Abstract
Ferredoxins comprise a large family of iron–sulfur (Fe–S) proteins that shuttle electrons in diverse biological processes. Human mitochondria contain two isoforms of [2Fe-2S] ferredoxins, FDX1 (aka adrenodoxin) and FDX2, with known functions in cytochrome P450-dependent steroid transformations and Fe–S protein biogenesis. Here, we show that only FDX2, but not FDX1, is involved in Fe–S protein maturation. Vice versa, FDX1 is specific not only for steroidogenesis, but also for heme a and lipoyl cofactor biosyntheses. In the latter pathway, FDX1 provides electrons to kickstart the radical chain reaction catalyzed by lipoyl synthase. We also identified lipoylation as a target of the toxic antitumor copper ionophore elesclomol. Finally, the striking target specificity of each ferredoxin was assigned to small conserved sequence motifs. Swapping these motifs changed the target specificity of these electron donors. Together, our findings identify new biochemical tasks of mitochondrial ferredoxins and provide structural insights into their functional specificity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The crystallographic dataset generated and analyzed during the current study is available in the PDB at www.rcsb.org (PDB ID of human FDX2, 2Y5C). All other datasets generated and analyzed during the current study are either shown in the source data files or are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Campbell, I. J., Bennett, G. N. & Silberg, J. J. Evolutionary relationships between low potential ferredoxin and flavodoxin electron carriers. Front. Energy Res. https://doi.org/10.3389/fenrg.2019.00079 (2019).
Hanke, G. & Mulo, P. Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ. 36, 1071–1084 (2013).
Miotto, O. et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat. Genet. 47, 226–234 (2015).
Changmai, P. et al. Both human ferredoxins equally efficiently rescue ferredoxin deficiency in Trypanosoma brucei. Mol. Microbiol. 89, 135–151 (2013).
Ewen, K. M., Ringle, M. & Bernhardt, R. Adrenodoxin–a versatile ferredoxin. IUBMB Life 64, 506–512 (2012).
Sheftel, A. D. et al. Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis. Proc. Natl Acad. Sci. USA 107, 11775–11780 (2010).
Lange, H., Kaut, A., Kispal, G. & Lill, R. A mitochondrial ferredoxin is essential for biogenesis of cellular iron-sulfur proteins. Proc. Natl Acad. Sci. USA 97, 1050–1055 (2000).
Shi, Y., Ghosh, M., Kovtunovych, G., Crooks, D. R. & Rouault, T. A. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Biophys. Acta 1823, 484–492 (2012).
Webert, H. et al. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat. Commun. 5, 5013 (2014).
Freibert, S. A. et al. Evolutionary conservation and in vitro reconstitution of microsporidian iron-sulfur cluster biosynthesis. Nat. Commun. 8, 13932 (2017).
Braymer, J. J., Freibert, S. A., Rakwalska-Bange, M. & Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Biophys. Acta, Mol. Cell. Res. 1868, 118863 (2021).
Kispal, G., Csere, P., Prohl, C. & Lill, R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 18, 3981–3989 (1999).
Boniecki, M. T., Freibert, S. A., Muhlenhoff, U., Lill, R. & Cygler, M. Structure and functional dynamics of the mitochondrial Fe/S cluster synthesis complex. Nat. Commun. 8, 1287 (2017).
Van Vranken, J. G. et al. The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis. eLife 5, e17828 (2016).
Kim, J. H., Frederick, R. O., Reinen, N. M., Troupis, A. T. & Markley, J. L. [2Fe-2S]-Ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J. Am. Chem. Soc. 15, 8117–8120 (2013).
Gervason, S. et al. Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin. Nat. Commun. 10, 3566 (2019).
Freibert, S. A. et al. N-terminal tyrosine of ISCU2 triggers [2Fe-2S] cluster synthesis by ISCU2 dimerization. Nat. Commun. 12, 6902 (2021).
