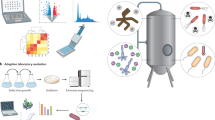
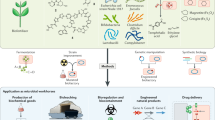
Abstract
Microbial chemical production is a rapidly growing industry, with much of the growth fueled by advances in synthetic biology. New approaches have enabled rapid strain engineering for the production of various compounds; however, translation to industry is often problematic because native phenotypes of model hosts prevent the design of new low-cost bioprocesses. Here, we argue for a new approach that leverages the native stress-tolerant phenotypes of non-conventional microbes that directly address design challenges from the outset. Growth at high temperature, high salt and solvent concentrations, and low pH can enable cost savings by reducing the energy required for product separation, bioreactor cooling, and maintaining sterile conditions. These phenotypes have the added benefit of allowing for the use of low-cost sugar and water resources. Non-conventional hosts are needed because these phenotypes are polygenic and thus far have proven difficult to recapitulate in the common hosts Escherichia coli and Saccharomyces cerevisiae.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carlson, R. Estimating the biotech sector’s contribution to the US economy. Nat. Biotechnol. 34, 247–255 (2016). This Perspective article provides a detailed analysis of the US industrial biotechnology sector.
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Caruthers, M. H. A brief review of DNA and RNA chemical synthesis. Biochem. Soc. Trans. 39, 575–580 (2011).
Chao, R., Mishra, S., Si, T. & Zhao, H. Engineering biological systems using automated biofoundries. Metab. Eng. 42, 98–108 (2017).
Hong, K. K. & Nielsen, J. Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries. Cell. Mol. Life Sci. 69, 2671–2690 (2012).
Pontrelli, S. et al. Escherichia coli as a host for metabolic engineering. Metab. Eng. 50, 16–46 (2018).
Shapouri, H. & Gallagher, P. USDA’s 2002 Ethanol Cost-of-Production Survey. Agricultural Economics Report Number 841 (US Department of Agriculture, Office of Energy Policy and New Uses, 2005).
Blanch, H.W. & Clark, D.S. Biochemical Engineering, xii (M. Dekker, 1996).
Lam, F. H., Ghaderi, A., Fink, G. R. & Stephanopoulos, G. Biofuels. Engineering alcohol tolerance in yeast. Science 346, 71–75 (2014).
Rich, J. O., Leathers, T. D., Bischoff, K. M., Anderson, A. M. & Nunnally, M. S. Biofilm formation and ethanol inhibition by bacterial contaminants of biofuel fermentation. Bioresour. Technol. 196, 347–354 (2015).
Löbs, A. K., Engel, R., Schwartz, C., Flores, A. & Wheeldon, I. CRISPR–Cas9-enabled genetic disruptions for understanding ethanol and ethyl acetate biosynthesis in Kluyveromyces marxianus. Biotechnol. Biofuels 10, 164 (2017).
Shui, W. et al. Understanding the mechanism of thermotolerance distinct from feat shock response through proteomic analysis of industrial strains of Saccharomyces cerevisiae. Mol. Cell. Proteom. 14, 1885–1897 (2015).
Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell 40, 253–266 (2010).
Phipps, B. M. et al. Structure of a molecular chaperone from a thermophilic Archaebacterium. Nature 361, 475–477 (1993).
Parsell, D. A. & Lindquist, S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496 (1993).
Strassburg, K., Walther, D., Takahashi, H., Kanaya, S. & Kopka, J. Dynamic transcriptional and metabolic responses in yeast adapting to temperature stress. OMICS 14, 249–259 (2010).
Balchin, D., Hayer-Hartl, M. & Hartl, F. U. In vivo aspects of protein folding and quality control. Science 353, aac4354 (2016).
Verghese, J., Abrams, J., Wang, Y. & Morano, K. A. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol. Mol. Biol. Rev. 76, 115–158 (2012).
Gasch, A. P. et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 (2000).
Rodrigues, J. L. & Rodrigues, L. R. Potential applications of the Escherichia coli heat shock response in synthetic biology. Trends Biotechnol. 36, 186–198 (2018).
Lewin, A., Wentzel, A. & Valla, S. Metagenomics of microbial life in extreme temperature environments. Curr. Opin. Biotechnol. 24, 516–525 (2013).
