Abstract
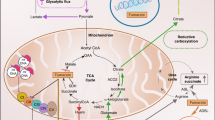
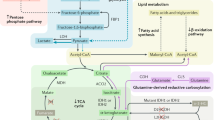
Hereditary cancer disorders often provide an important window into novel mechanisms supporting tumor growth. Understanding these mechanisms thus represents a vital goal. Toward this goal, here we report a chemoproteomic map of fumarate, a covalent oncometabolite whose accumulation marks the genetic cancer syndrome hereditary leiomyomatosis and renal cell carcinoma (HLRCC). We applied a fumarate-competitive chemoproteomic probe in concert with LC–MS/MS to discover new cysteines sensitive to fumarate hydratase (FH) mutation in HLRCC cell models. Analysis of this dataset revealed an unexpected influence of local environment and pH on fumarate reactivity, and enabled the characterization of a novel FH-regulated cysteine residue that lies at a key protein–protein interface in the SWI-SNF tumor-suppressor complex. Our studies provide a powerful resource for understanding the covalent imprint of fumarate on the proteome and lay the foundation for future efforts to exploit this distinct aspect of oncometabolism for cancer diagnosis and therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the identifiers PXD009378 (Supplementary Datasets 1–4) and PXD009202 (Supplementary Dataset 5). All of the data are accessible in the supplemental data sets (Supplementary Datasets 1–7) and can further be explored using our web-based resource (https://ccr2.cancer.gov/resources/Cbl/proteomics/fumarate).
References
Launonen, V. et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc. Natl Acad. Sci. USA 98, 3387–3392 (2001).
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 (2002).
Meier, J. L. Metabolic mechanisms of epigenetic regulation. ACS. Chem. Biol. 8, 2607–2621 (2013).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Pollard, P. J. et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 (2005).
MacKenzie, E. D. et al. Cell-permeating α-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol. Cell. Biol. 27, 3282–3289 (2007).
Sciacovelli, M. et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547 (2016).
Alderson, N. L. et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch. Biochem. Biophys. 450, 1–8 (2006).
Sullivan, L. B. et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 (2013).
Sudarshan, S. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 29, 4080–4090 (2009).
Kinch, L., Grishin, N. V. & Brugarolas, J. Succination of Keap1 and activation of Nrf2-dependent antioxidant pathways in FH-deficient papillary renal cell carcinoma type 2. Cancer Cell 20, 418–420 (2011).
Bardella, C. et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J. Pathol. 225, 4–11 (2011).
Piroli, G. G. et al. Succination is increased on select proteins in the brainstem of the NADH dehydrogenase (ubiquinone) Fe-S protein 4 (Ndufs4) knockout mouse, a model of leigh syndrome. Mol. Cell. Proteomics 15, 445–461 (2016).
Adam, J. et al. Fumarate hydratase deletion in pancreatic β cells leads to progressive diabetes. Cell Rep. 20, 3135–3148 (2017).
Ternette, N. et al. Inhibition of mitochondrial aconitase by succination in fumarate hydratase deficiency. Cell Rep. 3, 689–700 (2013).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Schmidt, T. J., Ak, M. & Mrowietz, U. Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine–preparation of S-substituted thiosuccinic acid esters. Bioorg. Med. Chem. 15, 333–342 (2007).
Blewett, M. M. et al. Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Sci. Signal. 9, rs10 (2016).
Zhou, Y. et al. Chemoproteomic strategy to quantitatively monitor transnitrosation uncovers functionally relevant S-nitrosation sites on cathepsin D and HADH2. CellChem. Biol. 23, 727–737 (2016).
Yang, Y. et al. UOK 262 cell line, fumarate hydratase deficient (FH – /FH –) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet. Cytogenet. 196, 45–55 (2010).
Zheng, L. et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 6, 6001 (2015).
Bar-Peled, L. et al. Chemical proteomics identifies druggable vulnerabilities in a genetically defined cancer. Cell 171, 696–709.e623 (2017).
Poole, L. B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 80, 148–157 (2015).
Wang, C., Weerapana, E., Blewett, M. M. & Cravatt, B. F. A chemoproteomic platform to quantitatively map targets of lipid-derived electrophiles. Nat. Methods 11, 79–85 (2014).
Zengeya, T. T. et al. Co-opting a bioorthogonal reaction for oncometabolite Detection. J. Am. Chem. Soc. 138, 15813–15816 (2016).
