed by time-resolved vibrational spectroscopy. J. Phys. Chem. Lett. 7, 3472–3476 (2016).
Peter, E., Dick, B. & Baeurle, S. A. Mechanism of signal transduction of the LOV2-Jα photosensor from Avena sativa. Nat. Commun. 1, 122–127 (2010).
He, L. et al. Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering. Nat. Chem. Biol. 17, 915–923 (2021).
Geng, L., Shen, J. & Wang, W. Circularly permuted AsLOV2 as an optogenetic module for engineering photoswitchable peptides. Chem. Commun. 57, 8051–8054 (2021).
Ruggiero, E., Alonso-de Castro, S., Habtemariam, A. & Salassa, L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: new opportunities and challenges in medicinal inorganic photochemistry. Dalt. Trans. 45, 13012–13020 (2016).
Stanton, B. Z., Chory, E. J. & Crabtree, G. R. Chemically induced proximity in biology and medicine. Science 359, eaao5902 (2018).
Wu, H. D. et al. Rational design and implementation of a chemically inducible heterotrimerization system. Nat. Methods 17, 928–936 (2020).
Karginov, A. V., Ding, F., Kota, P., Dokholyan, N. V. & Hahn, K. M. Engineered allosteric activation of kinases in living cells. Nat. Biotechnol. 28, 743–747 (2010).
Farrants, H. et al. Chemogenetic control of nanobodies. Nat. Methods 17, 279–282 (2020).
Clackson, T. et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl Acad. Sci. USA 95, 10437–10442 (1998).
Lamming, D. W. Inhibition of the mechanistic target of rapamycin (mTOR)—rapamycin and beyond. Cold Spring Harb. Perspect. Med. 6, a025924 (2016).
Banaszynski, L. A., Sellmyer, M. A., Contag, C. H., Wandless, T. J. & Thorne, S. H. Chemical control of protein stability and function in living mice. Nat. Med. 14, 1123–1127 (2008).
Wang, W. et al. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol. 35, 864–871 (2017).
Lee, D. et al. Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain. Nat. Methods 14, 495–503 (2017).
Kapust, R. B., Tözsér, J., Copeland, T. D. & Waugh, D. S. The P1′ specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun. 294, 949–955 (2002).
Shenoy, S. K. & Lefkowitz, R. J. Multifaceted roles of β-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem. J. 375, 503–515 (2003).
Vega, F. M. & Ridley, A. J. Rho GTPases in cancer cell biology. FEBS Lett. 582, 2093–2101 (2008).
Mellor, H. & Parker, P. J. The extended protein kinase C superfamily. Biochem. J. 332, 281–292 (1998).
Hogan, P. G. The STIM1–ORAI1 microdomain. Cell Calcium 58, 357–367 (2015).
Nguyen, T., Pappireddi, N. & Wühr, M. Proteomics of nucleocytoplasmic partitioning. Curr. Opin. Chem. Biol. 48, 55–63 (2019).
Wacker, D., Stevens, R. C. & Roth, B. L. How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–427 (2017).
Mansour, A., Hoversten, M. T., Taylor, L. P., Watson, S. J. & Akil, H. The cloned μ, δ and κ receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 700, 89–98 (1995).
Koehl, A. et al. Structure of the µ-opioid receptor–Gi protein complex. Nature 558, 547–552 (2018).
Guan, X. M., Kobilka, T. S. & Kobilka, B. K. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J. Biol. Chem. 267, 21995–21998 (1992).
Fan, F. et al. Novel genetically encoded biosensors using firefly luciferase. ACS Chem. Biol. 3, 346–351 (2008).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Che, T., Dwivedi-Agnihotri, H., Shukla, A. K. & Roth, B. L. Biased ligands at opioid receptors: current status and future directions. Sci. Signal. 14, 1–11 (2021).
Ptashne, M. & Gann, A. Transcriptional activation by recruitment. Nature 386, 569–577 (1997).
Richman, S. A. et al. Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo. Mol. Ther. 28, 1600–1613 (2020).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Pham, E., Mills, E. & Truong, K. A synthetic photoactivated protein to generate local or global Ca2+ signals. Chem. Biol. 18, 880–890 (2011).
Baarlink, C., Wang, H. & Grosse, R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 340, 864–867 (2013).
Cosentino, C. et al. Engineering of a light-gated potassium channel. Science 348, 707–710 (2015).
