Abstract
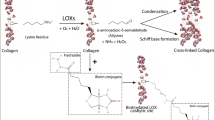
Collagens are fibrous proteins that are integral to the strength and stability of connective tissues. During collagen maturation, lysyl oxidases (LOX) initiate the cross-linking of fibers, but abnormal LOX activity is associated with impaired tissue function as seen in fibrotic and malignant diseases. Visualizing and targeting this dynamic process in healthy and diseased tissue is important, but so far not feasible. Here we present a probe for the simultaneous monitoring and targeting of LOX-mediated collagen cross-linking that combines a LOX-activity sensor with a collagen peptide to chemoselectively target endogenous aldehydes generated by LOX. This synergistic probe becomes covalently anchored and lights up in vivo and in situ in response to LOX at the sites where cross-linking occurs, as demonstrated by staining of normal skin and cancer sections. We anticipate that our reactive collagen-based sensor will improve understanding of collagen remodeling and provide opportunities for the diagnosis of fibrotic and malignant diseases.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information. Expression data for LOX and LOX-like isoforms is available from the Hair-GEL database (E14.5 and P5) (http://hair-gel.net/).
References
Shoulders, M. D. & Raines, R. T. Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 (2009).
Eming, S. A., Martin, P. & Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr266 (2014).
Rockey, D. C., Bell, P. D. & Hill, J. A. Fibrosis—a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 (2015).
Kagan, H. M. & Li, W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88, 660–672 (2003).
Vallet, S. D. & Ricard-Blum, S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. 63, 349–364 (2019).
Ramshaw, J. A. M., Shah, N. K. & Brodsky, B. Gly-X-Y tripeptide frequencies in collagen: a context for host–guest triple-helical peptides. J. Struct. Biol. 122, 86–91 (1998).
Rodriguez-Pascual, F. & Slatter, D. A. Collagen cross-linking: insights on the evolution of metazoan extracellular matrix. Sci. Rep. 6, 37374 (2016).
Piersma, B. & Bank, R. A. Collagen cross-linking mediated by lysyl hydroxylase 2: an enzymatic battlefield to combat fibrosis. Essays Biochem. 63, 377–387 (2019).
Levene, C. I. & Gross, J. Alterations in state of molecular aggregation of collagen induced in chick embryos by beta-aminopropionitrile (lathyrus factor). J. Exp. Med. 110, 771–790 (1959).
Cox, T. R. & Erler, J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Models Mech. 4, 165–178 (2011).
Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Montesi, S. B., Desogere, P., Fuchs, B. C. & Caravan, P. Molecular imaging of fibrosis: recent advances and future directions. J. Clin. Invest. 129, 24–33 (2019).
Baues, M. et al. Fibrosis imaging: current concepts and future directions. Adv. Drug. Deliv. Rev. 121, 9–26 (2017).
Palamakumbura, A. H. & Trackman, P. C. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal. Biochem. 300, 245–251 (2002).
Pinnell, S. R. & Martin, G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to α-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc. Natl Acad. Sci. USA 61, 708–716 (1968).
Holt, A. & Palcic, M. M. A peroxidase-coupled continuous absorbance plate-reader assay for flavin monoamine oxidases, copper-containing amine oxidases and related enzymes. Nat. Protoc. 1, 2498–2505 (2006).
Trackman, P. C., Zoski, C. G. & Kagan, H. M. Development of a peroxidase-coupled fluorometric assay for lysyl oxidase. Anal. Biochem. 113, 336–342 (1981).
Rodriguez, H. M. et al. Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J. Biol. Chem. 285, 20964–20974 (2010).
Aslam, T. et al. Optical molecular imaging of lysyl oxidase activity—detection of active fibrogenesis in human lung tissue. Chem. Sci. 6, 4946–4953 (2015).
Chen, G., Yee, D. J., Gubernator, N. G. & Sames, D. Design of optical switches as metabolic indicators: new fluorogenic probes for monoamine oxidases (MAO A and B). J. Am. Chem. Soc. 127, 4544–4545 (2005).
Albers, A. E., Rawls, K. A. & Chang, C. J. Activity-based fluorescent reporters for monoamine oxidases in living cells. Chem. Commun. 44, 4647–4649 (2007).
Wu, X., Shi, W., Li, X. & Ma, H. A. Strategy for specific fluorescence imaging of monoamine oxidase a in living cells. Angew. Chem. Int. Ed. 56, 15319–15323 (2017).
Sun, W.-C., Gee, K. R. & Haugland, R. P. Synthesis of novel fluorinated coumarins: excellent UV-light excitable fluorescent dyes. Bioorg. Med. Chem. Lett. 8, 3107–3110 (1998).
Cohen, J. D., Thompson, S. & Ting, A. Y. Structure-guided engineering of a pacific blue fluorophore ligase for specific protein imaging in living cells. Biochemistry 50, 8221–8225 (2011).
Lee, M. M., Gao, Z. & Peterson, B. R. Synthesis of a fluorescent analogue of paclitaxel that selectively binds microtubules and sensitively detects efflux by P-glycoprotein. Angew. Chem. Int. Ed. 56, 6927–6931 (2017).
Chang, D., Kim, K. T., Lindberg, E. & Winssinger, N. Accelerating turnover frequency in nucleic acid templated reactions. Bioconj. Chem. 29, 158–163 (2018).
Klein, G. & Reymond, J. L. An enantioselective fluorimetric assay for alcohol dehydrogenases using albumin-catalyzed beta-elimination of umbelliferone. Bioorg. Med. Chem. Lett. 8, 1113–1116 (1998).
Csiszar, K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 70, 1–32 (2001).
