Abstract
Much of the human proteome is involved in mRNA homeostasis, but most RNA-binding proteins lack chemical probes. Here we identify electrophilic small molecules that rapidly and stereoselectively decrease the expression of transcripts encoding the androgen receptor and its splice variants in prostate cancer cells. We show by chemical proteomics that the compounds engage C145 of the RNA-binding protein NONO. Broader profiling revealed that covalent NONO ligands suppress an array of cancer-relevant genes and impair cancer cell proliferation. Surprisingly, these effects were not observed in cells genetically disrupted for NONO, which were instead resistant to NONO ligands. Reintroduction of wild-type NONO, but not a C145S mutant, restored ligand sensitivity in NONO-disrupted cells. The ligands promoted NONO accumulation in nuclear foci and stabilized NONO–RNA interactions, supporting a trapping mechanism that may prevent compensatory action of paralog proteins PSPC1 and SFPQ. These findings show that NONO can be co-opted by covalent small molecules to suppress protumorigenic transcriptional networks.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw proteomic data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifiers PXD039283 (cysteine MS-ABPP with 6 h of compound treatment and FLAG–NONO coimmunoprecipitation–MS) and PXD032087 (all other experiments). Raw RNA-seq and eCLIP–seq data have been deposited to NCBI under Gene Expression Omnibus accession codes GSE222217 and GSE198212, respectively. Processed proteomic data are provided in Supplementary Datasets 1 and 3, RNA-seq data are provided in Supplementary Datasets 2 and 6, and eCLIP–seq data are provided in Supplementary Datasets 4 and 5. Source data are provided with this paper.
Code availability
All software used for data analysis is publicly available and detailed in the Methods. Custom scripts that were used for analyzing eCLIP data are linked in the Methods and Reporting Summary.
References
Bentley, D. L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 15, 163–175 (2014).
Gerstberger, S., Hafner, M. & Tuschl, T. A census of human RNA-binding proteins. Nat. Rev. Genet. 15, 829–845 (2014).
Gebauer, F., Schwarzl, T., Valcárcel, J. & Hentze, M. W. RNA-binding proteins in human genetic disease. Nat. Rev. Genet. 22, 185–198 (2021).
Pereira, B., Billaud, M. & Almeida, R. RNA-binding proteins in cancer: old players and new actors. Trends Cancer 3, 506–528 (2017).
Julio, A. R. & Backus, K. M. New approaches to target RNA binding proteins. Curr. Opin. Chem. Biol. 62, 13–23 (2021).
Wu, P. Inhibition of RNA-binding proteins with small molecules. Nat. Rev. Chem. 4, 441–458 (2020).
D’Agostino, V. G. et al. Screening approaches for targeting ribonucleoprotein complexes: a new dimension for drug discovery. SLAS Discov. 24, 314–331 (2019).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 (2021).
Han, T. et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 356, eaal3755 (2017).
Kotake, Y. et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 3, 570–575 (2007).
Naineni, S. K. et al. Functional mimicry revealed by the crystal structure of an eIF4A:RNA complex bound to the interfacial inhibitor, desmethyl pateamine A. Cell Chem. Biol. 28, 825–834 (2021).
Iwasaki, S. et al. The translation inhibitor rocaglamide targets a bimolecular cavity between eIF4A and polypurine RNA. Mol. Cell 73, 738–748 (2019).
Palacino, J. et al. SMN2 splice modulators enhance U1–pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 11, 511–517 (2015).
Lonergan, P. & Tindall, D. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 10, 20 (2011).
Sanford, M. Enzalutamide: a review of its use in metastatic, castration-resistant prostate cancer. Drugs 73, 1723–1732 (2013).
Kregel, S. et al. Androgen receptor degraders overcome common resistance mechanisms developed during prostate cancer treatment. Neoplasia 22, 111–119 (2020).
Salami, J. et al. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun. Biol. 1, 100 (2018).
Joseph, J. D. et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 3, 1020–1029 (2013).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Andersen, R. J. et al. Regression of castrate-recurrent prostate cancer by a amall-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 (2010).
Xie, J. et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat. Chem. Biol. 18,1341–1350 (2022).
Luna Velez, M. V., Verhaegh, G. W., Smit, F., Sedelaar, J. P. M. & Schalken, J. A. Suppression of prostate tumor cell survival by antisense oligonucleotide-mediated inhibition of AR-V7 mRNA synthesis. Oncogene 38, 3696–3709 (2019).
