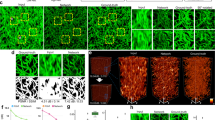
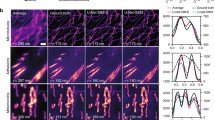
Abstract
We present deep-learning-enabled super-resolution across different fluorescence microscopy modalities. This data-driven approach does not require numerical modeling of the imaging process or the estimation of a point-spread-function, and is based on training a generative adversarial network (GAN) to transform diffraction-limited input images into super-resolved ones. Using this framework, we improve the resolution of wide-field images acquired with low-numerical-aperture objectives, matching the resolution that is acquired using high-numerical-aperture objectives. We also demonstrate cross-modality super-resolution, transforming confocal microscopy images to match the resolution acquired with a stimulated emission depletion (STED) microscope. We further demonstrate that total internal reflection fluorescence (TIRF) microscopy images of subcellular structures within cells and tissues can be transformed to match the results obtained with a TIRF-based structured illumination microscope. The deep network rapidly outputs these super-resolved images, without any iterations or parameter search, and could serve to democratize super-resolution imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
We declare that all the data supporting the findings of this work are available within the manuscript and Supplementary Information files. Raw images can be requested from the corresponding author. Deep learning models reported in this work used standard libraries and scripts that are publicly available in TensorFlow. The instruction manual for our Fiji/ImageJ plugin and trained models (available online as Supplementary Software 1–7) is provided as a Supplementary Protocol.
References
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Hess, S. T., Girirajan, T. P. K. & Mason, M. D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 6, 991–1009 (2011).
Hell, S. W. & Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994).
Gustafsson, M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Cox, S. Super-resolution imaging in live cells. Dev. Biol. 401, 175–181 (2015).
Gustafsson, M. G. L. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 102, 13081–13086 (2005).
Henriques, R. et al. QuickPALM: 3D real-time photoactivation nanoscopy image processing in ImageJ. Nat. Methods 7, 339–340 (2010).
Small, A. & Stahlheber, S. Fluorophore localization algorithms for super-resolution microscopy. Nat. Methods 11, 267–279 (2014).
Abraham, A. V., Ram, S., Chao, J., Ward, E. S. & Ober, R. J. Quantitative study of single molecule location estimation techniques. Opt. Express 17, 23352–23373 (2009).
Dempsey, G. T., Vaughan, J. C., Chen, K. H., Bates, M. & Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 8, 1027–1036 (2011).
Culley, S. et al. Quantitative mapping and minimization of super-resolution optical imaging artifacts. Nat. Methods 15, 263–266 (2018).
Sage, D. et al. Quantitative evaluation of software packages for single-molecule localization microscopy. Nat. Methods 12, 717–724 (2015).
Almada, P., Culley, S. & Henriques, R. PALM and STORM: into large fields and high-throughput microscopy with sCMOS detectors. Methods 88, 109–121 (2015).
Goodfellow, I. J. et al. Generative adversarial networks. arXiv Preprint at https://arxiv.org/abs/1406.2661 (2014).
Wilson, T. & Masters, B. R. Confocal microscopy. Appl. Opt. 33, 565–566 (1994).
Li, D. et al. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 349, aab3500 (2015).
Richardson, W. H. Bayesian-based iterative method of image restoration. J. Opt. Soc. Am. 62, 55 (1972).
Lucy, L. B. An iterative technique for the rectification of observed distributions. Astron. J. 79, 745 (1974).
Landweber, L. An iteration formula for Fredholm integral equations of the first kind. Am. J. Math. 73, 615–624 (1951).
Farahani, J. N., Schibler, M. J. & Bentolila, L. A. Stimulated emission depletion (STED)microscopy: from theory to practice. Microsc. Sci. Technol. Appl. Educ. 2, 1539–1547 (2010).
Hamel, P., Davies, M. E. P., Yoshii, K. & Goto, M. Transfer learning in MIR: sharing learned latent representations for music audio classification and similarity. Google AI https://ai.google/research/pubs/pub41530 (2013).
Wäldchen, S., Lehmann, J., Klein, T., van de Linde, S. & Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 5, 15348 (2015).
