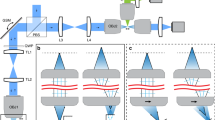
Abstract
Temporal focusing, with its ability to focus light in time, enables scanless illumination of large surface areas at the sample with micrometer axial confinement and robust propagation through scattering tissue. In conventional two-photon microscopy, widely used for the investigation of intact tissue in live animals, images are formed by point scanning of a spatially focused pulsed laser beam, resulting in limited temporal resolution of the excitation. Replacing point scanning with temporally focused widefield illumination removes this limitation and represents an important milestone in two-photon microscopy. Temporal focusing uses a diffusive or dispersive optical element placed in a plane conjugate to the objective focal plane to generate position-dependent temporal pulse broadening that enables axially confined multiphoton absorption, without the need for tight spatial focusing. Many techniques have benefitted from temporal focusing, including scanless imaging, super-resolution imaging, photolithography, uncaging of caged neurotransmitters and control of neuronal activity via optogenetics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Göppert-Mayer, M. Elementary processes with two quantum transitions. Ann. Phys. 18, 466–479 (2009).
Kaiser, W. & Garrett, C. G. B. Two-photon excitation in CaF2:Eu2+. Phys. Rev. Lett. 7, 229–232 (1961).
Abella, I. D. Optical double-photon absorption in cesium vapor. Phys. Rev. Lett. 9, 453–455 (1962).
Peticolas, W. L., Goldsborough, J. P. & Rieckhoff, K. E. Double photon excitation in organic crystals. Phys. Rev. Lett. 10, 43–45 (1963).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005). Demonstration of scanless TF microscopy for imaging and demonstration of two-photon widefield fluorescence images with optical sectioning. The technique was presented at the same time by the Xu lab (see ref. 6).
Zhu, G., van Howe, J., Durst, M., Zipfel, W. & Xu, C. Simultaneous spatial and temporal focusing of femtosecond pulses. Opt. Express 13, 2153–2159 (2005). Presentation of the technique of TF together with Silberberg lab (see ref. 5). Theoretical analysis and experimental characterization of the evolution of the temporally focused pulses along the propagation direction.
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
König, K. Multiphoton microscopy in life sciences. J. Microsc. 200, 83–104 (2000).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Göbel, W. & Helmchen, F. In vivo calcium imaging of neural network function. Physiology (Bethesda) 22, 358–365 (2007).
Pettit, D. L., Wang, S. S., Gee, K. R. & Augustine, G. J. Chemical two-photon uncaging: a novel approach to mapping glutamate receptors. Neuron 19, 465–471 (1997).
Oron, D., Papagiakoumou, E., Anselmi, F. & Emiliani, V. Two-photon optogenetics. Prog. Brain Res. 196, 119–143 (2012).
Conchello, J. A. & Lichtman, J. W. Optical sectioning microscopy. Nat. Methods 2, 920–931 (2005).
Salomé, R. et al. Ultrafast random-access scanning in two-photon microscopy using acousto-optic deflectors. J. Neurosci. Methods 154, 161–174 (2006).
Duemani Reddy, G., Kelleher, K., Fink, R. & Saggau, P. Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity. Nat. Neurosci. 11, 713–720 (2008).
Cheng, A., Gonçalves, J. T., Golshani, P., Arisaka, K. & Portera-Cailliau, C. Simultaneous two-photon calcium imaging at different depths with spatiotemporal multiplexing. Nat. Methods 8, 139–142 (2011).
Ducros, M., Goulam Houssen, Y., Bradley, J., de Sars, V. & Charpak, S. Encoded multisite two-photon microscopy. Proc. Natl Acad. Sci. USA 110, 13138–13143 (2013).
Theer, P., Hasan, M. T. & Denk, W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Barretto, R. P. J., Messerschmidt, B. & Schnitzer, M. J. In vivo fluorescence imaging with high-resolution microlenses. Nat. Methods 6, 511–512 (2009).