Weiler, B. D. et al. Mitochondrial [4Fe-4S] protein assembly involves reductive [2Fe-2S] cluster fusion on ISCA1-ISCA2 by electron flow from ferredoxin FDX2. Proc. Natl Acad. Sci. USA 117, 20555–20565 (2020).
Lill, R. & Freibert, S. A. Mechanisms of mitochondrial iron-sulfur protein biogenesis. Annu. Rev. Biochem. 89, 471–499 (2020).
Zhang, Y. et al. Ferredoxin reductase is critical for p53-dependent tumor suppression via iron regulatory protein 2. Genes Dev. 31, 1243–1256 (2017).
Barros, M. H., Carlson, C. G., Glerum, D. M. & Tzagoloff, A. Involvement of mitochondrial ferredoxin and Cox15p in hydroxylation of heme O. FEBS Lett. 492, 133–138 (2001).
Bareth, B. et al. The heme a synthase Cox15 associates with cytochrome c oxidase assembly intermediates during Cox1 maturation. Mol. Cell. Biol. 33, 4128–4137 (2013).
Swenson, S. A. et al. From synthesis to utilization: the ins and outs of mitochondrial heme. Cells 9, 579 (2020).
Ozeir, M. et al. Coenzyme Q biosynthesis: Coq6 is required for the C5-hydroxylation reaction and substrate analogs rescue Coq6 deficiency. Chem. Biol. 18, 1134–1142 (2011).
Cai, K., Tonelli, M., Frederick, R. O. & Markley, J. L. Human mitochondrial Ferredoxin 1 (FDX1) and Ferredoxin 2 (FDX2) both bind cysteine desulfurase and donate electrons for iron-sulfur cluster biosynthesis. Biochemistry 56, 487–499 (2017).
Landgraf, B. J., McCarthy, E. L. & Booker, S. J. Radical S-adenosylmethionine enzymes in human health and disease. Annu. Rev. Biochem. 85, 485–514 (2016).
Cronan, J. E. Assembly of lipoic acid on its cognate enzymes: an extraordinary and essential biosynthetic pathway. Microbiol Mol. Biol. Rev. 80, 429–450 (2016).
Zhu, J. & Thompson, C. B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 20, 436–450 (2019).
Tsvetkov, P. et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 15, 681–689 (2019).
Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261 (2022).
Campbell, I. J. et al. Recombination of 2Fe-2S ferredoxins reveals differences in the inheritance of thermostability and midpoint potential. ACS Synth. Biol. 9, 3245–3253 (2020).
Antonicka, H. et al. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 72, 101–114 (2003).
Sheftel, A. D. et al. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol. Biol. Cell 23, 1157–1166 (2012).
McCarthy, E. L. & Booker, S. J. Destruction and reformation of an iron-sulfur cluster during catalysis by lipoyl synthase. Science 358, 373–377 (2017).
Nagai, M. et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic. Biol. Med 52, 2142–2150 (2012).
Soma, S. et al. Elesclomol restores mitochondrial function in genetic models of copper deficiency. Proc. Natl Acad. Sci. USA 115, 8161–8166 (2018).
Hasinoff, B. B., Yadav, A. A., Patel, D. & Wu, X. The cytotoxicity of the anticancer drug elesclomol is due to oxidative stress indirectly mediated through its complex with Cu(II). J. Inorg. Biochem. 137, 22–30 (2014).
Yadav, A. A., Patel, D., Wu, X. & Hasinoff, B. B. Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J. Inorg. Biochem. 126, 1–6 (2013).
Vallieres, C., Holland, S. L. & Avery, S. V. Mitochondrial ferredoxin determines vulnerability of cells to copper excess. Cell Chem. Biol. 24, 1228–1237 e3 (2017).
Mühlenhoff, U. et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 12, 373–385 (2010).
Schiffler, B. et al. The adrenodoxin-like ferredoxin of Schizosaccharomyces pombe mitochondria. J. Inorg. Biochem. 98, 1229–1237 (2004).