McCauley, J. A. & Rudd, M. T. Hepatitis C virus NS3/4a protease inhibitors. Curr. Opin. Pharmacol. 30, 84–92 (2016).
Cunningham-Bryant, D. et al. A chemically disrupted proximity system for controlling dynamic cellular processes. J. Am. Chem. Soc. 141, 3352–3355 (2019).
Rose, J. C. et al. A computationally engineered RAS rheostat reveals RAS–ERK signaling dynamics. Nat. Chem. Biol. 13, 119–126 (2017).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
Abraham, M. J. et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinforma. 8, 1950–1958 (2010).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926 (1983).
Li, P. et al. Brain circuit of claustrophobia-like behavior in mice identified by upstream tracing of sighing. Cell Rep. 31, 107779 (2020).
Acknowledgements
H. Jiang helped with TEV protease expression and purification and neuronal culture preparation. L. Wen helped with cloning and imaging. FACS was performed at the Flow Cytometry Core at the University of Michigan. This study is supported by the University of Michigan and National Institutes of Health (NIH) grants DP2MH132939, R01AT011652, and R01HL156989. M.H. gratefully acknowledges financial support from Research Corporation for Science Advancement (#27360). K.K. is supported by NIH F31MH12915001.
Author information
Authors and Affiliations
Contributions
W.W., J.S., L.G., and P.L. conceived the project idea. W.W., J.S., and L.G. designed the yeast cell-based and cell culture experiments. P.L., X.L., C.E., W.W., J.S., and L.G. designed the animal experiments. J.S. performed the CapN evolution and L.G. performed the CapC evolution. L.G. performed cell culture application of CAPs caging TEVcs, and characterization of shield-1 concentration and incubation time in the transgene transcription system. J.S., L.G., and K.L. performed application of CapC caging enkephalin. J.S., K.K., and G.S. performed application of CAPs caging SsrA for translocation. J.S. performed all other cell culture application and characterization. X.L. and C.E. performed the animal experiments. M.H. performed the simulation experiment. All authors analyzed data, wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing interests. A patent application has been filed by W.W., P.L., J.S., and L.G. with title ‘Chemogenetic Regulation of Peptide Function,’ U.S. Provisional Patent Application No. 63/329,736, filed April 11, 2022; applicants: the Regents of the University of Michigan; patent pending. All other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks John Ngo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Ligand binding site of FKBP and directed evolution results of CapN.
Related to main Fig. 1. a, Crystal structure of FKBP12 (PDB:1FAP). The hydrophobic residues around the ligand binding site are shown in yellow and stick representation. b, Sequences of forty clones from the post 4th round CapN library as shown in main Fig. 1e. Twenty-three distinct sequences were identified. Clone #1 is the final CapN used for the rest of this study. c, FACS analysis of the most enriched eight clones, corresponding to clones #1-#8 shown in b. Values are median HA intensity of FLAG-positive cells (Q2 + Q4). All eight clones showed similar results. This experiment was performed once.
Extended Data Fig. 2 All-atom molecular dynamics simulations.
Results from a 2 microsecond molecular dynamics simulations of FKBP and a capped ArgTyrSerProAsnLeu peptide in 150 mM buffer. a, the central configurations for the top 5 clusters (Rank 1–5) obtained from RMSD clustering indicate direct interactions between Leu6 of the peptide (shown in a’licorice’ representation; cap residues are shown in green, other atoms in CPK colors with gray carbons) and the F36V binding site of FKBP (shown as van-der-Waals spheres). The secondary structure of the FKBP protein is shown in a cartoon representation with red 𝛼-helices and yellow 𝛽-sheets. b, RMSD time traces with respect to the structures shown in a indicate the longevity of the respective conformations within the simulations. RMSD’s of 0 indicate the simulation time points corresponding to the structures in a. A horizontal dashed line indicates the 1.5 Å cutoff used for clustering. c, time traces of the center of mass distances between each individual sidechain of the peptide and the sidechain of the F36V binding site indicate a persistent proximity of Leu6 to the binding site for a large fraction of the simulation trajectory (distances of 5–6 Å). Fractions of the simulation trajectory with close proximity of Leu6 to the F36V binding site include all configurations associated with the top 5 clusters shown in a.
Extended Data Fig. 3 Directed evolution results of CapC.