Wang, K. et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nat. Methods 11, 625–628 (2014).
Wang, K. et al. Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nat. Commun. 6, 7276 (2015).
Ji, N., Freeman, J. & Smith, S. L. Technologies for imaging neural activity in large volumes. Nat. Neurosci. 19, 1154–1164 (2016).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat. Methods 9, 201–208 (2012).
Nadella, K. M. N. S. et al. Random-access scanning microscopy for 3D imaging in awake behaving animals. Nat. Methods 13, 1001–1004 (2016).
Stirman, J. N., Smith, I. T., Kudenov, M. W. & Smith, S. L. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 (2016).
Sofroniew, N. J., Flickinger, D., King, J. & Svoboda, K. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. eLife 5, e14472 (2016).
Tal, E., Oron, D. & Silberberg, Y. Improved depth resolution in video-rate line-scanning multiphoton microscopy using temporal focusing. Opt. Lett. 30, 1686–1688 (2005).
Oron, D. & Silberberg, Y. Harmonic generation with temporally focused ultrashort pulses. J. Opt. Soc. Am. B 22, 2660–2663 (2005).
Oron, D. & Silberberg, Y. Spatiotemporal coherent control using shaped, temporally focused pulses. Opt. Express 13, 9903–9908 (2005).
Durst, M. E., Straub, A. A. & Xu, C. Enhanced axial confinement of sum-frequency generation in a temporal focusing setup. Opt. Lett. 34, 1786–1788 (2009).
Vaziri, A., Tang, J., Shroff, H. & Shank, C. V. Multilayer three-dimensional super resolution imaging of thick biological samples. Proc. Natl Acad. Sci. USA 105, 20221–20226 (2008).
Prevedel, R. et al. Fast volumetric calcium imaging across multiple cortical layers using sculpted light. Nat. Methods 13, 1021–1028 (2016).
Weisenburger, S. et al. Volumetric Ca2+ imaging in the mouse brain using hybrid multiplexed sculpted light microscopy. Cell 177, 1050–1066.e14 (2019). Demonstration of fast (17-Hz), volumetric (1 × 1 × 1.2 mm 3) in-depth in vivo functional imaging using a hybrid microscope including 2P-TF and 3P scanning imaging.
Rickgauer, J. P., Deisseroth, K. & Tank, D. W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824 (2014). Demonstration of in vivo all-optical neuronal circuits manipulation using temporally focused Gaussian beams.
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008). Demonstration of the combination of TF with holographic light shaping.
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010). Demonstration of in vitro scanless 2P optogenetic activation of multiple cells and multiple cell processes combining TF and the generalized phase-contrast method.
Chen, I. W., Papagiakoumou, E. & Emiliani, V. Towards circuit optogenetics. Curr. Opin. Neurobiol. 50, 179–189 (2018).
Vitek, D. N. et al. Spatio-temporally focused femtosecond laser pulses for nonreciprocal writing in optically transparent materials. Opt. Express 18, 24673–24678 (2010).
Vitek, D. N. et al. Temporally focused femtosecond laser pulses for low numerical aperture micromachining through optically transparent materials. Opt. Express 18, 18086–18094 (2010).
He, F. et al. Fabrication of microfluidic channels with a circular cross section using spatiotemporally focused femtosecond laser pulses. Opt. Lett. 35, 1106–1108 (2010).
Block, E. et al. Simultaneous spatial and temporal focusing for tissue ablation. Biomed. Opt. Express 4, 831–841 (2013).
Kim, D. & So, P. T. C. High-throughput three-dimensional lithographic microfabrication. Opt. Lett. 35, 1602–1604 (2010).
Spesyvtsev, R., Rendall, H. A. & Dholakia, K. Wide-field three-dimensional optical imaging using temporal focusing for holographically trapped microparticles. Opt. Lett. 40, 4847–4850 (2015).