Hein, B., Willig, K. I. & Hell, S. W. Stimulated emission depletion (STED) nanoscopy of a fluorescent protein-labeled organelle inside a living cell. Proc. Natl. Acad. Sci. USA 105, 14271–14276 (2008).
Hein, B. et al. Stimulated emission depletion nanoscopy of living cells using SNAP-tag fusion proteins. Biophys. J. 98, 158–163 (2010).
Dyba, M. & Hell, S. W. Photostability of a fluorescent marker under pulsed excited-state depletion through stimulated emission. Appl. Opt. 42, 5123–5129 (2003).
Kner, P., Chhun, B. B., Griffis, E. R., Winoto, L. & Gustafsson, M. G. L. Super-resolution video microscopy of live cells by structured illumination. Nat. Methods 6, 339–342 (2009).
Leyton-Puig, D. et al. Flat clathrin lattices are dynamic actin-controlled hubs for clathrin-mediated endocytosis and signalling of specific receptors. Nat. Commun. 8, 16068 (2017).
Fiolka, R., Shao, L., Rego, E. H., Davidson, M. W. & Gustafsson, M. G. L. Time-lapse two-color 3D imaging of live cells with doubled resolution using structured illumination. Proc. Natl. Acad. Sci. USA 109, 5311–5315 (2012).
Ferguson, J. P. et al. Deciphering dynamics of clathrin-mediated endocytosis in a living organism. J. Cell. Biol. 214, 347–358 (2016).
Forster, B. et al. M. Complex wavelets for extended depth-of-field: a new method for the fusion of multichannel microscopy images. Microsc. Res. Tech. 65, 33–42 (2004).
Liu, R. & Jia, J. Reducing boundary artifacts in image deconvolution. in 2008 15th IEEE International Conference on Image Processing 505–508 (IEEE, New York, 2008).
Cox, I. J. & Sheppard, C. J. R. Information capacity and resolution in an optical system. J. Opt. Soc. Am. A. 3, 1152–1158 (1986).
Katznelson, Y. An Introduction to Harmonic Analysis (Dover Publications, New York, 1976).
Bentolila, L. A. et al. Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci. Rep. 6, 23834 (2016).
Aguet, F. et al. Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418–3435 (2016).
Willy, N. M. et al. Membrane mechanics govern spatiotemporal heterogeneity of endocytic clathrin coat dynamics. Mol. Biol. Cell 28, 3480–3488 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Sage, D., Prodanov, D., Tinevez, J.-Y. & Schindelin, J. MIJ: making interoperability between ImageJ and Matlab possible. Poster presented at the ImageJ User & Developer Conference, Mondorf-les-Bains, Luxembourg, 24–26 October, 2012.
Rivenson, Y. et al. Deep learning enhanced mobile-phone microscopy. ACS Photonics 5, 2354–2364 (2018).
Rivenson, Y. et al. Deep learning-based virtual histology staining using auto-fluorescence of label-free tissue. arXiv Preprint at https://arxiv.org/abs/1803.11293 (2018).
Wang, Z., Bovik, A. C., Sheikh, H. R. & Simoncelli, E. P. Image quality assessment: from error visibility to structural similarity. IEEE Trans. Image Process. 13, 600–612 (2004).
Ronneberger, O., Fischer, P. & Brox, T. U-Net: convolutional networks for biomedical image segmentation. arXiv Preprint at https://arxiv.org/abs/1505.04597 (2015).
Wu, Y. et al. Extended depth-of-field in holographic imaging using deep-learning-based autofocusing and phase recovery. Optica 5, 704–710 (2018).
Kingma, D. P. & Ba, J. Adam: a method for stochastic optimization. arXiv Preprint at https://arxiv.org/abs/1412.6980 (2014).
Abadi, M. et al. TensorFlow: a system for large-scale machine learning. arXiv Preprint at https://arxiv.org/abs/1605.08695 (2016).
Aguet, F., Van De Ville, D. & Unser, M. Model-based 2.5-d deconvolution for extended depth of field in brightfield microscopy. IEEE Trans. Image Process. 17, 1144–1153 (2008).
Born, M., Wolf, E. & Bhatia, A. B. Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light (Cambridge University Press, 1999).
Kirshner, H., Aguet, F., Sage, D. & Unser, M. 3-D PSF fitting for fluorescence microscopy: implementation and localization application. J. Microsc. 249, 13–25 (2013).