Melnyk, J. E. et al. Targeting a splicing-mediated drug resistance mechanism in prostate cancer by inhibiting transcriptional regulation by PKCβ1. Oncogene 41, 1536–1549 (2022).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Vinogradova, E. V. et al. An activity-guided map of electrophile-cysteine interactions in primary human T Cells. Cell 182, 1009–1026 (2020).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Knott, G. J., Bond, C. S. & Fox, A. H. The DBHS proteins SFPQ, NONO and PSPC1: a multipurpose molecular scaffold. Nucleic Acids Res. 44, 3989–4004 (2016).
Emili, A. et al. Splicing and transcription-associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD. RNA 8, 1102–1111 (2002).
Passon, D. M. et al. Structure of the heterodimer of human NONO and paraspeckle protein component 1 and analysis of its role in subnuclear body formation. Proc. Natl Acad. Sci. USA 109, 4846–4850 (2012).
Ishitani, K. et al. p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem. Biophys. Res. Commun. 306, 660–665 (2003).
Dong, X., Sweet, J., Challis, J. R. G., Brown, T. & Lye, S. J. Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol. Biol. Cell 27, 4863–4875 (2007).
Takayama, K.-I. et al. Dysregulation of spliceosome gene expression in advanced prostate cancer by RNA-binding protein PSF. Proc. Natl Acad. Sci. USA 114, 10461–10466 (2017).
Mathieson, T. et al. Systematic analysis of protein turnover in primary cells. Nat. Commun. 9, 689 (2018).
Witwicki, R. M. et al. TRPS1 is a lineage-specific transcriptional dependency in breast cancer. Cell Rep. 25, 1255–1267 (2018).
Lefèvre, L. et al. Combined transcriptome studies identify AFF3 as a mediator of the oncogenic effects of β-catenin in adrenocortical carcinoma. Oncogenesis 4, e161 (2015).
Grand, R. S. et al. BANP opens chromatin and activates CpG-island-regulated genes. Nature 596, 133–137 (2021).
Young, L. C., McDonald, D. W. & Hendzel, M. J. Kdm4b histone demethylase is a DNA damage response protein and confers a survival advantage following γ irradiation. J. Biol. Chem. 288, 21376–21388 (2013).
Li, S. et al. Double-strand break repair deficiency in NONO knockout murine embryonic fibroblasts and compensation by spontaneous upregulation of the PSPC1 paralog. Nucleic Acids Res. 42, 9771–9780 (2014).
Stagsted, L. V. W., O’Leary, E. T., Ebbesen, K. K. & Hansen, T. B. The RNA-binding protein SFPQ preserves long-intron splicing and regulates circRNA biogenesis in mammals. eLife 10, e63088 (2021).
Kim, S., Kim, D., Cho, S. W., Kim, J. & Kim, J.-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Fox, A. H., Bond, C. S. & Lamond, A. L. P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner. Mol. Biol. Cell 16, 5304–5315 (2005).
Fox, A. H. et al. Paraspeckles: a novel nuclear domain. Curr. Biol. 12, 13–25 (2002).
Prasanth, K. V. et al. Regulating gene expression through RNA nuclear retention. Cell 123, 249–263 (2005).
Van Nostrand, E. L. et al. A large-scale binding and functional map of human RNA-binding proteins. Nature 583, 711–719 (2020).
Van Nostrand, E. L. et al. Robust transcriptome-wide discovery of RNA-binding protein binding sites with enhanced CLIP (eCLIP). Nat. Methods 13, 508–514 (2016).
Salton, M., Lerenthal, Y., Wang, S.-Y., Chen, D. J. & Shiloh, Y. Involvement of matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle 9, 1568–1576 (2010).
Her, H., Boyle, E. & Yeo, G. W. Metadensity: a background-aware Python pipeline for summarizing CLIP signals on various transcriptomic sites. Bioinform. Adv. 2, vbac083 (2022).
Briata, P. et al. Diverse roles of the nucleic acid-binding protein KHSRP in cell differentiation and disease. Wiley Interdiscip. Rev. RNA 7, 227–240 (2016).
Schreiber, S. L. The rise of molecular glues. Cell 184, 3–9 (2021).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Verbist, B. et al. Analyzing magnetic bead QuantiGene Plex 2.0 gene expression data in high throughput mode using QGprofiler. BMC Bioinformatics 20, 378 (2019).