Muller, J. J. et al. Structural and thermodynamic characterization of the adrenodoxin-like domain of the electron-transfer protein Etp1 from Schizosaccharomyces pombe. J. Inorg. Biochem. 105, 957–965 (2011).
Omura, T. & Gotoh, O. Evolutionary origin of mitochondrial cytochrome P450. J. Biochem. 161, 399–407 (2017).
Kimura, T. & Suzuki, K. Components of the electron transport system in adrenal steroid hydroxylase. Isolation and properties of non-heme iron protein (adrenodoxin). J. Biol. Chem. 242, 485–491 (1967).
Swift, R. P., Rajaram, K., Elahi, R., Liu, H. B. & Prigge, S. T. Roles of ferredoxin-dependent proteins in the apicoplast of Plasmodium falciparum parasites. mBio e0302321 (2022).
Arcinas, A. J., Maiocco, S. J., Elliott, S. J., Silakov, A. & Booker, S. J. Ferredoxins as interchangeable redox components in support of MiaB, a radical S-adenosylmethionine methylthiotransferase. Protein Sci. 28, 267–282 (2019).
Liao, W. L., Dodder, N. G., Mast, N., Pikuleva, I. A. & Turko, I. V. Steroid and protein ligand binding to cytochrome P450 46A1 as assessed by hydrogen-deuterium exchange and mass spectrometry. Biochemistry 48, 4150–4158 (2009).
Zhang, W. et al. Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners. ACS Catal. 8, 9992–10003 (2018).
Atkinson, J. T. et al. Metalloprotein switches that display chemical-dependent electron transfer in cells. Nat. Chem. Biol. 15, 189–195 (2019).
Wei, F. Y. et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 21, 428–442 (2015).
Stehling, O. et al. Function and crystal structure of the dimeric P-loop ATPase CFD1 coordinating an exposed [4Fe-4S] cluster for transfer to apoproteins. Proc. Natl Acad. Sci. USA 115, E9085–E9094 (2018).
Heigwer, F., Kerr, G. & Boutros, M. E-CRISP: fast CRISPR target site identification. Nat. Methods 11, 122–123 (2014).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Mühlenhoff, U., Richhardt, N., Ristow, M., Kispal, G. & Lill, R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet 11, 2025–2036 (2002).
Diekert, K., deKroon, A. I. P. M., Kispal, G. & Lill, R. Isolation and sub-fractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell. Biol. 65, 37–51 (2001).
Molik, S., Lill, R. & Mühlenhoff, U. Methods for studying iron metabolism in yeast mitochondria. Methods Cell. Biol. 80, 261–280 (2007).
McCarthy, E. L. & Booker, S. J. Biochemical approaches for understanding iron-sulfur cluster regeneration in Escherichia coli lipoyl synthase during catalysis. Methods Enzymol. 606, 217–239 (2018).
Zollner, A. et al. Purification and functional characterization of human 11beta hydroxylase expressed in Escherichia coli. FEBS J. 275, 799–810 (2008).
Stehling, O. et al. in Methods in Molecular Biology Vol. 372 (eds. Leister, D. & Herrmann, J. M.) 325–342 (Humana Press, 2007).
Stehling, O., Sheftel, A. D. & Lill, R. Chapter 12 Controlled expression of iron-sulfur cluster assembly components for respiratory chain complexes in mammalian cells. Methods Enzymol. 456, 209–231 (2009).