Jacquemet, A., Barbeau, J., Lemiègre, L. & Benvegnu, T. Archaeal tetraether bipolar lipids: structures, functions and applications. Biochimie 91, 711–717 (2009).
Li, P. et al. The transcription factors Hsf1 and Msn2 of thermotolerant Kluyveromyces marxianus promote cell growth and ethanol fermentation of Saccharomyces cerevisiae at high temperatures. Biotechnol. Biofuels 10, 289 (2017).
Seong, Y. J. et al. Physiological and metabolomic analysis of Issatchenkia orientalis MTY1 with multiple tolerance for cellulosic bioethanol production. Biotechnol. J. 12, 1700110 (2017).
Blaby, I. K. et al. Experimental evolution of a facultative thermophile from a mesophilic ancestor. Appl. Environ. Microbiol. 78, 144–155 (2012).
Rudolph, B., Gebendorfer, K. M., Buchner, J. & Winter, J. Evolution of Escherichia coli for growth at high temperatures. J. Biol. Chem. 285, 19029–19034 (2010).
Caspeta, L. et al. Biofuels. Altered sterol composition renders yeast thermotolerant. Science 346, 75–78 (2014).
Shi, D. J., Wang, C. L. & Wang, K. M. Genome shuffling to improve thermotolerance, ethanol tolerance and ethanol productivity of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 36, 139–147 (2009).
Bhandiwad, A. et al. Metabolic engineering of Thermoanaerobacterium saccharolyticum for n-butanol production. Metab. Eng. 21, 17–25 (2014).
Lin, P. P. et al. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum. Metab. Eng. 31, 44–52 (2015).
Cripps, R. E. et al. Metabolic engineering of Geobacillus thermoglucosidasius for high yield ethanol production. Metab. Eng. 11, 398–408 (2009).
Yue, H. T. et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates. Biotechnol. Biofuels 7, 108 (2014). This paper shows the power of stress-tolerant microbes. Engineered halophilic H. campaniensis was used in non-aseptic bioreactors to produce high titers of polyhydroxybutyrate from mixed substrate feedstocks.
Voronovsky, A. Y., Rohulya, O. V., Abbas, C. A. & Sibirny, A. A. Development of strains of the thermotolerant yeast Hansenula polymorpha capable of alcoholic fermentation of starch and xylan. Metab. Eng. 11, 234–242 (2009).
Löbs, A. K., Lin, J. L., Cook, M. & Wheeldon, I. High throughput, colorimetric screening of microbial ester biosynthesis reveals high ethyl acetate production from Kluyveromyces marxianus on C5, C6, and C12 carbon sources. Biotechnol. J. 11, 1274–1281 (2016).
Xiao, H., Shao, Z., Jiang, Y., Dole, S. & Zhao, H. Exploiting Issatchenkia orientalis SD108 for succinic acid production. Microb. Cell Fact. 13, 121 (2014).
Löbs, A. K., Schwartz, C., Thorwall, S. & Wheeldon, I. Highly multiplexed CRISPRi repression of respiratory functions enhances mitochondrial localized ethyl acetate biosynthesis in Kluyveromyces marxianus. ACS Synth. Biol. 7, 2647–2655 (2018). This paper demonstrates the power of CRISPR technologies for rapidly engineering non-conventional hosts. Here, multiplexed CRISPR interference (CRISPRi) was used to screen various transcriptional programs to optimize a native biosynthetic pathway in the thermotolerant yeast K. marxianus.
Abdel-Banat, B. M., Hoshida, H., Ano, A., Nonklang, S. & Akada, R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 85, 861–867 (2010).
Cernak, P. et al. Engineering Kluyveromyces marxianus as a robust synthetic biology platform host. MBio 9, e01410–e01418 (2018).
Cheon, Y. et al. A biosynthetic pathway for hexanoic acid production in Kluyveromyces marxianus. J. Biotechnol. 182-183, 30–36 (2014).
Dmytruk, K., Kurylenko, O., Ruchala, J., Ishchuk, O. & Sibirny, A. in Yeast Diversity in Human Welfare (eds Satyanarayana, T. & Kunze, G.) 257–282 (Springer, 2017).