Related to main Fig. 2. a, Sequences of twenty clones from the post 2nd round CapC library as shown in main Fig. 2c. Eighteen distinct sequences were identified and characterized. One sequence with early stop codon is not shown. Clone #18 is the final CapC used for the rest of this study. b, FACS analysis of the eighteen clones shown in a. Values are median HA intensity of FLAG-positive cells (Q2 + Q4). This experiment was performed once.
Extended Data Fig. 4 Comparison of single CapN, single CapC, and tandem CAPs in caging TEVcs.
a, Scheme of the three constructs tested. CapN-TEVcs-CapC is the combined use of both post-evolution CAPs. b, FACS plots of the three constructs shown in a. Values are median biotin intensity of FLAG-positive cells (Q2 + Q4). This experiment used a stronger TEV protease condition than main Figs. 1e and 1f. See method section for details. Protease cleavage (‘+ shield-1’ or ‘− shield-1’) is defined as the difference of the median HA signal between the + protease and – protease conditions. The dynamic ranges are calculated by the ratio of protease cleavage of the ‘+ shield-1’ and ‘− shield-1’ conditions. This experiment was performed once.
Extended Data Fig. 5 µOR peptide ligands and binding pocket, and effect of different drugs in the control study.
Related to main Fig. 4c, d. a, Left: Structure of [Met5]-enkephalin and its analog DAMGO. Right: Crystal structure of µOR binding pocket. PDB: 6DDF. b, Scheme and sequence of the constructs. SS, signal sequence (KTIIALSYIFCLVFA) is cleaved before the protein is trafficked to plasma membrane. Enkephalin (YGGFM). Left: Construct of CapC caged enkephalin fused to µOR. Right: Construct of µOR only used for control study. c, Cells transfected with µOR construct were stimulated with forskolin (1 µM) at 15 min, and then different drugs (10 µM) at 45 min. Top: Four conditions plotted on the same graph. Bottom: Four conditions plotted on separate graphs. From left to right: no drug, naloxone, shield-1, DAMGO. n = 3 wells from one replicate for all conditions. Errors are standard error of the mean. This experiment was performed once.
Extended Data Fig. 6 Effect of shield-1 on transcription-activation domain expression.
Related to main Fig. 5. a, Quantification of reporter gene activation level (mCherry, left) and transcription-activation domain expression marker level (EGFP, right). n = 12 fields of view from one replicate for all conditions. b, No difference in reporter expression was found between the + shield-1 and – shield-1 conditions in the control study with SspB-EGFP-VP16 only. n = 12 fields of view from one replicate for all conditions. All scale bars, 50 µm. P values are determined by unpaired two-tailed t-tests. **P < 0.01; ***P < 0.001; ****P < 0.0001; NS, not significant. This experiment was performed three times with similar results.
Extended Data Fig. 7 Dose response curve of CapC and CAPs caged SsrA.
The mean mCherry intensities of EGFP positive cell population are plotted against shield-1 concentration. Half maximum response was observed at 21 nM for CapC single-caged SsrA (95% confidence interval = 19 nM ~ 24 nM) and 55 nM for CAPs double-caged SsrA (95% confidence interval = 51 nM ~ 59 nM). n = 3 technical replicates for all conditions. Errors are standard error of the mean. Source FACS data is shown in Supplementary Fig. 8. This experiment was performed once.
Supplementary information
Supplementary Information
Supplementary Figs 1–14, Supplementary Method 1, Supplementary Table, References.
Supplementary Video
Video of shield-1 induced protein translocation to plasma membrane.
Supplementary Data
Statistical data of Supplementary Figs 1, 7, 9, 12.
Source data
Source Data Fig. 3
Statistical data in graphs.
Source Data Fig. 4
Statistical data in graphs.
Source Data Fig. 5
Statistical data in graphs.
Source Data Fig. 6
Statistical data in graphs.
Source Data Extended Data Fig. 2b
Simulation data in graphs.
Source Data Extended Data Fig. 2c
Simulation data in graphs.
Source Data Extended Data Fig. 5
Statistical data in graphs.
Source Data Extended Data Fig. 6
Statistical data in graphs.
Source Data Extended Data Fig. 7
Statistical data in graphs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Geng, L., Li, X. et al. A general method for chemogenetic control of peptide function. Nat Methods 20, 112–122 (2023). https://doi.org/10.1038/s41592-022-01697-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-022-01697-8