Brown-Augsburger, P., Tisdale, C., Broekelmann, T., Sloan, C. & Mecham, R. P. Identification of an elastin cross-linking domain that joins three peptide chains: possible role in nucleated assembly. J. Biol. Chem. 270, 17778–17783 (1995).
Sanada, H. et al. Changes in collagen cross-linking and lysyl oxidase by estrogen. Biochim. Biophys. Acta, Gen. Subj. 541, 408–413 (1978).
Szauter, K. M., Cao, T., Boyd, C. D. & Csiszar, K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol. Biol. (Paris) 53, 448–456 (2005).
Chattopadhyay, S. & Raines, R. T. Collagen-based biomaterials for wound healing. Biopolymers 101, 821–833 (2014).
Bennink, L. L. et al. Visualizing collagen proteolysis by peptide hybridization: from 3D cell culture to in vivo imaging. Biomaterials 183, 67–76 (2018).
Dones, J. M. et al. Optimization of interstrand interactions enables burn detection with a collagen-mimetic peptide. Org. Biomol. Chem. 17, 9906–9912 (2019).
Li, Y. et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl Acad. Sci. USA 109, 14767–14772 (2012).
Takita, K. K., Fujii, K. K., Kadonosono, T., Masuda, R. & Koide, T. Cyclic peptides for efficient detection of collagen. Chem. Bio. Chem. 19, 1613–1617 (2018).
Kalia, J. & Raines, R. T. Hydrolytic stability of hydrazones and oximes. Angew. Chem. Int. Ed. 47, 7523–7526 (2008).
Kölmel, D. K. & Kool, E. T. Oximes and hydrazones in bioconjugation: mechanism and catalysis. Chem. Rev. 117, 10358–10376 (2017).
Waghorn, P. A. et al. Molecular magnetic resonance imaging of lung fibrogenesis with an oxyamine-based probe. Angew. Chem. Int. Ed. 56, 9825–9828 (2017).
Hentzen, N. B., Smeenk, L. E. J., Witek, J., Riniker, S. & Wennemers, H. Cross-linked collagen triple helices by oxime ligation. J. Am. Chem. Soc. 139, 12815–12820 (2017).
Merkel, J. R., DiPaolo, B. R., Hallock, G. G. & Rice, D. C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc. Soc. Exp. Biol. Med. 187, 493–497 (1988).
Volk, S. W., Wang, Y., Mauldin, E. A., Liechty, K. W. & Adams, S. L. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 194, 25–37 (2011).
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4, 119–136 (2015).
Wietecha, M. S. et al. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat. Commun. 11, 2604 (2020).
Rucklidge, G. J., Milne, G., McGaw, B. A., Milne, E. & Robins, S. P. Turnover rates of different collagen types measured by isotope ratio mass spectrometry. Biochim. Biophys. Acta 1156, 57–61 (1992).
Campagnola, P. J. et al. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 82, 493–508 (2002).
Zoumi, A., Yeh, A. & Tromberg, B. J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl Acad. Sci. USA 99, 11014–11019 (2002).
Perryman, L. & Erler, J. T. Lysyl oxidase in cancer research. Future Oncol. 10, 1709–1717 (2014).
Cangkrama, M. et al. A paracrine activin A–mDia2 axis promotes squamous carcinogenesis via fibroblast reprogramming. EMBO Mol. Med. 12, e11466 (2020).
Pensalfini, M. et al. The mechanical fingerprint of murine excisional wounds. Acta Biomater. 65, 226–236 (2018).
Acknowledgements
We thank P. Bruckner, Freiburg, Germany, for critical comments on the manuscript, M. Wietecha, ETH Zurich, for discussions and assistance compiling LOX expression data, M. Cangkrama, ETH Zurich, for the skin tumor samples and J. Kusch for microscopy assistance. SHG imaging was carried out at the Scientific Center for Optical and Electron Microscopy (ScopeM) at the ETH Zurich. This work was funded by the ETH Zurich and the European Union’s Seventh Framework Program with an ETH Postdoctoral Fellowship (to M.R.A.), the Fonds National de la Recherche Luxembourg with an AFR Ph.D. Fellowship (to N.B.H.), the Swiss National Science Foundation (SNF grant nos. 31003B-189364 (to S.W.) and 2000020_178805 (to H.W.)), University Medicine Zurich (Flagship project SKINTEGRITY to S.W. and H.W.) and by the ETH Zurich OpenETH Project SKINTEGRITY.CH (to S.W. and H.W.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.R.A. and H.W. conceived the project. M.R.A. performed the chemical syntheses together with N.B.H., and carried out the spectroscopic analyses and the ex vivo and in situ assays after P.H. collected the tissue samples. P.H. performed the in vivo experiments and histological/immunofluorescence analyses. S.W. designed the in vivo experiments together with P.H. M.R.A. and H.W. drafted the paper. All authors contributed to data analysis and writing of the paper.
Corresponding author
Ethics declarations
Competing interests
ETH Zurich has applied for a patent (EP19213787.5) related to technology described in this publication with M.R.A and H.W. listed as inventors.
Additional information
Peer review information Nature Chemical Biology thanks Fernando Rodriguez-Pascual and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–35, Table 1 and Note: Synthetic protocols and characterization.
Rights and permissions
About this article
Cite this article
Aronoff, M.R., Hiebert, P., Hentzen, N.B. et al. Imaging and targeting LOX-mediated tissue remodeling with a reactive collagen peptide. Nat Chem Biol 17, 865–871 (2021). https://doi.org/10.1038/s41589-021-00830-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00830-6
This article is cited by
-
Glycosylation of Collagen Provokes Diabetic Wound Ulcers
Biomedical Materials & Devices (2024)