Cornelissen, F., Cik, M. & Gustin, E. Phaedra, a protocol-driven system for analysis and validation of high-content imaging and flow cytometry. J. Biomol. Screen. 17, 496–506 (2012).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Love, M. I. et al. Tximeta: reference sequence checksums for provenance identification in RNA-seq. PLoS Comput. Biol. 16, e1007664 (2020).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Shen, S. et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-seq data. Proc. Natl Acad. Sci. USA 111, E5593–E5601 (2014).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Yee, B. A., Pratt, G. A., Graveley, B. R., Van Nostrand, E. L. & Yeo, G. W. RBP-Maps enables robust generation of splicing regulatory maps. RNA 25, 193–204 (2019).
Acknowledgements
This work was supported by the NIH (R35 CA231991), a fellowship to S.G.K. from the National Cancer Institute (CA228436), a fellowship to H.-L.H. from the J. Yang Foundation Fellowship, a fellowship to H.L. from the Damon Runyon Foundation and a fellowship to J.R.R. from the American Cancer Society (PF-18-217-01-CDD). Part of this work was supported by Janssen R&D Integrated Technology Strategy Funding. We thank G. Van Hecke, H. De Wolf, G. Laenen and H. Murray for technical help, R. de Hoogt for providing the engineered cell line and S. Allen, I. Heo, H. Murray, P. Abeywickrema, A. Thompson, S. Sharma, P. J. Connolly and J. P. Edwards for insightful discussions. G.W.Y. is supported by NIH grants U41 HG009889, U01 HG009417, R01 HL137223 and R01 HG004659. G.W.Y. is supported as an Allen Distinguished Investigator, a Paul G. Allen Frontier Group advised grant to the Paul G. Allen Family Foundation.
Author information
Authors and Affiliations
Contributions
S.G.K., S.J.K., K.A., G.W.Y., B.G. and B.F.C. conceived the study. S.J.K. and S.J. conducted the initial screen for AR mRNA and protein depletion, and S.J.K. conducted all subsequent QuantiGene and high-content imaging studies. S.J.K., S.G.K. and G.L.L. prepared knockout and mutant cell lines and conducted cell growth assays. S.G.K., G.L.L. and J.R.R. performed all MS-ABPP experiments, and S.G.K. and G.L.L. conducted all other proteomics experiments. S.G.K. and G.L.L. prepared RNA-seq samples, and S.M.B. prepared eCLIP samples. H.-L.H. and H.L. analyzed RNA-seq data, and H.-L.H. analyzed and visualized splicing and eCLIP–seq data. S.G.K. performed NONO immunofluorescence studies. B.G. directed chemical synthesis. The paper was written by S.G.K. and B.F.C. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
B.F.C. is a founder and scientific advisor to Vividion Therapeutics. G.W.Y. is an SAB member of Jumpcode Genomics and a cofounder, a member of the Board of Directors, on the SAB, an equity holder and a paid consultant for Locanabio and Eclipse BioInnovations. G.W.Y. is a visiting professor at the National University of Singapore. G.W.Y.’s interests have been reviewed and approved by the University of California, San Diego, in accordance with its conflict-of-interest policies. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Benjamin Blencowe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Discovery of electrophilic compounds that deplete AR mRNA and protein.
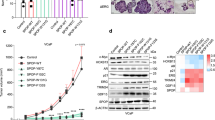
a, AR-FL and AR-V7 mRNA content in parental 22Rv1 cells and 22Rv1 cells stably expressing an shRNA targeting AR-FL (shRNA_AR-FL cells) as quantified by QuantiGene assays and normalized relative to a set of housekeeping genes. Data are mean values ± s.e.m. for 96 data points from two independent experiments. p values from unpaired t-test (two-tailed) are indicated above the bars. b, Western blot showing AR-FL and AR-V7 protein content in parental 22Rv1 cells and shRNA_AR-FL 22Rv1 cells. Data are from a single experiment representative of at least two independent experiments. c, AR protein content and cell counts (used as a cytotoxicity estimate counter screen) measured by high content imaging (HCI) after treatment of parental (upper) or shRNA_AR-FL (lower) 22Rv1 cells with electrophilic compounds (10 µM, 24 h). Hit compounds depleting AR protein content by ≥ 50% while maintaining cell counts ≥ 50% relative to DMSO are highlighted in red. See Supplementary Table 3 for structures of other hit compounds in addition to B21. d, Representative HCI images of time-dependent effects of hit compound B21 (25 µM) on total AR protein in 22Rv1 cells. See Fig. 1e for quantification of these data. Scale bar: 50 µM. e, Concentration-dependent effects of active (R)-10 and inactive (S)-10 enantiomers (see Table 1 for compound structures) on AR-FL and AR-V7 mRNA in 22Rv1 cells. Compound treatments were for 6 h, and Data are mean values ± s.e.m. for four replicates of a single experiment representative of two independent experiments. f, Structures of B21 (1) and analogues where the α-chloroacetamide reactive group was replaced with an acrylamide (12) or non-electrophilic propanamide (13). g, Concentration-dependent effects of B21, 12, and 13 on AR-FL and AR-V7 mRNA in 22Rv1 cells. Compound treatments were for 6 h, and Data are mean values for two replicates of a single experiment representative of two independent experiments.