Sun, B. et al. Four-dimensional light shaping: manipulating ultrafast spatiotemporal foci in space and time. Light Sci. Appl. 7, 17117 (2018).
Zhang, S., Asoubar, D., Kammel, R., Nolte, S. & Wyrowski, F. Analysis of pulse front tilt in simultaneous spatial and temporal focusing. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 31, 2437–2446 (2014).
Du, R. et al. Analysis of fast axial scanning scheme using temporal focusing with acousto-optic deflectors. J. Mod. Opt. 56, 81–84 (2009).
Da Sie, Y. et al. Bioimaging via temporal focusing multiphoton excitation microscopy with binary digital-micromirror-device holography. J. Biomed. Opt. 1604, 18086–18094 (2011).
Papagiakoumou, E., de Sars, V., Emiliani, V. & Oron, D. Temporal focusing with spatially modulated excitation. Opt. Express 17, 5391–5401 (2009).
Vaziri, A. & Shank, C. V. Ultrafast widefield optical sectioning microscopy by multifocal temporal focusing. Opt. Express 18, 19645–19655 (2010).
Choi, H. et al. Improvement of axial resolution and contrast in temporally focused widefield two-photon microscopy with structured light illumination. Biomed. Opt. Express 4, 995–1005 (2013).
Cheng, L.-C. et al. Nonlinear structured-illumination enhanced temporal focusing multiphoton excitation microscopy with a digital micromirror device. Biomed. Opt. Express 5, 2526–2536 (2014).
Hu, Y. S., Zimmerley, M., Li, Y., Watters, R. & Cang, H. Single-molecule super-resolution light-sheet microscopy. Chemphyschem. 15, 577–586 (2014).
Bovetti, S. et al. Simultaneous high-speed imaging and optogenetic inhibition in the intact mouse brain. Sci. Rep. 7, 40041 (2017).
Dana, H. et al. Hybrid multiphoton volumetric functional imaging of large-scale bioengineered neuronal networks. Nat. Commun. 5, 3997 (2014). Application of line-scanning TF for volumetric functional imaging in bioengineered neuronal tissue.
Picot, A. et al. Temperature rise under two-photon optogenetic brain stimulation. Cell Reports 24, 1243–1253 (2018).
Therrien, O. D., Aubé, B., Pagès, S., Koninck, P. D. & Côté, D. Wide-field multiphoton imaging of cellular dynamics in thick tissue by temporal focusing and patterned illumination. Biomed. Opt. Express 2, 696–704 (2011).
Schrödel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nat. Methods 10, 1013–1020 (2013).
Padgett, M. J. & Boyd, R. W. An introduction to ghost imaging: quantum and classical. Philos. Trans. A Math. Phys. Eng. Sci. 375, 20160233, https://doi.org/10.1098/rsta.2016.0233 (2017).
Toda, K. et al. Temporal focusing microscopy using three-photon excitation fluorescence with a 92-fs Yb-fiber chirped pulse amplifier. Biomed. Opt. Express 8, 2796–2806 (2017).
Rowlands, C. J. et al. Wide-field three-photon excitation in biological samples. Light Sci. Appl. 6, e16255 (2017).
Feldbauer, K. et al. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl Acad. Sci. USA 106, 12317–12322 (2009).
Andrasfalvy, B. K., Zemelman, B. V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl Acad. Sci. USA 107, 11981–11986 (2010).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 1171–1179 (2012).
Packer, A. M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1205 (2012).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl Acad. Sci. USA 106, 15025–15030 (2009).
Yang, W., Carrillo-Reid, L., Bando, Y., Peterka, D. S. & Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife 7, e32671 (2018).
Marshel, J. H. et al. Cortical layer–specific critical dynamics triggering perception. Science 365, eaaw5202 (2019).
Bègue, A. et al. Two-photon excitation in scattering media by spatiotemporally shaped beams and their application in optogenetic stimulation. Biomed. Opt. Express 4, 2869–2879 (2013).