Reisz, J. A., Bechtold, E., King, S. B., Poole, L. B. & Furdui, C. M. Thiol-blocking electrophiles interfere with labeling and detection of protein sulfenic acids. FEBS. J. 280, 6150–6161 (2013).
Doorn, J. A. & Petersen, D. R. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 15, 1445–1450 (2002).
Winterbourn, C. C. & Hampton, M. B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561 (2008).
Reva, B., Antipin, Y. & Sander, C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 39, e118 (2011).
DelBove, J. et al. Identification of a core member of the SWI/SNF complex, BAF155/SMARCC1, as a human tumor suppressor gene. Epigenetics. 6, 1444–1453 (2011).
Brugarolas, J. PBRM1 and BAP1 as novel targets for renal cell carcinoma. Cancer J. 19, 324–332 (2013).
Sohn, D. H. et al. SRG3 interacts directly with the major components of the SWI/SNF chromatin remodeling complex and protects them from proteasomal degradation. J. Biol. Chem. 282, 10614–10624 (2007).
Yan, L., Xie, S., Du, Y. & Qian, C. Structural insights into BAF47 and BAF155 complex formation. J. Mol. Biol. 429, 1650–1660 (2017).
Keppler, B. R. & Archer, T. K. Ubiquitin-dependent and ubiquitin-independent control of subunit stoichiometry in the SWI/SNF complex. J. Biol. Chem. 285, 35665–35674 (2010).
Knutson, S. K. et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl Acad. Sci. USA 110, 7922–7927 (2013).
Harmel, R. & Fiedler, D. Features and regulation of non-enzymatic post-translational modifications. Nat. Chem. Biol. 14, 244–252 (2018).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Erez, A. & DeBerardinis, R. J. Metabolic dysregulation in monogenic disorders and cancer - finding method in madness. Nat. Rev. Cancer 15, 440–448 (2015).
Yang, J., Gupta, V., Carroll, K. S. & Liebler, D. C. Site-specific mapping and quantification of protein S-sulphenylation in cells. Nat. Commun. 5, 4776 (2014).
Strelko, C. L. et al. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 133, 16386–16389 (2011).
Kulkarni, R. A. et al. Discovering targets of non-enzymatic acylation by thioester reactivity profiling. Cell Chem. Biol. 24, 231–242 (2017).
Wagner, G. R. et al. A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell. Metab. 25, 823–837.e828 (2017).
Frizzell, N., Thomas, S. A., Carson, J. A. & Baynes, J. W. Mitochondrial stress causes increased succination of proteins in adipocytes in response to glucotoxicity. Biochem. J. 445, 247–254 (2012).
Agaimy, A. et al. SWI/SNF protein expression status in fumarate hydratase-deficient renal cell carcinoma: immunohistochemical analysis of 32 tumors from 28 patients. Hum. Pathol. 77, 139–146 (2018).
Reddie, K. G. & Carroll, K. S. Expanding the functional diversity of proteins through cysteine oxidation. Curr. Opin. Chem. Biol. 12, 746–754 (2008).
Li, R. & Kast, J. Biotin switch assays for quantitation of reversible cysteine Oxidation. Methods Enzymol. 585, 269–284 (2017).
Jost, M. & Weissman, J. S. CRISPR approaches to small molecule target identification. ACS. Chem. Biol. 13, 366–375 (2018).
Candiano, G. et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 25, 1327–1333 (2004).
Eng, J. K., McCormack, A. L. & Yates, J. R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass. Spectrom. 5, 976–989 (1994).
Tabb, D. L., McDonald, W. H. & Yates, J. R. III. DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J. Proteome. Res. 1, 21–26 (2002).
Bak, D. W., Pizzagalli, M. D. & Weerapana, E. Identifying functional cysteine residues in the mitochondria. ACS. Chem. Biol. 12, 947–957 (2017).
Lee, B. & Richards, F. M. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55, 379–400 (1971).
Florens, L. & Washburn, M. P. Proteomic analysis by multidimensional protein identification technology. Methods Mol. Biol. 328, 159–175 (2006).
Zhang, Y., Wen, Z., Washburn, M. P. & Florens, L. Effect of dynamic exclusion duration on spectral count based quantitative proteomics. Anal. Chem. 81, 6317–6326 (2009).
Zhang, Y., Wen, Z., Washburn, M. P. & Florens, L. Improving proteomics mass accuracy by dynamic offline lock mass. Anal. Chem. 83, 9344–9351 (2011).