Extended Data Fig. 2 Active compounds suppress AR expression by targeting NONO.
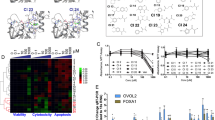
a, Global cysteine reactivity profiles of 22Rv1 cells treated with DMSO or (R)-SKBG-1 (20 µM, 1 h) as determined by MS-ABPP. Data are mean values from three independent experiments, where cysteines were quantified in at least two experiments. Maximum quantifiable ratio values were set at 10 and 0.1. The ratio for NONO_C145 is marked in red. b, Quantification of NONO_C145 engagement by active (2-5, (R)-10 and (R)-SKBG-1) and inactive (6-9, (S)-10 and (S)-SKBG-1) compounds as determined by cysteine-directed ABPP. Data are mean values ± s.e.m for three independent experiments. c Heat map showing cysteines substantially (> 75%) engaged by (R)-SKBG-1 (20 µM) at 6 h in 22Rv1 cells and their corresponding engagement by (S)-SKBG-1 as determined by cysteine-directed ABPP. Blue and white respectively designate cysteines engaged ≥ 50% or < 50% by the indicated compounds. d, Engagement of the stereoselective targets of (R)-SKBG-1 from (c) by other active compounds 2-5 (20 µM, 1 h) in cysteine-directed ABPP experiments. For c, d, data represent mean values from three independent experiments. For a-d, see Supplementary Data 1 for full proteomic data. e, Western blot showing NONO and AR-FL and V7 protein content of sgControl and sgNONO 22Rv1 cell populations generated by CRISPR-Cas9. sgControl 1 and sgNONO 5 cells were used for subsequent experiments. Data are from a single experiment representative of two independent experiments. f, AR-FL and AR-V7 mRNA content of sgControl and sgNONO cells. Data are mean values ± s.e.m. for 14 data points from two independent experiments. p values from unpaired t-tests. g, Concentration-dependent effects of active compound 5 and inactive control 8 (6 h treatments) on AR-FL (left) and AR-V7 (right) mRNA content of sgControl and sgNONO cells. Data are mean values for two replicates of a single experiment representative of two independent experiments. h, Western blots showing AR-FL and AR-V7 protein content in sgControl (NONO+) or sgNONO (NONO-) cells treated with active compound 5 and inactive control 9 (20 µM, 24 h). Also shown are western blotting signals for NONO and PSPC1. i, Quantification of western blotting data for AR-FL and AR-V7 in h and Fig. 2e. Data are mean values ± s.e.m. for four independent experiments. p values from two-way ANOVA. j, Concentration-dependent effects of compound 5 (6 h treatment) on AR-FL and AR-V7 mRNA content of sgNONO cells expressing WT-NONO or C145S-NONO. Data are mean values for two replicates of a single experiment representative of two independent experiments. For g and j, AR mRNAs were quantified by QuantiGene. k, l, Western blots showing AR-FL and AR-V7 protein content of sgNONO cells expressing WT-NONO or C145S-NONO treated with active compounds (R)-SKBG-1 (k) or 5 (l) and inactive controls (S)-SKBG-1 (k) or 9 (l) (20 µM compounds, 24 h). m, Quantification of western blotting data for AR-FL and AR-V7 in k and l. Data are mean values ± s.e.m. for four independent experiments. p values from two-way ANOVA.
