Abstract
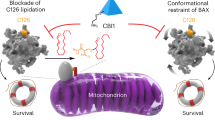
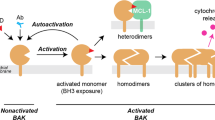
BAX is a critical effector of the mitochondrial cell death pathway in response to a diverse range of stimuli in physiological and disease contexts. Upon binding by BH3-only proteins, cytosolic BAX undergoes conformational activation and translocation, resulting in mitochondrial outer-membrane permeabilization. Efforts to rationally target BAX and develop inhibitors have been elusive, despite the clear therapeutic potential of inhibiting BAX-mediated cell death in a host of diseases. Here, we describe a class of small-molecule BAX inhibitors, termed BAIs, that bind directly to a previously unrecognized pocket and allosterically inhibit BAX activation. BAI binding around the hydrophobic helix α5 using hydrophobic and hydrogen bonding interactions stabilizes key areas of the hydrophobic core. BAIs inhibit conformational events in BAX activation that prevent BAX mitochondrial translocation and oligomerization. Our data highlight a novel paradigm for effective and selective pharmacological targeting of BAX to enable rational development of inhibitors of BAX-mediated cell death.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data generated or analyzed during the study and included in this published article are available from the corresponding author on reasonable request.
References
Fuchs, Y. & Steller, H. Programmed cell death in animal development and disease. Cell 147, 742–758 (2011).
Whelan, R. S., Kaplinskiy, V. & Kitsis, R. N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 72, 19–44 (2010).
Bredesen, D. E., Rao, R. V. & Mehlen, P. Cell death in the nervous system. Nature 443, 796–802 (2006).
Panganiban, R. A., Snow, A. L. & Day, R. M. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int. J. Mol. Sci. 14, 15931–15958 (2013).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 (2008).
Chipuk, J. E., Moldoveanu, T., Llambi, F., Parsons, M. J. & Green, D. R. The BCL-2 family reunion. Mol. Cell 37, 299–310 (2010).
Shamas-Din, A., Kale, J., Leber, B. & Andrews, D. W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 5, a008714 (2013).
Moldoveanu, T., Follis, A. V., Kriwacki, R. W. & Green, D. R. Many players in BCL-2 family affairs. Trends. Biochem. Sci. 39, 101–111 (2014).
Luna-Vargas, M. P. & Chipuk, J. E. The deadly landscape of pro-apoptotic BCL-2 proteins in the outer mitochondrial membrane. FEBS. J. 283, 2676–2689 (2016).
Tait, S. W. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632 (2010).
Edlich, F. et al. Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 (2011).
Garner, T. P. et al. An autoinhibited dimeric form of BAX regulates the BAX activation pathway. Mol. Cell 63, 485–497 (2016).
Dewson, G. & Kluck, R. M. Mechanisms by which bak and bax permeabilise mitochondria during apoptosis. J. Cell Sci. 122, 2801–2808 (2009).
Whelan, R. S. et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc. Natl Acad. Sci. USA 109, 6566–6571 (2012).
Karch, J. et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife 2, e00772 (2013).
Perez, G. I. et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 21, 200–203 (1999).
Libby, R. T. et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS. Genet. 1, 17–26 (2005).
Ben-Ari, Z. et al. Bax ablation protects against hepatic ischemia/reperfusion injury in transgenic mice. Liver Transpl. 13, 1181–1188 (2007).
Hochhauser, E. et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem. Biophys. 47, 11–20 (2007).
Garner, T. P., Lopez, A., Reyna, D. E., Spitz, A. Z. & Gavathiotis, E. Progress in targeting the BCL-2 family of proteins. Curr. Opin. Chem. Biol. 39, 133–142 (2017).
Ashkenazi, A., Fairbrother, W. J., Leverson, J. D. & Souers, A. J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug. Discov. 16, 273–284 (2017).
Bombrun, A. et al. 3,6-Dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J. Med. Chem. 46, 4365–4368 (2003).
Hetz, C. et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 280, 42960–42970 (2005).
Peixoto, P. M., Ryu, S. Y., Bombrun, A., Antonsson, B. & Kinnally, K. W. MAC inhibitors suppress mitochondrial apoptosis. Biochem. J. 423, 381–387 (2009).
Niu, X. et al. A small-molecule inhibitor of Bax and Bak oligomerization prevents genotoxic cell death and promotes neuroprotection. Cell Chem. Biol. 24, 493–506.e5 (2017).
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Czabotar, P. E. et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 (2013).
Barclay, L. A. et al. Inhibition of pro-apoptotic BAX by a noncanonical interaction mechanism. Mol. Cell 57, 873–886 (2015).
Ma, J. et al. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl Acad. Sci. USA 109, 20901–20906 (2012).
Gavathiotis, E., Reyna, D. E., Bellairs, J. A., Leshchiner, E. S. & Walensky, L. D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol. 8, 639–645 (2012).
Reyna, D. E. et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505.e10 (2017).
