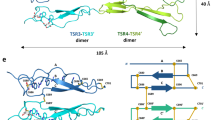
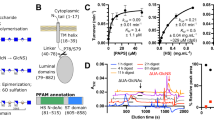
Abstract
Chondroitin sulfate (CS) and heparan sulfate (HS) are glycosaminoglycans that both bind the receptor-type protein tyrosine phosphatase PTPRσ, affecting axonal regeneration. CS inhibits axonal growth, while HS promotes it. Here, we have prepared a library of HS octasaccharides and, together with synthetic CS oligomers, we found that PTPRσ preferentially interacts with CS-E—a rare sulfation pattern in natural CS—and most HS oligomers bearing sulfate and sulfamate groups. Consequently, short and long stretches of natural CS and HS, respectively, bind to PTPRσ. CS activates PTPRσ, which dephosphorylates cortactin—herein identified as a new PTPRσ substrate—and disrupts autophagy flux at the autophagosome–lysosome fusion step. Such disruption is required and sufficient for dystrophic endball formation and inhibition of axonal regeneration. Therefore, sulfation patterns determine the length of the glycosaminoglycan segment that bind to PTPRσ and define the fate of axonal regeneration through a mechanism involving PTPRσ, cortactin and autophagy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Iozzo, R. V. & Schaefer, L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 (2015).
Margolis, R. K. & Margolis, R. U. Nervous tissue proteoglycans. Experientia 49, 429–446 (1993).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002).
Inatani, M., Irie, F., Plump, A. S., Tessier-Lavigne, M. & Yamaguchi, Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 302, 1044–1046 (2003).
Moon, L. D., Asher, R. A., Rhodes, K. E. & Fawcett, J. W. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat. Neurosci. 4, 465–466 (2001).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Imagama, S. et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J. Neurosci. 31, 17091–17102 (2011).
Wang, L. & Denburg, J. L. A role for proteoglycans in the guidance of a subset of pioneer axons in cultured embryos of the cockroach. Neuron 8, 701–714 (1992).
Shen, Y. et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596 (2009).
Aricescu, A. R., McKinnell, I. W., Halfter, W. & Stoker, A. W. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol. Cell Biol. 22, 1881–1892 (2002).
Fisher, D. et al. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J. Neurosci. 31, 14051–14066 (2011).
Johnson, K. G. et al. The HSPGs syndecan and dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49, 517–531 (2006).
Coles, C. H. et al. Proteoglycan-specific molecular switch for RPTPσ clustering and neuronal extension. Science 332, 484–488 (2011).
Katagiri, Y. et al. Identification of novel binding sites for heparin in receptor protein-tyrosine phosphatase (RPTPσ): implications for proteoglycan signaling. J. Biol. Chem. 293, 11639–11647 (2018).
Tonks, N. K. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7, 833–846 (2006).
Xu, D. & Esko, J. D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 83, 129–157 (2014).
Hu, Y. P. et al. Synthesis of 3-O-sulfonated heparan sulfate octasaccharides that inhibit the herpes simplex virus type 1 host-cell interaction. Nat. Chem. 3, 557–563 (2011).
Zulueta, M. M. et al. α-Glycosylation by d-glucosamine-derived donors: synthesis of heparosan and heparin analogues that interact with mycobacterial heparin-binding hemagglutinin. J. Am. Chem. Soc. 134, 8988–8995 (2012).
Hu, Y. P. et al. Divergent synthesis of 48 heparan sulfate-based disaccharides and probing the specific sugar-fibroblast growth factor-1 interaction. J. Am. Chem. Soc. 134, 20722–20727 (2012).
Brown, J. M. et al. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc. Natl Acad Sci. USA 109, 4768–4773 (2012).
Dickendesher, T. L. et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 15, 703–712 (2012).
Tamura, J., Neumann, K. W., Kurono, S. & Ogawa, T. Synthetic approach towards sulfated chondroitin di-, tri- and tetrasaccharides corresponding to the repeating unit. Carbohydr. Res. 305, 43–63 (1997).
Tamura, J. et al. Synthesis and interaction with midkine of biotinylated chondroitin sulfate tetrasaccharides. Bioorg. Med. Chem. Lett. 22, 1371–1374 (2012).
Tamura, J., Nakada, Y., Taniguchi, K. & Yamane, M. Synthesis of chondroitin sulfate E octasaccharide in a repeating region involving an acetamide auxiliary. Carbohydr. Res. 343, 39–47 (2008).
Properzi, F. et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur. J. Neurosci. 21, 378–390 (2005).
Properzi, F. et al. Heparan sulphate proteoglycans in glia and in the normal and injured CNS: expression of sulphotransferases and changes in sulphation. Eur. J. Neurosci. 27, 593–604 (2008).
Tom, V. J., Steinmetz, M. P., Miller, J. H., Doller, C. M. & Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004).
Ramón y Cajal, S. Degeneration and Regeneration of the Nervous System. (Oxford Univ. Press, 1928).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007).
Maday, S., Wallace, K. E. & Holzbaur, E. L. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell. Biol. 196, 407–417 (2012).
Itakura, E., Kishi-Itakura, C. & Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012).
Lee, S., Sato, Y. & Nixon, R. A. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J. Neurosci. 31, 7817–7830 (2011).