Extended Data Fig. 3 Confirming direct engagement of NONO_C145 by active compounds using alkyne probe 14.
a, Structure of alkyne probe 14. b, Western blots showing effects of alkyne probe 14 on AR-FL and AR-V7 protein content of 22Rv1 cells. Active compounds 5 and (R)-SKBG-1 and inactive control compounds 8 and (S)-SKBG-1 are shown for comparison. Cells were treated with 20 µM of each compound for 24 h. Also shown are western blotting signals for PSPC1. Data are from a single experiment representative of two independent experiments. c, Waterfall plot showing global protein enrichment profiles from MS-ABPP experiments performed with alkyne 14-treated sgNONO 22Rv1 cells expressing FLAG-WT- or C145S-NONO. Cells were treated with 20 µM of 14 for 1 h prior to lysis and enrichment and quantification of 14-labeled proteins (see Methods section for more details). Data are presented as the ratio of enrichment of proteins from WT-NONO/C145S-NONO cells. Data are average values for all quantified peptides for each protein from two independent replicates. d, Gel-based ABPP experiment showing that alkyne 14 labels WT-NONO, but not C145S-NONO expressed as FLAG epitope-tagged proteins in sgNONO 22Rv1 cells. sgNONO cells (with or without exogenous expression of WT- or C145S-NONO) were pretreated with DMSO or (R)-SKBG-1 (20 µM, 2 h) followed by DMSO or alkyne 14 (20 µM, 1 h), lysed, conjugated to a rhodamine-azide (Rh-N3) by copper-catalyzed azide-alkyne cycloaddition chemistry, and analyzed by SDS-PAGE and in-gel fluorescence. e, Concentration-dependent effects of (R)-SKBG-1 and (S)-SKBG-1 on alkyne 14 labeling of FLAG-tagged WT-NONO expressed in HEK cells. Cells were treated with (R)-SKBG-1 or (S)-SKBG-1 for 2 h, followed by 14 (20 µM, 1 h), and then processed as described in d. Mock (empty vector-transfected) cells and cells expressing FLAG-tagged C145S-NONO are shown as controls. For d and e, red asterisk marks band corresponding to the MW of FLAG-NONO. IB, immunoblot. f, Quantification of blockade of 14 labeling of recombinant FLAG-WT-NONO by (R)-SKBG-1. Data are mean values ± s.e.m. for four independent experiments. 95% confidence interval or calculated IC50 value is shown in brackets.
Extended Data Fig. 4 Global effects of NONO ligands on the transcriptomes and proteomes of cancer cells.
a, Volcano plot of mRNA log2 fold changes in (R)-SKBG-1-treated sgControl cells/(S)-SKBG-1-treated sgControl cells. Dotted lines indicate cutoffs for genes with significantly decreased (log2 fold change ≤ −0.5, padj ≤ 0.01) or increased (log2 fold change ≥ 0.5, padj ≤ 0.01) expression. log2 fold change values are mean values from three independent replicates. Plotted are protein-coding genes with >50 counts in each of three independent replicates and a coefficient of variation for gene counts < 0.5. b, Volcano plot of genes significantly decreased by (R)-SKBG-1-treated sgControl cells relative to DMSO-treated sgControl cells (left) and of genes significantly decreased by DMSO-treated sgNONO cells relative to DMSO-treated sgControl cells (right). Significantly decreased was defined as log2 fold change ≤ −0.5, padj ≤ 0.01. log2 fold change values are mean values from three replicates. Plotted are protein-coding genes with at least 50 counts in at least three samples, and a coefficient of variation for gene counts < 0.5 among the three replicates. c, Volcano plot of mRNA log2 fold change in (R)-SKBG-1-treated sgControl cells / DMSO-treated sgControl cells. d, Volcano plot of mRNA log2 fold change in (S)-SKBG-1-treated sgControl cells / DMSO-treated sgControl cells. For a-d, differential expression p values were adjusted with the Benjamini-Hochberg procedure for multiple comparisons. e, Scatter plot comparing mRNA log2 fold changes (x-axis) versus protein log2 fold changes (y-axis) in (R)-SKBG-1-treated sgControl / (R)-SKBG-1-treated sgNONO 22Rv1 cells for proteins found by MS-based proteomics to be specifically decreased by (R)-SKBG-1 in a NONO-dependent manner (see Fig. 3d). Gene products inside the red dotted brackets show consistent mRNA and protein changes. f, g, Comparison of mRNA and protein content for AR (f) and SRSF4 (g) in the indicated treatment groups as measured by RNA-seq (4 h post-treatment with compounds) or MS-based proteomics (24 h post-treatment with compounds), respectively. Data are mean values ± s.e.m. for three (RNA-seq) or four (MS-based proteomics) independent replicates. p values from one-way ANOVA analysis are indicated above the bars.