Acknowledgements
We thank R. Rösser and S. Hanschke for excellent technical assistance, U. Linne and J. Bamberger from the Core Facility ‘Mass spectrometry and Elemental analysis’ of Philipps University of Marburg for mass spectrometry, and F. Hannemann and R. Bernhardt (Saarbrücken) for help with cortisol formation assays. We acknowledge the contribution of the Core Facility ‘Protein Biochemistry and Spectroscopy’ of the Philipps University of Marburg. R.L. received generous financial support from Deutsche Forschungsgemeinschaft (grant no. SPP 1927) and COST Action FeSBioNet (Contract no. CA15133). S.J.B. acknowledges support from the National Science Foundation (grant no. MCB-1716686) and the Eberly Family Distinguished Chair in Science. S.J.B. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
V.S., S.B., S.A.-F., H.W., U.M., O.S. and R.L. designed the concept. V.S., S.B., S.A.-F., H.W., L.B., U.M., F.P. and O.S. conducted the experiments. L.-O.E. provided advice for crystallography. D.M.W. and S.J.B. provided materials and advice for the LIAS experiments. V.S., S.A.-F., O.S. and R.L. wrote the original draft. All authors reviewed and edited the manuscript. S.J.B. and R.L. acquired the funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Nicolas Rouhier, Jonathan Silberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Growth phenotypes and mitochondrial Fe/S protein status of FDX1 knockout cells.
a HEK293 cells were subjected to CRISPR-Cas9 FDX1 gene knockout using CC1-CC3 guide RNAs. Cumulative growth of cells treated as in Fig. 1b was calculated from cell counts at various harvesting time points after puromycin removal. In detail, individual knockout cell lines were sub-cultured by harvesting an entire culture vessel and subsequent re-seeding of a defined cell number into a new culture device. Remaining cells were collected, and the total protein content of this sample was determined. This protein amount was then used as a denominator for the calculation of the specific (that is total protein-related) enzymes activities measured in the respective sample. This tissue culture regime involving the re-seeding of aliquots of harvested cells produced sufficient cell material to conduct multiple analyses, even in case of an experimentally elicited growth retardation. Based on the cell counting performed at each harvest, the cumulative growth was calculated for each cell line from the total cell yield as well as the portion of cells needed for re-seeding during sub-culturing. Values are presented relative to those of control cells (set to 100%, dashed line; n = 4, days 3 + 4; n = 6, days 7 + 8; n = 5, days 10-12; n = 2, day 14; n = 4, days 16-17; mean ± SEM). b Densitometric quantification of immunostains from cells treated as in Figs. 1b, 2a, 3a (that is harvested 7 or 8 days after puromycin removal) using Image studio lite 5.2. Error bars indicate SEM (n = 4, FDX2, NDUFS1, LIAS; n = 5, all others). c-e Total aconitase (Aco), succinate dehydrogenase (SDH) and citrate synthase (CS) activities were determined in cell samples obtained at the indicated time points after puromycin removal as in Fig. 1c. Values were presented relative to those of control cells (set to 100%, dashed line; n = 6, days 7-8 [CC1, CC3]; n = 9, days 7-8 [CC2]; n = 5, days 16-17; n = 3, all others; mean ± SEM).
Extended Data Fig. 2 Even high excess of FDX1 does not support enzymatic [2Fe-2S] cluster reconstitution on ISCU2.
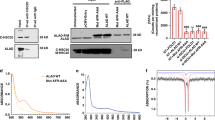
Enzymatic synthesis of [2Fe-2S] clusters on ISCU2 in vitro was monitored by the CD signal at 431 nm for 10 min. Reactions included NIA, FXN, FDXR and the indicated equivalent amounts (eq.) of (a) FDX2 or (b) FDX1 relative to NIA. Representative experiments are shown and initial rates are presented in Fig. 1d.
Extended Data Fig. 3 FDX1 knockout cells are deficient in cytochrome oxidase activity.