Yethon, J. A., Epand, R. F., Leber, B., Epand, R. M. & Andrews, D. W. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 278, 48935–48941 (2003).
Suzuki, M., Youle, R. J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654 (2000).
Pritz, J. R. et al. Allosteric sensitization of proapoptotic BAX. Nat. Chem. Biol. 13, 961–967 (2017).
Deschamps, M. L., Pilka, E. S., Potts, J. R., Campbell, I. D. & Boyd, J. Probing protein-peptide binding surfaces using charged stable free radicals and transverse paramagnetic relaxation enhancement (PRE). J. Biomol. NMR 31, 155–160 (2005).
Gavathiotis, E., Reyna, D. E., Davis, M. L., Bird, G. H. & Walensky, L. D. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell 40, 481–492 (2010).
Kim, H. et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol. Cell 36, 487–499 (2009).
Zhao, G. et al. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol. Cell Biol. 34, 1198–1207 (2014).
Uchime, O. et al. Synthetic antibodies inhibit Bcl-2-associated X protein (BAX) through blockade of the N-terminal activation site. J. Biol. Chem. 291, 89–102 (2016).
Leshchiner, E. S., Braun, C. R., Bird, G. H. & Walensky, L. D. Direct activation of full-length proapoptotic BAK. Proc. Natl Acad. Sci. USA 110, E986–E995 (2013).
Iyer, S. et al. Identification of an activation site in Bak and mitochondrial Bax triggered by antibodies. Nat. Commun. 7, 11734 (2016).
Follis, A. V. et al. Regulation of apoptosis by an intrinsically disordered region of Bcl-xL. Nat. Chem. Biol. 14, 458–465 (2018).
Follis, A. V. et al. PUMA binding induces partial unfolding within BCL-xL to disrupt p53 binding and promote apoptosis. Nat. Chem. Biol. 9, 163–168 (2013).
Lee, S. et al. Allosteric inhibition of antiapoptotic MCL-1. Nat. Struct. Mol. Biol. 23, 600–607 (2016).
Hwang, R. L. & Shaka, A. J. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. A 112, 275–279 (1995).
Mayer, M. & Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Edn Engl. 38, 1784–1788 (1999).
Hajduk, P. J., Meadows, R. P. & Fesik, S. W. Discovering high-affinity ligands for proteins. Science 278, 497–499 (1997).
Marintchev, A., Frueh, D. & Wagner, G. NMR methods for studying protein-protein interactions involved in translation initiation. Methods Enzymol. 430, 283–331 (2007).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Halgren, T. A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 49, 377–389 (2009).
Marsh, J. J. et al. Structural insights into fibrinogen dynamics using amide hydrogen/deuterium exchange mass spectrometry. Biochemistry 52, 5491–5502 (2013).
Kuzmic, P. Program DYNAFIT for the analysis of enzyme kinetic data: application to HIV proteinase. Anal. Biochem. 237, 260–273 (1996).
Acknowledgements
We thank B. Agianian and A. Haimowitz for assistance with MST controls and BAX C62S/C126S/S3C mutant preparation. Studies were supported by an American Heart Association Collaborative Science Award (15CSA26240000) to E.G. and R.N.K. Support was also provided by NIH award 1R01CA178394 to E.G. and the Fondation Leducq Transatlantic Network of Excellence grant (RA15CVD04) to E.G. and R.N.K. E.G. is supported by the Pershing Square Sohn Cancer Research Alliance and the Irma T. Hirschl Trust Career Award. NMR data were collected with support from NIH awards 1S10OD016305, P30 CA013330 a grant from NYSTAR.
Author information
Authors and Affiliations
Contributions
T.P.G. performed NMR, biochemical and molecular modeling studies. D.E.R. performed cell-based studies, and S.L. performed mass spectrometry studies. D.A. and R.N.K. performed cell-based studies. E.G. conceived the study, designed experiments and wrote the manuscript, which was edited by all authors.
Corresponding author
Ethics declarations
Competing interests
E.G., R.N.K., T.P.G, and D.A. are inventors on a patent application PCT/US2018/021644 submitted by Albert Einstein College of Medicine that covers compounds, compositions and methods for BAX inhibition for the treatment of diseases and disorders.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1, Supplementary Figures 1–21
Rights and permissions
About this article
Cite this article
Garner, T.P., Amgalan, D., Reyna, D.E. et al. Small-molecule allosteric inhibitors of BAX. Nat Chem Biol 15, 322–330 (2019). https://doi.org/10.1038/s41589-018-0223-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0223-0
This article is cited by
-
Covalent inhibition of pro-apoptotic BAX
Nature Chemical Biology (2024)
-
Mechanisms of BCL-2 family proteins in mitochondrial apoptosis
Nature Reviews Molecular Cell Biology (2023)
-
TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models
Nature Communications (2023)
-
Chemical modulation of cytosolic BAX homodimer potentiates BAX activation and apoptosis
Nature Communications (2023)
-
Apoptotic stress causes mtDNA release during senescence and drives the SASP
Nature (2023)