Extended Data Fig. 5 (R)-SKBG-1 causes similar transcriptomic changes in human prostate and breast cancer cells.
a, Western blot showing NONO content of sgControl and sgNONO MCF7 cell populations generated by CRISPR-Cas9. sgControl 1 and sgNONO 1 or 3 cells were used for subsequent experiments. b, Quantification of NONO_C145 engagement by (R)-SKBG-1 versus (S)-SKBG-1 in MCF7 cells determined by cysteine-directed ABPP (indicated compound concentrations, 3 h). Data are mean values for two independent cell treatments analyzed in a single cysteine-directed ABPP experiment. See Supplementary Data 1 for complete proteomic data. c, Scatter plot of mRNA log2 fold changes for sgControl MCF7 cells treated with (R)-SKBG-1 or (S)-SKBG-1 (x-axis) versus mRNA log2fold changes for sgControl or sgNONO cells each treated with (R)-SKBG-1 (y-axis). Cells were treated with compounds (5 µM) for 4 h prior to RNA-seq analysis. Genes inside the red dotted brackets were designated as decreased (log2 fold change ≤ −0.5) or increased (log2 fold change ≥ 0.5) by (R)-SKBG-1 in a NONO-dependent manner. Data are mean values for protein-coding genes with at least 50 counts in each of two-three independent replicates. d, Scatter plot of mRNA log2fold changes in (R)-SKBG-1-treated sgControl / (R)-SKBG-1-treated sgNONO 22Rv1 cells (x-axis) versus (R)-SKBG-1-treated sgControl / (R)-SKBG-1-treated sgNONO MCF7 cells (y-axis). MCF7 and 22Rv1 cells were treated with 5 and 20 µM of compounds, respectively, for 4 h, to match the respective potency of NONO_C145 engagement by (R)-SKBG-1 in each cell line (see panel b and Extended Data Fig. 2b). Data are mean values for protein-coding genes with >50 counts in each of two-three independent replicates. e, f, Quantification of AR (e) and RXRA (f) mRNA from RNA-seq experiments performed in (R)-SKBG-1 or (S)-SKBG-1-treated sgControl or sgNONO 22Rv1 and MCF7 cells. Data are mean values ± s.e.m. for two-three independent replicates. TPM, transcripts per million reads. p values from one-way ANOVA analysis are indicated above the corresponding bars, with relative percent decreases in mRNA caused by (R)-SKBG-1 in sgControl cells shown in parentheses.
Extended Data Fig. 6 NONO ligand (R)-SKBG-1 impairs cancer cell growth in a stereoselective and NONO C145-dependent manner.
a, Effects of (R)-SKBG-1 and (S)-SKBG-1 on the growth of sgControl and sgNONO MCF7 cells. b, Effects of (R)-SKBG-1 and (S)-SKBG-1 on the growth of sgNONO 22Rv1 cells expressing WT-NONO or C145S-NONO. c, Effects of (R)-SKBG-1 and (S)-SKBG-1 on the growth of HeLa and HT1080 cells. Cells were treated with DMSO or (R)-SKBG-1 or (S)-SKBG-1. After 6 days (22Rv1), 5 days (MCF7) or 4 days (HeLa and HT1080), cell growth was determined by CellTiterGlo, normalized to cells treated with only DMSO. Data are mean values ± s.e.m. normalized to DMSO-treated control cells for four independent replicates. EC50 values are reported with 95% confidence intervals shown in brackets. d, sgControl and sgNONO 22Rv1 cells show similar growth rates. 10,000 cells were seeded overnight, and then cell growth was measured by CellTiterGlo, normalized to day 0 signal. Data are mean values ± s.e.m. for four independent replicates.
Extended Data Fig. 7 Crosstalk between NONO and DBHS paralogs PSPC1 and SFPQ in cells genetically or chemically disrupted for NONO.