Ronzitti, E. et al. Submillisecond optogenetic control of neuronal firing with two-photon holographic photoactivation of Chronos. J. Neurosci. 37, 10679–10689 (2017).
Chen, I.-W. et al. In vivo submillisecond two-photon optogenetics with temporally focused patterned light. J. Neurosci. 39, 3484–3497 (2019).
Dana, H., Kruger, N., Ellman, A. & Shoham, S. Line temporal focusing characteristics in transparent and scattering media. Opt. Express 21, 5677–5687 (2013).
Sun, B., Salter, P. S. & Booth, M. J. Effects of aberrations in spatiotemporal focusing of ultrashort laser pulses. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 31, 765–772 (2014).
Katz, O. et al. Focusing and compression of ultrashort pulses through scattering media. Nat. Photonics 5, 372–377 (2011).
Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J. Neurosci. Methods 111, 29–37 (2001).
Dana, H. & Shoham, S. Numerical evaluation of temporal focusing characteristics in transparent and scattering media. Opt. Express 19, 4937–4948 (2011).
Papagiakoumou, E. et al. Functional patterned multiphoton excitation deep inside scattering tissue. Nat. Photonics 7, 274–278 (2013). Theoretical and experimental analysis of the propagation of temporally focused shaped patterns through scattering media.
Escobet-Montalbán, A. et al. Wide-field multiphoton imaging through scattering media without correction. Sci. Adv. 4, eaau1338 (2018).
Wijesinghe, P., Escobet-Montalbán, A., Chen, M., Munro, P. R. T. & Dholakia, K. Optimal compressive multiphoton imaging at depth using single-pixel detection. Opt. Lett. 44, 4981–4984 (2019).
Durst, M. E., Zhu, G. & Xu, C. Simultaneous spatial and temporal focusing for axial scanning. Opt. Express 14, 12243–12254 (2006).
Dana, H. & Shoham, S. Remotely scanned multiphoton temporal focusing by axial grism scanning. Opt. Lett. 37, 2913–2915 (2012).
Straub, A., Durst, M. E. & Xu, C. High speed multiphoton axial scanning through an optical fiber in a remotely scanned temporal focusing setup. Biomed. Opt. Express 2, 80–88 (2010).
Leshem, B., Hernandez, O., Papagiakoumou, E., Emiliani, V. & Oron, D. When can temporally focused excitation be axially shifted by dispersion? Opt. Express 22, 7087–7098 (2014).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7, 11928 (2016). Demonstration of optical generation of spatiotemporally shaped patterns at axially distinct axial planes.
Di Leonardo, R., Ianni, F. & Ruocco, G. Computer generation of optimal holograms for optical trap arrays. Opt. Express 15, 1913–1922 (2007).
Pégard, N. M., Oldenburg, I., Sridharan, S., Waller, L. & Adesnik, H. Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT). Nat. Commun. 8, 1228 (2017).
Mardinly, A. R. et al. Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 21, 881–893 (2018). Demonstration of a three-dimensional all-optical read-write interface, for in vivo simultaneous photostimulation of multiple neurons in mice in a volume.
Accanto, N. et al. Multiplexed temporally focused light shaping for high-resolution multi-cell targeting. Optica 5, 1478–1491 (2018).
Ronzitti, E., Emiliani, V. & Papagiakoumou, E. Methods for three-dimensional all-optical manipulation of neural circuits. Front. Cell. Neurosci. 12, 469 (2018).
Spampinato, G. et al. All-optical interrogation of a direction selective retinal circuit by holographic wave front shaping. Preprint at https://www.biorxiv.org/content/10.1101/513192 (2019).
Xu, C. Cross-Sections of Fluorescence Molecules in Multiphoton Microscopy (John Wiley & Sons, 2002).
Xu, C. & Webb, W. W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13, 481 (1996).
Sheppard, C. J. R. & Wilson, T. Depth of field in the scanning microscope. Opt. Lett. 3, 115 (1978).