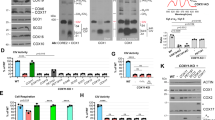
a Tissue culture media of HEK293 control and FDX1 knockout cells (cf. Figure 2a and Extended Data Fig. 1a) were collected at the indicated time points. Cells were harvested, counted, and processed as required. The color change of the pH indicator phenol red in the culture medium from reddish toward yellowish, particularly for FDX1-CC1 and -CC2 knockout cells, revealed a pH drop despite lower cell numbers in these cultures (given at the top; in millions). b Cytochrome c oxidase (COX) activities were determined in total membrane fractions from cells harvested at the indicated time points after puromycin removal (cf. Figure 2b). COX activities were expressed relative to citrate synthase (CS) activities (Extended Data Fig. 1e), and values are presented relative to those of control cells (set to 100%, dashed line; n = 2, days 16-17; n = 6, days 7-8 [CC2]; n = 3, all others; mean ± SEM). c HEK293 cells (see Fig. 2f) were chemically transfected with control plasmid PX459 or the PX459-derived plasmid FDX1-CC2 and selected by puromycin for 3 days as in Fig. 2a. 10 to 15 days after selection cells were transiently transfected by electroporation with plasmids coding for either a PEST-destabilized EGFP or FDX1, each fused to the N-terminal mitochondrial Su9 targeting sequence (from Neurospora crassa subunit 9 of mitochondrial F1Fo ATP synthase). Three days after transfection cells were harvested and analyzed for COX and CS activities. Enzyme activities are presented relative to the values for Su9-EGFP control cells (n = 4; SEM). d Cell samples from (c) were subjected to immunostaining against the indicated proteins. Observed molecular masses (kDa) for proteins are given in parentheses. Representative blots are shown.
Extended Data Fig. 4 Combined FDX1 deletion and FDX2 depletion elicits severe defects in growth and mitochondrial Fe/S proteins.
a Cumulative growth of HEK293 cells from Fig. 2f was calculated from cell counts at the three harvests on day 3, (n = 6 each), day 6 (n = 5, Control(CC)/FDX2-si; n = 6, FDX1-CC2/FDX2-si) and day 9 (n = 2, Control(CC)/FDX2-si; n = 4, FDX1-CC2/FDX2-si) after the first electroporation. Values were presented relative to those of mock control cells (no CRISPR, no RNAi treatment; set to 100%, dashed line; mean ± SEM). b HEK293 cells were transfected with FDX1-directed gRNA-encoding plasmids (CC1 to CC3) and subsequently with FDX2-directed siRNAs similar to Fig. 2f. Cell samples were obtained at the specified time points and subjected to immunostaining of the indicated mitochondrial proteins or lipoyl cofactor. The observed molecular weights are given in parentheses. C-I, C-II, C-III, respiratory complexes I, II, and III. C-V, F1Fo ATP synthase. The immunoblot signals from cell samples with combined FDX1-CC2 and FDX2-si deficiency are representative for at least three independent experiments, and are here presented conjointly with FDX1-CC1 / FDX2-si and FDX1-CC1 / FDX2-si treated cells, respectively.
Extended Data Fig. 5 The lipoylation defect in FDX1 knockout cells can be complemented by FDX1.
HEK293 cells from Extended Data Fig. 3c, d knocked out for FDX1 (by FDX1-CC2 gRNA) and complemented with Su9-EGFP-PEST or Su9-FDX1 plasmids were subjected to immunostaining against the indicated proteins or lipoyl cofactor. Observed molecular masses (kDa) for proteins are given in parentheses. Representative blots are shown.
Extended Data Fig. 6 In vitro synthesis of 6-thiooctanoyl intermediate and lipoyl product by human lipoyl synthase LIAS requires ferredoxin FDX1 as an electron donor.