a, Quantification of PSPC1 (left) and SFPQ (right) mRNA from RNA-seq experiments performed in (R)-SKBG-1 or (S)-SKBG-1-treated sgControl or sgNONO 22Rv1 and MCF7 cells. See Fig. 3a for more details on RNA-seq experiments. Data are mean values ± s.e.m. shown as % of the DMSO-treated sgControl groups for two-three independent replicates. p values from one-way ANOVA analysis are indicated above the bars. b, Quantification of PSPC1 (left) and SFPQ (right) protein from MS-based proteomic experiments performed in (R)-SKBG-1 or (S)-SKBG-1 -treated sgControl or sgNONO 22Rv1 and MCF7 cells. MCF7 and 22Rv1 cells were treated with 2.5 and 20 µM of compounds, respectively, for 24 h. Data are mean values ± s.e.m. for four independent replicates. p values from one-way ANOVA analysis are indicated above the bars. c, Dependency Map data showing that the three human cancer cell lines showing greatest dependency on NONO have predicted deleterious mutations in SFPQ or PSPC1. d, Western blot showing efficiency of disruption of PSPC1 and SFPQ expression in sgControl and sgNONO 22Rv1 cells following 72 h treatment with sgRNAs targeting PSPC1 or SFPQ using the Alt-R CRISPR-Cas9 nucleofection system. e, Number of transcripts that were strongly decreased (log2 fold change ≤ −0.75, padj ≤ 0.01) in the indicated sgRNA cell models relative to sgControl_sgControl cells. Red and blue bars correspond to transcripts decreased in sgControl_sgXXX (sgControl_sgPSPC1, sgControl_sgSFPQ) and sgNONO_sgXXX (sgNONO_sgControl, sgNONO_sgPSPC1, sgNONO_sgSFPQ) cell models, respectively. f, Cell growth of indicated sgRNA cell models measured by seeding 10,000 cells overnight (72 h post-nucleofection) and following growth over a five-day additional time period as determined by CellTiterGlo, normalized to day 0 signal. Data are mean values ± s.e.m. for five independent replicates. g, Bar graph showing the percentage of overall transcripts or transcripts stereoselectively decreased by (R)-SKBG-1 (log2 fold change (R)-SKBG-1 / (S)-SKBG-1 ≤ −0.75, padj ≤ 0.01) that were also decreased in the indicated sgRNA cell models.
Extended Data Fig. 8 NONO ligands induce accumulation of NONO and the paralogous proteins PSPC1 and SFPQ into nuclear foci.
a, Immunofluorescence data showing time-dependent effects of active compound 5 and inactive control 8 (20 µM) on the localization of NONO in nuclear foci (yellow wedge) in 22Rv1 cells. NONO was imaged with primary antibody (Bethyl Laboratories, A300-587A) and an Alexa-488 secondary antibody. Scale bar: 20 µm. b, c, NONO foci induced by compounds 5 (b) and (R)-SKBG-1 (c) localize proximal to the nucleolar marker fibrillarin (20 µM compound treatment, 24 h). NONO was imaged with primary antibody (BD Biosciences, 611278) and an Alexa-488 secondary antibody. Fibrillarin was imaged with primary antibody (Cell Signaling, 2639 S) and an Alexa-647 secondary. Scale bar: 20 µm. d, e, Zoomed-in merged images of NONO (green) and fibrillarin (red) localization from compounds 5 (d) or (R)-SKBG-1 (e) treated 22Rv1 cells (24 h time point). DAPI stain, blue. Scale bar: 10 µm. f, g, Immunofluorescence data showing effects of active compounds 5 (f) and (R)-SKBG-1 (g) and inactive controls 8 and (S)-SKBG-1 on the localization of SFPQ in nuclear foci (yellow wedge) in 22Rv1 cells (20 µM of each compound, 24 h). NONO was imaged with primary antibody (Bethyl Laboratories, A300-587A) and an Alexa-488 secondary antibody. SFPQ was imaged with primary antibody (Sigma-Aldrich, WH0006421M2) and an Alexa-647 secondary. Scale bar: 20 µm. h, Immunofluorescence data showing effects of active compounds 5 and (R)-SKBG-1 and inactive controls 8 and (S)-SKBG-1 on the localization of PSPC1 in nuclear foci (yellow wedge) in 22Rv1 cells (20 µM of each compound, 24 h). NONO was imaged with primary antibody (Bethyl Laboratories, A300-587A) and an Alexa-488 secondary antibody. PSPC1 was imaged with primary antibody (Sigma-Aldrich, SAB4200503) and an Alexa-647 secondary. Scale bar: 20 µm.
Extended Data Fig. 9 Effects of NONO ligands on NONO-protein and NONO-mRNA interactions.