Wilson, T. Resolution and optical sectioning in the confocal microscope. J. Microsc. 244, 113–121 (2011).
Durst, M. E., Zhu, G. & Xu, C. Simultaneous spatial and temporal focusing in nonlinear microscopy. Opt. Commun. 281, 1796–1805 (2008).
Gerchberg, R. W. & Saxton, W. O. A practical algorithm for the determination of the phase from image and diffraction pictures. Optik (Stuttg.) 35, 237–246 (1972).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008).
Papagiakoumou, E. et al. Two-photon optogenetics by computer-generated holography. Neuromethods 133, 175–197 (2018).
Glückstad, J. & Palima, D. Generalized Phase Contrast: Applications in Optics and Photonics (Springer, 2009).
Acknowledgements
We thank R. Sims for proofreading of the manuscript and fruitful discussions on 2PE-based imaging and C. Molinier for the preparation of the supplementary video. We thank the Agence Nationale de la Recherche (grant ANR-15-CE19-0001-01, 3DHoloPAc), the Human Frontiers Science Program (Grant RGP0015/2016), the European Research Council SYNERGY Grant Scheme (HELMHOLTZ, ERC Grant Agreement # 610110), the Fondation Bettencourt Schueller (Prix Coups d’élan pour la recherche française), the Getty Lab, the National Institute of Health (grant NIH 1UF1NS107574-01) and the Axa research funding for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Fig. 1 Axial propagation of spatially and temporally focused beams.
a, Spatial focusing of a Gaussian high-NA (0.8) and b, low-NA (0.2) beam and corresponding lateral intensity profiles, I, at different axial positions. c, Temporal focusing of a non-spatially focused Gaussian beam and corresponding pulse intensity profile, I, (at z = 0 μm, 1.5 μm and 3 μm), showing the shortening of the pulse duration at the sample plane (NA=0.8).
Supplementary Fig. 2 Comparison of the axial resolution between 2P and 3P temporally focused excited fluorescence.
Reproduced from Toda et al. Measured signal distributions for the 2P-TF (red curve) and 3P-TF (blue curve) excited fluorescence, recorded using a PMT. Fluorescence was excited in a layer of 200-nm fluorescent beads. The exposure times were 100 ms. The input powers for 2P-TF and 3P-TF microscopes were 5.5 mW and 55 mW, and the FWHMs of the signal distributions were 2.1 µm and 1.6 µm, respectively. The out-of-focus excitation for 2P-TF and 3P-TF was estimated as the full width at 1/100 maxima of the signal distributions, which were 69.2 µm and 11.8 µm, respectively, showing a higher out-of-focus signal suppression in 3P-TF by a factor of 5.9. Toda, K. et al. Temporal focusing microscopy using three-photon excitation fluorescence with a 92-fs Yb-fiber chirped pulse amplifier. Biomed. Opt. Express 8, 2796–2806 (2017).
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2 and Supplementary Note 1
Supplemental Video 1
An ultrashort pulse impinges on a dispersive grating at an angle γ (top panel). At each point in time, the intersection between the pulse and the dispersive element is a line, which is scanning the grating surface at a speed of c/sinγ (left, bottom panel) and the sample plane at a de-magnified speed of (c/sinγ)/M (right, bottom panel).
Rights and permissions
About this article
Cite this article
Papagiakoumou, E., Ronzitti, E. & Emiliani, V. Scanless two-photon excitation with temporal focusing. Nat Methods 17, 571–581 (2020). https://doi.org/10.1038/s41592-020-0795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-020-0795-y
This article is cited by
-
More than double the fun with two-photon excitation microscopy
Communications Biology (2024)
-
Ultrafast light targeting for high-throughput precise control of neuronal networks
Nature Communications (2023)
-
The future of brain–machine interfaces is optical
Nature Electronics (2023)
-
All-optical interrogation of neural circuits in behaving mice
Nature Protocols (2022)