Lenton, S., Walsh, D. L., Rhys, N. H., Soper, A. K. & Dougan, L. Structural evidence for solvent-stabilisation by aspartic acid as a mechanism for halophilic protein stability in high salt concentrations. Phys. Chem. Chem. Phys. 18, 18054–18062 (2016).
Oren, A. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst. 4, 2 (2008).
Padan, E., Venturi, M., Gerchman, Y. & Dover, N. Na+/H+ antiporters. Biochim. Biophys. Acta 1505, 144–157 (2001).
Purvis, J. E., Yomano, L. P. & Ingram, L. O. Enhanced trehalose production improves growth of Escherichia coli under osmotic stress. Appl. Environ. Microbiol. 71, 3761–3769 (2005).
Yang, L. et al. A primary sodium pump gene of the moderate halophile Halobacillus dabanensis exhibits secondary antiporter properties. Biochem. Biophys. Res. Commun. 346, 612–617 (2006).
Ekberg, J. et al. Adaptive evolution of the lager brewing yeast Saccharomyces pastorianus for improved growth under hyperosmotic conditions and its influence on fermentation performance. FEMS Yeast Res. 13, 335–349 (2013).
Schweikhard, E. S., Kuhlmann, S. I., Kunte, H. J., Grammann, K. & Ziegler, C. M. Structure and function of the universal stress protein TeaD and its role in regulating the ectoine transporter TeaABC of Halomonas elongata DSM 2581(T). Biochemistry 49, 2194–2204 (2010).
Kunte, H., Lentzen, G. & Galinski, E. Industrial production of the cell protectant ectoine: protection mechanisms, processes, and products. Curr. Biotechnol. 3, 10–25 (2014).
Chen, X., Yu, L., Qiao, G. & Chen, G. Q. Reprogramming Halomonas for industrial production of chemicals. J. Ind. Microbiol. Biotechnol. 45, 545–554 (2018).
Qin, Q. et al. CRISPR/Cas9 editing genome of extremophile Halomonas spp. Metab. Eng. 47, 219–229 (2018).
Chen, R. et al. Optimization of the extraction and purification of the compatible solute ectoine from Halomonas elongate in the laboratory experiment of a commercial production project. World J. Microbiol. Biotechnol. 33, 116 (2017).
Zaky, A. S., Greetham, D., Tucker, G. A. & Du, C. The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Sci. Rep. 8, 12127 (2018).
Yuan, W. J., Zhao, X. Q., Ge, X. M. & Bai, F. W. Ethanol fermentation with Kluyveromyces marxianus from Jerusalem artichoke grown in salina and irrigated with a mixture of seawater and freshwater. J. Appl. Microbiol. 105, 2076–2083 (2008).
Ramos, J. L., Duque, E., Godoy, P. & Segura, A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J. Bacteriol. 180, 3323–3329 (1998).
Rühl, J., Schmid, A. & Blank, L. M. Selected Pseudomonas putida strains able to grow in the presence of high butanol concentrations. Appl. Environ. Microbiol. 75, 4653–4656 (2009).
Ramos, J. L. et al. Mechanisms for solvent tolerance in bacteria. J. Biol. Chem. 272, 3887–3890 (1997).
Rojas, A. et al. Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J. Bacteriol. 183, 3967–3973 (2001).
Terán, W. et al. Complexity in efflux pump control: cross-regulation by the paralogues TtgV and TtgT. Mol. Microbiol. 66, 1416–1428 (2007).
Segura, A. et al. Proteomic analysis reveals the participation of energy- and stress-related proteins in the response of Pseudomonas putida DOT-T1E to toluene. J. Bacteriol. 187, 5937–5945 (2005).
Dunlop, M. J. et al. Engineering microbial biofuel tolerance and export using efflux pumps. Mol. Syst. Biol. 7, 487 (2011).
Tan, Z., Yoon, J. M., Nielsen, D. R., Shanks, J. V. & Jarboe, L. R. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables. Metab. Eng. 35, 105–113 (2016).
Zingaro, K. A. & Terry Papoutsakis, E. GroESL overexpression imparts Escherichia coli tolerance to i-, n-, and 2-butanol, 1,2,4-butanetriol and ethanol with complex and unpredictable patterns. Metab. Eng. 15, 196–205 (2013).