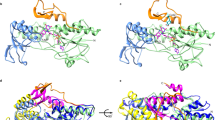
a Model of the multi-step reaction mechanism of lipoyl formation by human lipoyl synthase (LIAS) based on bacterial LipA34. The LIAS enzyme contains two [4Fe-4S] clusters, the catalytic and auxiliary cluster, needed for reductive cleavage of S-adenosylmethionine (SAM) and as a source for sulfur insertion into the octanoyl precursor, respectively. (1) The reaction starts with the binding of SAM and the octanoyl substrate which is covalently attached to a lysinyl residue of a lipoyl carrier domain (LCD) of the H protein of the glycine cleavage system (GCS)26,34. An electron donor (orange) transfers a single electron to the catalytic cluster which mediates reductive cleavage of SAM to methionine and a 5’-deoxyadenosyl radical (5’-dA·). This work identified human FDX1 as the physiological electron donor, efficiently replacing dithionite that typically is used as an artificial electron donor34. (2) The radical abstracts a hydrogen atom from the octanoyl C6 carbon, forming 5’-deoxyadenosine (5’-dAH). (3) The octanoyl C6 carbon in turn forms a covalent bond with a sulfur atom of the auxiliary cluster, concomitant with partial degradation of the cluster. (4) A second SAM molecule binds to LIAS, and upon electron supply from FDX1 (or DT) again leads to 5’-dA· radical formation by the catalytic cluster and abstraction of a proton from the terminal C8 carbon of the octanoyl moiety. (5) A second sulfur atom is covalently attached to the thiooctanoyl molecule, (6) leading to formation of the mature lipoyl cofactor and further degradation of the Fe/S cluster. To enable multiple reaction cycles, the auxiliary cluster must be regenerated by a still unclear mechanism. In humans, the mature lipoyl cofactor is finally transferred to the target proteins, for example, the E2 subunits of pyruvate (PDH) and α-ketoglutarate (KGDH) dehydrogenase complexes. BCKDH, branched-chain ketoacid dehydrogenase; OADH, 2-oxoadipate dehydrogenase. b Time courses of 6-thiooctanoyl intermediate and lipoyl product formation in FDX1-catalyzed reactions (see Fig. 3b, c). Samples included 0.5 mM peptide substrate, 35 µM LIAS, 2 mM NADPH, 20 µM FDXR, FDX1 as indicated and 1 mM SAM. Formation of the 6-thiooctanoyl intermediate proceeded significantly faster than lipoyl formation, indicating the second sulfur insertion step to be rate-limiting under the experimental conditions. The result suggested to record the data of other experiments after 150 min incubation.
Extended Data Fig. 7 Differential growth complementation of human LIAS-containing Gal-YAH1-lip5Δ cells by human FDX1 and FDX2 reflects their distinct functions.
Gal-YAH1-lip5Δ yeast cells were transformed with a plasmid encoding human LIAS plus vectors containing no gene (-), FDX1 and/or FDX2 as indicated. a Cells were grown in liquid media for 4 days. Optical density (OD) at 600 nm was measured every 30 min. Growth in minimal lactate medium (SLac) is indicated by dashed lines, in rich yeast peptone lactate (YPLac) medium by solid lines. Grey dashed lines indicate standard errors (n = 4) using a microplate reader. b Serial dilutions of the indicated yeast strains were spotted onto agar plates containing minimal (S) or yeast peptone rich (YP) medium plus the indicated carbon sources. Plates were incubated at 30 °C for 3 days. The growth results fit to distinct functions of human FDX1 and FDX2 in lipoylation/heme a synthesis and Fe/S protein biogenesis, respectively. Complementation of the LIAS-expressing Gal-YAH1-lip5Δ cells with FDX2 but not FDX1 supported growth due to Fe/S protein biogenesis restoration. The residual growth on non-fermentable carbon sources (lactate or glycerol) was due to the leaky GAL promoter allowing residual amounts of Yah1, and hence lipoate/heme a, being produced. Growth under these conditions was increased to normal levels by the combined expression of both human FDXs because FDX1 regenerated synthesis of both lipoate and heme a, and FDX2 supported Fe/S protein biogenesis.
Extended Data Fig. 8 Multi-sequence alignment of mitochondrial ferredoxins.