Xu, T. et al. ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteomics. 129, 16–24 (2015).
Zhang, Y., Wen, Z., Washburn, M. P. & Florens, L. Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal. Chem. 82, 2272–2281 (2010).
Anders, S., Pyl, P. T. & Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Alexander Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2012).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
The authors thank C. Grose (Protein Expression Laboratory) for cloning and preparation of plasmid DNA, T. Archer (NIEHS) for the gift of the SMARCC1 and SNF5 plasmids, B. Weinberg (MIT) for the gift of the pLKO.1 puro plasmid (Addgene plasmid # 8453), A. Roberts, J. Garlick, and T. Zengeya (NCI) for assisting with preliminary studies. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (ZIA BC011488-05, ZIA BC011038-10) and the CCR FLEX Program. Support for E.W. was provided by the NIH (1R01GM117004 and 1R01GM118431-01A1).
Author information
Authors and Affiliations
Contributions
R.A.K., D.W.B., E.W. and J.L.M. designed experiments. R.A.K. and D.W.B. performed all chemical proteomic labeling and enrichment experiments. D.W.B. and E.W. performed all LC–MS/MS studies and relative stoichiometry analyses of IA-alkyne enrichment experiments. R.A.K. and A.E.B. synthesized compounds. R.A.K., S.E.B. and J.H.S. performed all cell-based analyses and co-immunoprecipitation experiments. C.A.B. assisted with cell-based analyses and performed S-succination reversibility studies. J.L.M., D.W.B., A.L.T. and R.A.K. performed bioinformatics analyses. D.W. and W.M.L. performed HLRCC spheroid growth inhibition studies and assisted with SWI–SNF analyses. A.A. and E.C.D. performed glycerol gradient fractionation analysis of the SWI–SNF complex and SNF5-dependent gene expression in HLRCC cell lines. N.F. provided the S-succination antibody and literature analysis. W.M.L. and D.R.C. provided HLRCC cell lines and advised experimental design. M.P.W., L.F. and M.L. performed whole-proteome MudPIT S-succination analyses of HLRCC cells. R.A.K. and J.L.M. analyzed data and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10
Supplementary Note 1
Synthetic Procedures
Supplementary Dataset 1
FH-regulated cysteines identified by comparative profiling of FH−/− HLRCC cell line (UOK262) and a FH+/+ rescue HLRCC cell line (UOK262WT); n = 3 independent experiments.
Supplementary Dataset 2
Compiled list of S-succinated cysteine residues previously characterized in the literature, and annotation with chemoproteomic data (if available).
Supplementary Dataset 3
Sequences used for motif analysis, as well as results for analyses of conservation based functional impact (FI), gene ontology (GO), and genomic lesions found in covalent fumarate targets in kidney cancer.
Supplementary Dataset 4
Fumarate-sensitive cysteines identified by competitive profiling of HEK-293 cells treated and untreated with fumarate (1 mM); n = 4 independent experiments.
Supplementary Dataset 5
Peptides identified as targets of S-succination in MudPIT LC–MS/MS analyses of HLRCC cell (UOK262 and UOK268) proteomes.
Supplementary Dataset 6
Analysis of transcripts co-regulated by FH and SNF5 in publicly accessible RNA-seq datasets.
Supplementary Dataset 7
Solvent-exposed surface area analysis of FH-regulated (Supplementary Dataset 1), exogenous fumarate sensitive (Supplementary Dataset 4), and hyperreactive cysteines.
Rights and permissions
About this article
Cite this article
Kulkarni, R.A., Bak, D.W., Wei, D. et al. A chemoproteomic portrait of the oncometabolite fumarate. Nat Chem Biol 15, 391–400 (2019). https://doi.org/10.1038/s41589-018-0217-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0217-y
This article is cited by
-
Metabolic alterations in hereditary and sporadic renal cell carcinoma
Nature Reviews Nephrology (2024)
-
Blocking reverse electron transfer-mediated mitochondrial DNA oxidation rescues cells from PANoptosis
Acta Pharmacologica Sinica (2024)
-
EZH1/2 as targets for cancer therapy
Cancer Gene Therapy (2023)
-
Learning the metabolic language of cancer
Nature (2023)
-
Fumarate suppresses B-cell activation and function through direct inactivation of LYN
Nature Chemical Biology (2022)