Cook, T. B. et al. Genetic tools for reliable gene expression and recombineering in Pseudomonas putida. J. Ind. Microbiol. Biotechnol. 45, 517–527 (2018).
Gong, T. et al. Metabolic engineering of Pseudomonas putida KT2440 for complete mineralization of methyl parathion and γ-hexachlorocyclohexane. ACS Synth. Biol. 5, 434–442 (2016).
Nikel, P. I. & de Lorenzo, V. Pseudomonas putida as a functional chassis for industrial biocatalysis: from native biochemistry to trans-metabolism. Metab. Eng. 50, 142–155 (2018).
Bormann, S. et al. Engineering Clostridium acetobutylicum for production of kerosene and diesel blendstock precursors. Metab. Eng. 25, 124–130 (2014).
Anbarasan, P. et al. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 491, 235–239 (2012).
Zhang, J., Wu, C. D., Du, G. C. & Chen, J. Enhanced acid tolerance in Lactobacillus casei by adaptive evolution and compared stress response during acid stress. Biotechnol. Bioprocess Eng. 17, 283–289 (2012).
Zhou, L. et al. Improvement of d-lactate productivity in recombinant Escherichia coli by coupling production with growth. Biotechnol. Lett. 34, 1123–1130 (2012).
Lee, J. Y., Kang, C. D., Lee, S. H., Park, Y. K. & Cho, K. M. Engineering cellular redox balance in Saccharomyces cerevisiae for improved production of l-lactic acid. Biotechnol. Bioeng. 112, 751–758 (2015).
Yan, D. et al. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour. Technol. 156, 232–239 (2014).
Kwon, Y. D., Kim, S., Lee, S. Y. & Kim, P. Long-term continuous adaptation of Escherichia coli to high succinate stress and transcriptome analysis of the tolerant strain. J. Biosci. Bioeng. 111, 26–30 (2011).
Wright, J. et al. Batch and continuous culture-based selection strategies for acetic acid tolerance in xylose-fermenting Saccharomyces cerevisiae. FEMS Yeast Res. 11, 299–306 (2011).
Sandoval, N. R., Mills, T. Y., Zhang, M. & Gill, R. T. Elucidating acetate tolerance in E. coli using a genome-wide approach. Metab. Eng. 13, 214–224 (2011).
Suominen, P. et al. Genetically modified yeast of the species Issatchenkia Orientalis and closely relates species, and fermentation processes using same (Cargill Inc., 2012).
Valdés, J. et al. Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9, 597 (2008).
Kernan, T. et al. Engineering the iron-oxidizing chemolithoautotroph Acidithiobacillus ferrooxidans for biochemical production. Biotechnol. Bioeng. 113, 189–197 (2016).
Inaba, Y., Banerjee, I., Kernan, T. & Banta, S. Transposase-mediated chromosomal integration of exogenous genes in Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 84, e01381–18 (2018).
Maezato, Y., Johnson, T., McCarthy, S., Dana, K. & Blum, P. Metal resistance and lithoautotrophy in the extreme thermoacidophile Metallosphaera sedula. J. Bacteriol. 194, 6856–6863 (2012).
Auernik, K. S., Maezato, Y., Blum, P. H. & Kelly, R. M. The genome sequence of the metal-mobilizing, extremely thermoacidophilic archaeon Metallosphaera sedula provides insights into bioleaching-associated metabolism. Appl. Environ. Microbiol. 74, 682–692 (2008).
Zeldes, B. M. et al. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front. Microbiol. 6, 1209 (2015).
Feist, A. M. et al. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol. Syst. Biol. 3, 121 (2007).
Förster, J., Famili, I., Fu, P., Palsson, B. O. & Nielsen, J. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 13, 244–253 (2003).
Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature 460, 894–898 (2009).
Warner, J. R., Reeder, P. J., Karimpour-Fard, A., Woodruff, L. B. & Gill, R. T. Rapid profiling of a microbial genome using mixtures of barcoded oligonucleotides. Nat. Biotechnol. 28, 856–862 (2010).
Lee, M. E., DeLoache, W. C., Cervantes, B. & Dueber, J. E. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth. Biol. 4, 975–986 (2015).