Sage, D. et al. DeconvolutionLab2: an open-source software for deconvolution microscopy. Methods 115, 28–41 (2017).
Acknowledgements
The Ozcan Research Group at UCLA acknowledges the support of NSF Engineering Research Center (ERC, PATHS-UP), the Army Research Office (ARO; W911NF-13-1-0419 and W911NF-13-1-0197), the ARO Life Sciences Division, the National Science Foundation (NSF) CBET Division Biophotonics Program, the NSF Emerging Frontiers in Research and Innovation (EFRI) Award, the NSF INSPIRE Award, NSF Partnerships for Innovation: Building Innovation Capacity (PFI:BIC) Program, the National Institutes of Health (NIH, R21EB023115), the Howard Hughes Medical Institute (HHMI), Vodafone Americas Foundation, the Mary Kay Foundation, and Steven & Alexandra Cohen Foundation. Yair Rivenson is partially supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No H2020-MSCA-IF-2014-659595 (MCMQCT). Confocal and STED laser scanning microscopy was performed at the California NanoSystems Institute (CNSI) Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA. We also thank the Advanced Imaging Center (AIC) at Janelia Research Campus for access to their TIRF-SIM microscope. The AIC is jointly supported by the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation. Finally, we thank H. Chang (Purdue University, West Lafayette, IN, USA) for sharing the CLC-mEmerald fly strain.
Author information
Authors and Affiliations
Contributions
H.W., Y.R., and A.O. conceived the research. H.W., Y.R., L.B., and C.K. contributed to the experiments. H.W., Y.J., Z.W., and H.G. processed the data. H.W. and Y.J. prepared the figures. H.W., Y.R., and A.O. prepared the manuscript, and all the authors contributed to the manuscript. H.W., Y.R., and R.G. developed the Fiji/ImageJ plugin. A.O. supervised the research.
Corresponding author
Ethics declarations
Competing interests
A.O., Y.R., and H.W. have a pending patent application on the contents of the presented results.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary text and figures
Supplementary Notes 1–10, Supplementary Figures 1–14 and Supplementary Table 1
Supplementary Protocol
SISR-Fluorescent Fiji/ImageJ plugin: User Manual
Supplementary Video 1
Deep-learning enabled cross-modality image transformation from TIRF to TIRF-SIM. A video of deep-learning-enabled cross-modality image transformation from TIRF to TIRF-SIM, corresponding to a gene-edited SUM159 cell expressing AP2-eGFP, revealing the temporal dynamics of endocytic protein structures within the cell. The highlighted frames in this video correspond to subpanels of Fig. 6 (main text). Experiments were repeated with >1,000 images/frames, achieving similar results.
Supplementary Software 1
SISR-Fluorescent Fiji/ImageJ plugin
Supplementary Software 2
Pre-trained model: wide-field DAPI
Supplementary Software 3
Pre-trained model: wide-field FITC
Supplementary Software 4
Pre-trained model: wide-field TxRed
Supplementary Software 5
Pre-trained model: confocal STED (nanobeads)
Supplementary Software 6
Pre-trained model: confocal STED (nuclei)
Supplementary Software 7
Pre-trained model: TIRF-SIM
Rights and permissions
About this article
Cite this article
Wang, H., Rivenson, Y., Jin, Y. et al. Deep learning enables cross-modality super-resolution in fluorescence microscopy. Nat Methods 16, 103–110 (2019). https://doi.org/10.1038/s41592-018-0239-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-018-0239-0
This article is cited by
-
Surmounting photon limits and motion artifacts for biological dynamics imaging via dual-perspective self-supervised learning
PhotoniX (2024)
-
Self-supervised denoising for multimodal structured illumination microscopy enables long-term super-resolution live-cell imaging
PhotoniX (2024)
-
Content-aware frame interpolation (CAFI): deep learning-based temporal super-resolution for fast bioimaging
Nature Methods (2024)
-
Revealing speckle obscured living human retinal cells with artificial intelligence assisted adaptive optics optical coherence tomography
Communications Medicine (2024)
-
Live-cell imaging powered by computation
Nature Reviews Molecular Cell Biology (2024)