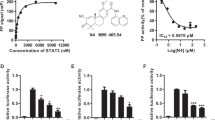
Abstract
Signal transducer and activator of transcription 5 (STAT5) is an attractive therapeutic target, but successful targeting of STAT5 has proved to be difficult. Here we report the development of AK-2292 as a first, potent and selective small-molecule degrader of both STAT5A and STAT5B isoforms. AK-2292 induces degradation of STAT5A/B proteins with an outstanding selectivity over all other STAT proteins and more than 6,000 non-STAT proteins, leading to selective inhibition of STAT5 activity in cells. AK-2292 effectively induces STAT5 depletion in normal mouse tissues and human chronic myeloid leukemia (CML) xenograft tissues and achieves tumor regression in two CML xenograft mouse models at well-tolerated dose schedules. AK-2292 is not only a powerful research tool with which to investigate the biology of STAT5 and the therapeutic potential of selective STAT5 protein depletion and inhibition but also a promising lead compound toward ultimate development of a STAT5-targeted therapy.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Co-crystal structures are available in the Protein Data Bank (7TVB for AK-305:STAT5A and 7TVA for AK-2292:STAT5A). Proteomics data are deposited and available in the Proteomics Identifications Database (PRIDE) (accession number PXD037895). Transcriptomic profiling data are available at Gene Expression Omnibus with accession GSE217647. Source data are provided with this paper.
References
Wang, Z. & Bunting, K. D. STAT5 in hematopoietic stem cell biology and transplantation. JAKSTAT 2, e27159 (2013).
Vainchenker, W. & Constantinescu, S. N. JAK/STAT signaling in hematological malignancies. Oncogene 32, 2601–2613 (2013).
Rani, A. & Murphy, J. J. STAT5 in cancer and immunity. J. Interferon Cytokine Res. 36, 226–237 (2016).
Walz, C. et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2V617F in mice. Blood 119, 3550–3560 (2012).
Yan, D., Hutchison, R. E. & Mohi, G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood 119, 3539–3549 (2012).
Jabbour, E. & Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 95, 691–709 (2020).
Nieborowska-Skorska, M. et al. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J. Exp. Med. 189, 1229–1242 (1999).
Carlesso, N., Frank, D. A. & Griffin, J. D. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J. Exp. Med. 183, 811–820 (1996).
Ilaria, R. L. Jr & Van, R. A. Etten, P210 and P190BCR/ABL induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J. Biol. Chem. 271, 31704–31710 (1996).
Shuai, K. et al. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene 13, 247–254 (1996).
Hoelbl, A. et al. Stat5 is indispensable for the maintenance of bcr/abl-positive leukaemia. EMBO Mol. Med. 2, 98–110 (2010).
Ye, D. et al. STAT5 signaling is required for the efficient induction and maintenance of CML in mice. Blood 107, 4917–4925 (2006).
Hantschel, O. et al. BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat. Chem. Biol. 8, 285–293 (2012).
Warsch, W., Walz, C. & Sexl, V. JAK of all trades: JAK2-STAT5 as novel therapeutic targets in BCR-ABL1+ chronic myeloid leukemia. Blood 122, 2167–2175 (2013).
Yoshimoto, G. et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood 114, 5034–5043 (2009).
Wingelhofer, B. et al. Pharmacologic inhibition of STAT5 in acute myeloid leukemia. Leukemia 32, 1135–1146 (2018).
Maurer, B. et al. High activation of STAT5A drives peripheral T-cell lymphoma and leukemia. Haematologica 105, 435–447 (2020).
Quentmeier, H. et al. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia 17, 120–124 (2003).
Orlova, A. et al. Direct targeting options for STAT3 and STAT5 in cancer. Cancers (Basel) 11, 1930 (2019).
Lai, A. C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2017).
Bai, L. et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell 36, 498–511 (2019).
Becker, S., Groner, B. & Muller, C. W. Three-dimensional structure of the Stat3β homodimer bound to DNA. Nature 394, 145–151 (1998).
Chen, X. et al. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93, 827–839 (1998).
Levy, D. E. & Darnell, J. E. Jr STATs: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Mandal, P. K. et al. Targeting the Src homology 2 (SH2) domain of signal transducer and activator of transcription 6 (STAT6) with cell-permeable, phosphatase-stable phosphopeptide mimics potently inhibits Tyr641 phosphorylation and transcriptional activity. J. Med. Chem. 58, 8970–8984 (2015).
Knight, J. M. et al. Small molecule targeting of the STAT5/6 Src homology 2 (SH2) domains to inhibit allergic airway disease. J. Biol. Chem. 293, 10026–10040 (2018).
Wang, S. et al. Small molecule degraders of stat3. WO2020198435 (2020).
Zhou, H. et al. SD-91 as a potent and selective STAT3 degrader capable of achieving complete and long-lasting tumor regression. ACS Med. Chem. Lett. 12, 996–1004 (2021).
Zhou, H. et al. Structure-based discovery of SD-36 as a potent, selective, and efficacious PROTAC degrader of STAT3 protein. J. Med. Chem. 62, 11280–11300 (2019).