Xu, P., Vansiri, A., Bhan, N. & Koffas, M. A. ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth. Biol. 1, 256–266 (2012).
Löbs, A. K., Schwartz, C. & Wheeldon, I. Genome and metabolic engineering in non-conventional yeasts: current advances and applications. Synth. Syst. Biotechnol. 2, 198–207 (2017).
Schwartz, C., Frogue, K., Ramesh, A., Misa, J. & Wheeldon, I. CRISPRi repression of nonhomologous end-joining for enhanced genome engineering via homologous recombination in Yarrowia lipolytica. Biotechnol. Bioeng. 114, 2896–2906 (2017).
Cao, M., Gao, M., Ploessl, D., Song, C. & Shao, Z. CRISPR-mediated genome editing and gene repression in Scheffersomyces stipitis. Biotechnol. J. 13, e1700598 (2018).
Schwartz, C., Shabbir-Hussain, M., Frogue, K., Blenner, M. & Wheeldon, I. Standardized markerless gene integration for pathway engineering in Yarrowia lipolytica. ACS Synth. Biol. 6, 402–409 (2017).
Schwartz, C. et al. Validating genome-wide CRISPR-Cas9 function improves screening in the oleaginous yeast Yarrowia lipolytica. Metab. Eng. 55, 102–110 (2019).
Schwartz, C. M., Hussain, M. S., Blenner, M. & Wheeldon, I. Synthetic RNA polymerase III promoters facilitate high-efficiency CRISPR-Cas9-mediated genome editing in Yarrowia lipolytica. ACS Synth. Biol. 5, 356–359 (2016).
Cao, M. et al. Centromeric DNA facilitates nonconventional yeast genetic engineering. ACS Synth. Biol. 6, 1545–1553 (2017).
Lewis, N. E., Nagarajan, H. & Palsson, B. O. Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 10, 291–305 (2012).
Schwalen, C. J., Hudson, G. A., Kille, B. & Mitchell, D. A. Bioinformatic expansion and discovery of thiopeptide antibiotics. J. Am. Chem. Soc. 140, 9494–9501 (2018).
Brophy, J. A. N. et al. Engineered integrative and conjugative elements for efficient and inducible DNA transfer to undomesticated bacteria. Nat. Microbiol. 3, 1043–1053 (2018). This work engineers the integrative and conjugative elements of Bacillus subtilis to create a strain technology called XPORT that can be used to rapidly engineer novel bacterial isolates.
Schwartz, C., Curtis, N., Löbs, A. K. & Wheeldon, I. Multiplexed CRISPR activation of cryptic sugar metabolism enables Yarrowia lipolytica growth on cellobiose. Biotechnol. J. 13, e1700584 (2018).
Boundy-Mills, K. L. et al. Yeast culture collections in the twenty-first century: new opportunities and challenges. Yeast 33, 243–260 (2016).
Solomon, K. V. et al. Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science 351, 1192–1195 (2016). This work uses ‘omics’-level analyses and biochemical assays to discover new biotechnology-relevant enzymes, including cellulases for the breakdown of lignocellulosic biomass in new isolates of anaerobic gut fungi.
Acknowledgements
This material is based upon work supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, Genomic Science Program under Award Number DE-SC0019093, Air Force Office of Scientific Research award FA9550-17-1-0270, Army Research Office MURI award W911NF1410263, and National Science Foundation award NSF-CBET 1706545 for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thorwall, S., Schwartz, C., Chartron, J.W. et al. Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis. Nat Chem Biol 16, 113–121 (2020). https://doi.org/10.1038/s41589-019-0452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0452-x
This article is cited by
-
Prospects of formamide as nitrogen source in biotechnological production processes
Applied Microbiology and Biotechnology (2024)
-
Strategies to increase the robustness of microbial cell factories
Advanced Biotechnology (2024)
-
General mechanisms of weak acid-tolerance and current strategies for the development of tolerant yeasts
World Journal of Microbiology and Biotechnology (2024)
-
Exploring microproteins from various model organisms using the mip-mining database
BMC Genomics (2023)
-
Engineering co-utilization of glucose and xylose for chemical overproduction from lignocellulose
Nature Chemical Biology (2023)