The multi-sequence alignment was generated by Multalin (http://multalin.toulouse.inra.fr/multalin). Secondary structure elements of the human FDX2 structure are shown above the alignment according to PROMOTIF (www.uoxray.uoregon.edu/local/manuals/promotif/document_2.html). Numbering is according to the full-length sequences of human (Hs) FDX1 and FDX2 retrieved from Uniprot (https://www.uniprot.org). The Fe/S cluster-coordinating cysteine residues are highlighted in yellow. Altered residues/regions of human FDX1 and FDX2 are highlighted in grey (see also Supplementary Table 3). Residues/regions mutated for interconversion of FDX1 and FDX2 functions (mutants M3, M5, M6, M7, R135E and C-terminal deletion/exchange) are additionally highlighted by colored boxes for animal FDX1/2-type sequences. Names of organisms are colored according to FDX-type: animal FDX2 (red), mitosomal (black), plant (green), fungal (orange), animal FDX1 (blue) and bacterial (grey). Two Trypanosoma brucei (Tb) FDXs best align between fungal and FDX1-type (FdxA) proteins or between FDX1-type and bacterial (FdxB) proteins4. FDXs from the following organisms were used (sequence identifiers in brackets): Homo sapiens (NP_001026904.2, NP_004100.1), Canis lupus familiaris (XP_038284595.1, XP_038367022.1), Bos taurus (NP_001073695.1, NP_851354.1), Rattus norvegicus (NP_001101472.1, NP_058822.2), Mus musculus (NP_001034913.1, NP_032022.1), Danio rerio (NP_001070132.1, XP_001922722.2), Drosophila melanogaster (NP_001189075.1, NP_647889.2), Encephalitozoon cuniculi GB-M1 (NP_585988.1), Trachipleistophora hominis OX = 72359 GN = THOM_0371 PE = 4 SV = 1 (L7K0F4_TRAHO), Arabidopsis thaliana (NP_001329852.1), Populus alba (XP_034922198.1), Oryza sativa Japonica Group (XP_015647182.1), Chaetomium thermophilum var. thermophilum DSM 1495 (XP_006692961.1), Chaetomium globosum CBS 148.51 (XP_001225251.1), Neurospora crassa OR74A (XP_958085.1), Aspergillus fumigatus Af293 (XP_747954.1), Yarrowia lipolytica CLIB122 (XP_500417.1), Saccharomyces cerevisiae S288C (NP_015071.1), Schizosaccharomyces pombe Etp1fd (2WLB_A), Trypanosoma brucei brucei (XP_845713.1 and XP_844647.1), Magnetospirillum magnetotacticum (WP_009867486.1), Rhodospirillum rubrum (WP_200292119.1), Rickettsia prowazekii (WP_004595967.1), Yersinia pestis (WP_015683702.1), Shigella flexneri (EHF1065008.1), Salmonella enterica (WP_001124473.1).
Extended Data Fig. 9 Site-directed mutagenesis identifies FDX2 regions that functionally distinguish from FDX1.
Gal-YAH1 yeast cells were transformed with vectors expressing the indicated FDXs and variants (see Supplementary Table 5). Growth of serial cell dilutions on minimal medium (S) agar plates containing glucose or galactose was at 30 °C for 3 days. FDX1-FDX2-discriminating regions critical for the in vivo function of human FDX2 were identified using FDX2 variants in which amino acids where exchanged to those of FDX1. A loss of cell growth identified the respective altered region as being important for FDX2 function. The broken lines separate independent plates.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–6 and References.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schulz, V., Basu, S., Freibert, SA. et al. Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2. Nat Chem Biol 19, 206–217 (2023). https://doi.org/10.1038/s41589-022-01159-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01159-4
This article is cited by
-
Targeting cuproplasia and cuproptosis in cancer
Nature Reviews Clinical Oncology (2024)
-
Mechanism and structural dynamics of sulfur transfer during de novo [2Fe-2S] cluster assembly on ISCU2
Nature Communications (2024)
-
Diabetic Cardiomyopathy and Cell Death: Focus on Metal-Mediated Cell Death
Cardiovascular Toxicology (2024)
-
A novel cuproptosis-related diagnostic gene signature and differential expression validation in atherosclerosis
Molecular Biomedicine (2023)
-
Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer
Journal of Experimental & Clinical Cancer Research (2023)