Peltola, K. J. et al. Pim-1 kinase inhibits STAT5-dependent transcription via its interactions with SOCS1 and SOCS3. Blood 103, 3744–3750 (2004).
Lord, J. D. et al. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J. Immunol. 164, 2533–2541 (2000).
Matsumoto, A. et al. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood 89, 3148–3154 (1997).
Hoermann, G. et al. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am. J. Pathol. 178, 2344–2356 (2011).
Ribeiro, D. et al. STAT5 is essential for IL-7-mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv. 2, 2199–2213 (2018).
Quintas-Cardama, A. et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109–3117 (2010).
Nagashima, S. et al. Synthesis and evaluation of 2-{[2-(4-hydroxyphenyl)-ethyl]amino}pyrimidine-5-carboxamide derivatives as novel STAT6 inhibitors. Bioorg. Med. Chem. 15, 1044–1055 (2007).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015).
Bondeson, D. P. et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 25, 78–87 (2018).
MOE 2020.09 (Chemical Computing Group ULC, 2020).
Gianti, E. & Zauhar, R. J. An SH2 domain model of STAT5 in complex with phospho-peptides define ‘STAT5 Binding Signatures’. J. Comput. Aided Mol. Des. 29, 451–470 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Bricogne, G. et al. BUSTER. Global Phasing Ltd.: Cambridge, United Kingdom (2017).
Smart, O. S. et al. GRADE. Global Phasing Ltd.: Cambridge, United Kingdom (2017).
Nikolovska-Coleska, Z. et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 332, 261–273 (2004).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Wingett, S. W. & Andrews, S. FastQ Screen: a tool for multi-genome mapping and quality control. F1000Res. 7, 1338 (2018).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Reich, M. et al. GenePattern 2.0. Nat. Genet. 38, 500–501 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Liberzon, A. et al. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
McAlister, G. C. et al. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 (2014).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Acknowledgements
We are grateful for financial support from Oncopia Therapeutics, Proteovant Therapeutics and Roivant Sciences. This study is also supported, in part, by the Cancer Center Support Grant (P30CA046592) from the National Cancer Institute, National Institutes of Health, to Rogel Cancer Center of the University of Michigan. Use of the Advanced Photon Source was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract number DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program (grant 085P1000817). Proteomic profiling was assisted by the Proteomics Resource Facility in the Department of Pathology of the University of Michigan. Transcriptomic profiling was assisted by the Advanced Genomics Core at Biomedical Research Core Facilities of the University of Michigan. Animal studies were assisted by the In-Vivo Animal Core of the University of Michigan. Pharmacokinetics studies were performed by the Pharmacokinetics Core at the University of Michigan. 19F and 31P NMR spectra were obtained by the University of Michigan BioNMR Core Facility. We thank G. W. A. Milne for critical proofreading of the manuscript.
Author information
Authors and Affiliations
Contributions
A.K., L.B., M.W. and S.W. conceived of the project idea, made intellectual contributions, designed experiments, performed experiments, analyzed and interpreted the data and wrote the manuscript. D.M., J.L.M., P.D.K., L.Z., M.W., B.W., D.S. and J.A.S. made intellectual contributions, designed experiments, performed experiments, analyzed and interpreted the data and wrote the manuscript. R.X., Y.W., W.J., H.M. and H.J. made intellectual contributions, designed experiments, performed experiments and analyzed and interpreted the data. Experimentally, A.K. synthesized all the final compounds. R.X. developed the key tyrosine mimetic group, which was used in all the final compounds. M.W., A.K. and L.B. evaluated the binding affinity of compounds. L.B. and A.K. evaluated the degradation efficiency of synthesized degraders and performed cell viability assay. P.D.K. performed the molecular modeling. L.B. performed the proteomic profiling and transcriptomic profiling. J.L.M. and J.A.S. solved co-crystal structures of the highlighted inhibitor and degrader. L.Z. performed the apoptosis assay. D.M., Y.W., W.J. and H.M. performed all the efficacy studies. D.M., Y.W., W.J. and H.M. dosed compounds to mice and prepared tissue samples for the pharmacodynamics and pharmacokinetics analyses. M.W., B.W. and D.S. performed all the pharmacokinetics studies. S.W. conceived experiments and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The University of Michigan has filed patent applications on inhibitors and degraders described in this study, which have been licensed to Oncopia Therapeutics, Proteovant Therapeutics and Roivant Sciences. S.W., A.K., L.B. M.W., D.M. and J.S. are co-inventors on one or more of these patent applications and receive royalties from the University of Michigan. The University of Michigan has received a research contract from Oncopia/Proteovant/Roivant, for which S.W. serves as the principal investigator. S.W. is a paid consultant to Proteovant/Roivant and owns equity in Roivant. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Andrea Testa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Binding affinities of STAT5/6 ligands.
Chemical structure and binding data of key representative ligands to STAT5A, STAT5B and STAT6. Data represent n = 3 independent experiments.
Extended Data Fig. 2 Structure and degradation efficiency relationship.