a, Quantification of PSPC1 and SFPQ from anti-FLAG immunoprecipitation-MS experiments of FLAG-NONO-expressing cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h). Data are mean values ± s.e.m.for four independent replicates. b, (R)-SKBG-1 effects on the abundance of transcripts previously shown in eCLIP experiments to bind NONO or NONO-associated RBPs (SFPQ, MATR3) versus transcripts bound to any RBP or not bound to RBPs45,46. Transcript abundances determined by RNA-seq of 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h). Transcripts stereoselectively decreased or increased by (R)-SKBG-1 determined as described in Fig. 3a. A bound transcript was defined as having ≥ 1 eCLIP IDR peak for a given RBP. c, Volcano plot comparing enrichment of mRNAs stereoselectively decreased by (R)-SKBG-1 in 22Rv1 cells among NONO and SFPQ-bound transcripts (in black) to 30 sets of randomly selected transcripts of matched number and expression levels (in red). Enrichment odds ratio and FDR values were determined as in Fig. 5c. d, Motifs enriched in NONO eCLIP-seq peaks from cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h) or DMSO. e, Scatter plot showing repetitive element distribution between (R)-SKBG-1 and (S)-SKBG-1-treated eCLIP-seq. f, eCLIP-seq relative information content determined for transcripts bound to NONO in 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h) that were stereoselectively decreased or not decreased by (R)-SKBG-1. g, h, eCLIP-seq reads for total mRNA for AR (g) or TAF4 (h) in 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h). TAF4 is a representative mRNA that was bound to NONO but not altered in abundance by (R)-SKBG-1. For f-h, relative information content was determined as described in Fig. 5e. i, Percent of transcripts from the indicated categories bound to NONO in 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h) or DMSO. d-i, results are from a single eCLIP-seq experiment representative of two independent replicates.
Extended Data Fig. 10 Effects of NONO ligands on mRNA splicing.
a, b, 5’ splice site sequence upstream (a) and 3’ splice site sequence downstream (b) of cassette exons in different contexts. Included, excluded or no change refers to the effect on cassette exons upon (R)-SKBG-1 treatment compared to (S)-SKBG-1 treatment in sgControl or sgNONO cells. c, Splice site strength of the splice sites surrounding the alternatively-spliced exons was determined using MaxEntScore. No difference was observed comparing the included/excluded group to the no change group. d, Motif enrichment comparing inclusion/exclusion events following (R)-SKBG-1 treatment to cassette exons with no change, spread across surrounding regions. Intronic sequences are defined as 500 b.p. surrounding the splice site e, Normalized eCLIP density around alternatively spliced exons for transcripts bound to NONO in 22Rv1 cells under the three indicated treatment conditions (DMSO or (R)-SKBG-1 or (S)-SKBG-1 (20 µM, 4 h treatment)), and control exons with various inclusion levels (ψ). f, Overlapping ENCODE HepG2 eCLIP IDR peaks with regions near cassette exons included after (R)-SKBG-1 treatment. Coloring shows the log2 (odds ratio) comparing included exons to regions with no change.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and synthesis and characterization of compounds.
Supplementary Data 1
Cysteine site-of-labeling MS-ABPP and alkyne enrichment MS-ABPP in 22Rv1 and MCF7 cells.
Supplementary Data 2
RNA-seq results from sgControl or sgNONO 22Rv1 or MCF7 cells treated with (R)-SKBG-1 or (S)-SKBG-1, from 22Rv1 cells coexpressing endogenous NONO and exogenous NONOWT or NONOC145S (or empty vector control) treated with (R)-SKBG-1 or (S)-SKBG-1 and from sgControl, sgPSC1 and sgSFPQ 22Rv1 cells generated in sgControl or sgNONO cell lines.
Supplementary Data 3
Whole proteomics of sgControl or sgNONO 22Rv1 or MCF7 cells treated with (R)-SKBG-1 or (S)-SKBG-1.
Supplementary Data 4
Comparison of transcripts bound to 104 RBPs in HepG2 cells to transcript changes from 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1.
Supplementary Data 5
NONO eCLIP–seq results from 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1.
Supplementary Data 6
rMATs alternative splicing analysis of RNA-seq results from sgControl or sgNONO 22Rv1 cells treated with (R)-SKBG-1 or (S)-SKBG-1.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gels and western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kathman, S.G., Koo, S.J., Lindsey, G.L. et al. Remodeling oncogenic transcriptomes by small molecules targeting NONO. Nat Chem Biol 19, 825–836 (2023). https://doi.org/10.1038/s41589-023-01270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01270-0
This article is cited by
-
TRIM25 promotes glioblastoma cell growth and invasion via regulation of the PRMT1/c-MYC pathway by targeting the splicing factor NONO
Journal of Experimental & Clinical Cancer Research (2024)
-
The pleiotropic nature of NONO, a master regulator of essential biological pathways in cancers
Cancer Gene Therapy (2024)
-
Harnessing innate immune pathways for therapeutic advancement in cancer
Signal Transduction and Targeted Therapy (2024)
-
Chemoproteomic capture of RNA binding activity in living cells
Nature Communications (2023)