Xie, Y. et al. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J. Biol. Chem. 281, 16482–16492 (2006).
Lang, B. T. et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518, 404–408 (2015).
Faux, C. et al. PTPsigma binds and dephosphorylates neurotrophin receptors and can suppress NGF-dependent neurite outgrowth from sensory neurons. Biochim. Biophys. Acta 1773, 1689–1700 (2007).
Tehrani, S., Tomasevic, N., Weed, S., Sakowicz, R. & Cooper, J. A. Src phosphorylation of cortactin enhances actin assembly. Proc. Natl Acad. Sci. USA 104, 11933–11938 (2007).
Hasegawa, J. et al. Autophagosome-lysosome fusion in neurons requires INPP5E, a protein associated with Joubert syndrome. EMBO J. 35, 1853–1867 (2016).
Lee, J. Y. et al. HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 29, 969–980 (2010).
He, Y. et al. Src and cortactin promote lamellipodia protrusion and filopodia formation and stability in growth cones. Mol. Biol. Cell 26, 3229–3244 (2015).
Leveugle, B. et al. Heparin oligosaccharides that pass the blood-brain barrier inhibit beta-amyloid precursor protein secretion and heparin binding to beta-amyloid peptide. J. Neurochem. 70, 736–744 (1998).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Lee, J. H. et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158 (2010).
Knöferle, J. et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl Acad Sci. USA 107, 6064–6069 (2010).
Ribas, V. T. & Lingor, P. Autophagy in degenerating axons following spinal cord injury: evidence for autophagosome biogenesis in retraction bulbs. Neural. Regen. Res 10, 198–200 (2015).
He, M. et al. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc. Natl Acad. Sci. USA 113, 11324–11329 (2016).
Martin, K. R. et al. Identification of PTPsigma as an autophagic phosphatase. J. Cell Sci. 124, 812–819 (2011).
Ohtake, Y. et al. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci. Rep. 6, 37152 (2016).
Ignelzi, M. A., Miller, D. R., Soriano, P. & Maness, P. F. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron 12, 873–884 (1994).
Ito, A. et al. The subcellular localization and activity of cortactin is regulated by acetylation and interaction with Keap1. Sci. Signal. 8, ra120 (2015).
Okada, M. et al. Biosynthesis of heparan sulfate in EXT1‐deficient cells. Biochem. J. 428, 463–471 (2010).
Acknowledgements
We thank T. Kuboyama, T. Tojima and H. Kamiguchi (RIKEN BSI) for their technical guidance with the primary culture of DRG neurons. The expression vector for cortactin was kindly provided by M. Yoshida (RIKEN). We also thank N. Sugiura (Aichi Medical University) for his critical comment on the biochemical data. We wish to acknowledge the Division for Medical Research Engineering, Nagoya University School of Medicine, for the technical support during the electron microscopy and SPR assay. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (KAKENHHI Grant No. 23110002 to K.K.) and a Grant-in-Aid for Scientific Research (KAKENHHI Grant No. 16H05139 to K.K.) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and by a Grant-in-Aid for Young Scientists (KAKENHI Grant No. 26860209 to K.S.) from the Japan Society for the Promotion of Science (JSPS), Japan and by the Ministry of Science and Technology, Taiwan (grant nos. MOST 106-2745-M-001-001-ASP, MOST 106-2113-M259-009, MOST 106-0210-01-15-02 and MOST 106-2113-M-001-009-MY2 to S.C.H) and Academia Sinica (grant no. AS-IA-104-L04 to S.C.H.).
Author information
Authors and Affiliations
Contributions
K.S., T.O. and K.K. designed and performed biological experiments. Y.-C.K., C.-F.T., M.M.L.Z. and A.B. synthesized HS oligosaccharides under supervision by S.C.H. Y.G., S.N. and H.K. contributed biochemical experiments. M.M., Y.I., and K.U. contributed in vivo SCI experiments. J.-I.T. synthesized CS oligosaccharides. K.S., T.O., M.M.L.Z. and K.K. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2, Supplementary Figs. 1–19, Supplementary Video captions.
Supplementary Note
Synthetic Procedures
Supplementary Video 1
Live imaging of growth cones.
Supplementary Video 2
Live imaging of a dystrophic endball.
Supplementary Video 3
Live imaging of retrograde transport of autophagosomes in growth cones.
Supplementary Video 4
Live imaging of retrograde transport of autophagosomes in a dystrophic endball.
Rights and permissions
About this article
Cite this article
Sakamoto, K., Ozaki, T., Ko, YC. et al. Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nat Chem Biol 15, 699–709 (2019). https://doi.org/10.1038/s41589-019-0274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-019-0274-x
This article is cited by
-
Inhibition of CSPG-PTPσ Activates Autophagy Flux and Lysosome Fusion, Aids Axon and Synaptic Reorganization in Spinal Cord Injury
Molecular Neurobiology (2024)
-
Identification of the growth cone as a probe and driver of neuronal migration in the injured brain
Nature Communications (2024)
-
New insights into glial scar formation after spinal cord injury
Cell and Tissue Research (2022)
-
Chondroitin 6-sulphate is required for neuroplasticity and memory in ageing
Molecular Psychiatry (2021)