(A). Structure and DC50 relationship of key degraders based on AK-013. (B) Structure and DC50 relationship of key degraders based on AK-305 and structurally similar analogues. Data represent at least n = 2 independent experiments.
Extended Data Fig. 4 Key parameter tables of proteomics analysis.
(A) Protein abundance table of STAT proteins in cells treated with AK-2292 under indicated concentrations and treatment durations, analyzed by multiplexed quantitative proteomics analysis (Fig. 2D). P-value: two-sided Student’s t-test. KU812 (>6,800 quantified proteins), PBMCs (>6,640 quantified proteins), NCO2 (>6,800 quantified proteins). N/D: not detected. Values are derived from three independent replicates. (B) KU812 cells were treated with AK-2292Me under indicated concentrations and treatment durations for multiplexed quantitative proteomics analysis. P-value: two-sided Student’s t-test. KU812 (>6,800 quantified proteins). Values are derived from three independent replicates.
Extended Data Fig. 5 All down- or up-regulated genes in RNAseq analysis.
List of all the down- or up-regulated genes ≥2-fold p-value < 0.05 in KU812 cells, treated with AK-2292 1 µM for 8 h and analyzed by RNA-seq. The experiment was performed in three independent replicates. Statistical testing was performed with two-sided Student’s t-test.
Extended Data Fig. 6 AK-2292 induces STAT5 degradation and exerts growth-inhibitory activity in CML cell lines.
Growth-inhibitory activities of AK-2292 were determined as IC50 by Cell Titer Glo (CTG) assay (3 days). IC50 data represent n= 3 independent experiments. STAT5 degradation efficiency of AK-2292 was calculated as DC50 by immunoblot analysis. DC50 data represent at least n = 2 independent experiments. AK-2292 responding cell lines are marked in bold.
Extended Data Fig. 7 PD effect in CD-1 mice.
Female CD-1 mice were treated with AK-2292 at 100 mg/kg or 200 mg/kg i.p. and tissues were analyzed by immunoblotting and a quantification table. Blots represent n = 3 independent experiments.
Extended Data Fig. 8 Weight change and blood chemistry in Balb/C mice toxicity study.
(A) Balb/C mice were treated with 100 mg/kg of AK-2292 and 200 mg/kg through I.P. 5 days per week for 2 weeks and the body weights of mice were measured every dosing day (n = 5). Values in the plots are shown as mean ± s.d. from n = 5 mice per treatment arm. (B) Balb/C mice were treated with 100 mg/kg of AK-2292 and 200 mg/kg through I.P. 5 days per week for 2 weeks and the blood was analyzed. Values in the table are derived from n = 5 mice per group. Statistical testing was performed with two-sided Student’s t-test.
Extended Data Fig. 9 Cell permeability and plasma protein binding evaluation.
(A) Permeability and Efflux Ratio Determination of AK-2292 & AK-305 in Caco-2 Cells. Papp: apparent permeability coefficient (cm/s). Data shown represent three independent replicates. See also method section. (B) Percentage of protein binding of AK-2292 in Human and Mice Plasma. Data shown represent two independent replicates.
Extended Data Fig. 10 Pharmacokinetics study of AK-2292 and predicted free drug concentration in plasma.
(A) SCID mouse was dosed with AK-2292 10 mg/kg through IP route and Vivo PK parameters were analyzed. Cmax = Maximum observed concentration, Tmax = Time to reach Cmax, AUC(0-tldc) = Area under the concentration-time curve from time zero to time of last detectable concentration, AUC(0-inf) = Area under the concentration-time curve from time zero to infinite, CL = Systemic clearance, CL: Apparent clearance, Vz/F: Volume of distribution associated with the terminal elimination phrase, Terminal elimination half-life (t½) was calculated based on data points (>= 3) in the terminal phase with correlation of coefficient > 0.90. Values in the plot are shown as mean ± s.d. from n = 3 independent experiments. (B) Predicted free drug concentrations in plasma in mice when dosed with AK-2292 at 100 mg/kg, using the data in Extended Data Fig. 9B and 10A. DC50 and DC90 values were calculated using the data in Supplementary Fig. 7. (C) Predicted free drug concentrations in plasma in mice when dosed with AK-2292 at 200 mg/kg, using the data in Extended Data Fig. 9B and 10A. DC50 and DC 90 values were calculated using the data in Supplementary Fig. 7.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, source data of supplementary figures and chemistry data.
Supplementary Data
Structures of the best-ranked homology models.
Source data
Source Data Fig. 2
Unprocessed western blots of Fig. 2 and proteomics data table.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaneshige, A., Bai, L., Wang, M. et al. A selective small-molecule STAT5 PROTAC degrader capable of achieving tumor regression in vivo. Nat Chem Biol 19, 703–711 (2023). https://doi.org/10.1038/s41589-022-01248-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01248-4
This article is cited by
-
Notch3 restricts metastasis of breast cancers through regulation of the JAK/STAT5A signaling pathway
BMC Cancer (2023)
-
The rise of targeting chimeras (TACs): next-generation medicines that preempt cellular events
Medicinal Chemistry Research (2023)